Introduction
According to the World Health Organization (WHO)
classification of digestive tract tumors, neuroendocrine
cell-derived tumors are frequently encountered in the lung,
digestive tract and pancreas and are roughly classified into
neuroendocrine tumor (NET) and neuroendocrine carcinoma (NEC) based
on proliferation kinetics (1). NECs
arising from the uterine endometrium account for <1% of all
uterine endometrial carcinomas. Hematogenous/lymphogenous
metastasis occurs early during the course of this disease and the
prognosis is poor (2). Large-cell
NEC (LCNEC) arising from the uterine endometrium is particularly
rare, with only 15 cases reported from 2000 onwards by Medline. We
herein report the case of a LCNEC patient exhibiting rapid disease
progression.
Case report
The patient was a 52-year-old woman, gravida 2 para
2, with a medical history of endometriosis, who had been followed
up for myoma every 6 months for several years. The patient sought
medical advice due to lower abdominal pain persisting for 1 month,
with rapid uterine enlargement. Suspecting a malignant tumor of the
uterine corpus, the patient was referred to the Department of
Obstetrics and Gynecology of Wakayama Medical University Hospital
(Wakayama, Japan).
On physical examination, the uterus had enlarged to
the size of a newborn's head with associated tenderness on
palpation, but no postmenopausal genital bleeding was observed.
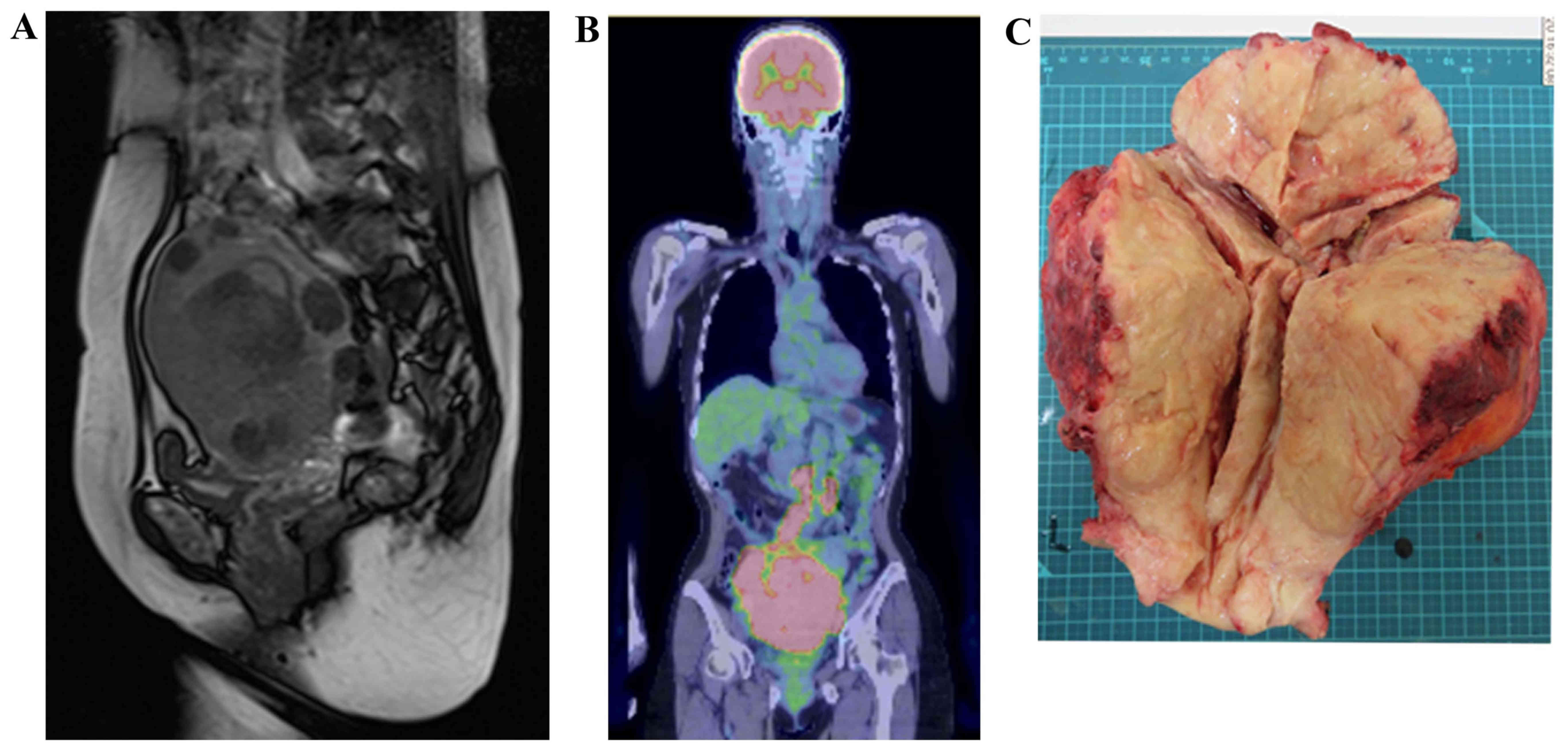
Transvaginal ultrasonography and a magnetic resonance imaging (MRI)
scan revealed irregular edema and marked hypertrophy of the uterine
muscle layer, which was suspected to be carcinoma or sarcoma of the
uterine corpus (Fig. 1A). On
fluorodeoxyglucose (FDG)-positron emission tomography
(PET)/computed tomography (CT) examination, FDG uptake was
increased in the uterus, bilateral ovaries and lymph nodes, from
the pelvic to the para-aortic nodes (Fig. 1B). The histopathological diagnosis
following endometrial biopsy was malignancy and the differential
diagnosis included small-cell (SC) and poorly differentiated
carcinoma, but a definitive diagnosis was difficult to make. The
serum level of lactate dehydrogenase was 1,213 IU/ml (normal range,
106–220 IU/l), of neuron-specific enolase (NSE) 84.6 ng/ml (normal
range, <10 ng/ml), of carbohydrate antigen (CA)-125 158.8 U/ml
(normal range, 0–35 U/ml), of carcinoembryonic antigen 33.3 ng/ml
(normal range, 0–5 ng/ml) and of CA19-9 72.6 U/ml (normal range,
0–37 U/ml). The levels of pro-gastrin-releasing peptide were
normal. Suspecting uterine sarcoma, carcinosarcoma or poorly
differentiated carcinoma, surgery was considered to be the
treatment of choice. The uterus was found to be enlarged and the
serosal surface was collapsed. In addition, the lymph nodes from
the pelvic to the para-aortic region were enlarged to 3 cm, in a
beaded pattern. Abdominal total hysterectomy, bilateral
salpingo-oophorectomy, pelvic lymph node dissection and para-aortic
lymph node biopsy were performed. The excised uterus weighed 1,500
g and the tumor extensively infiltrated the cervix, parametrium and
bilateral ovaries (Fig. 1C). On
histopathological examination, the lesions were accompanied by
extensive necrosis on hematoxylin and eosin staining, and tumor
cells with a high nuclear/cytoplasmic (N/C) ratio proliferated
forming mostly solid lesions (Fig.
2A). The nucleoplasm was pale, mitoses were abundant, and the
N/C ratio was lower compared with that of the SC type, suggesting
the LC type tumor (Fig. 2B). On
immunostaining, the tumor cells were positive for the
neuroendocrine markers synaptophysin, chromogranin A and CD56
(Fig. 2C-E). Based on these
findings, the tumor was diagnosed as LCNEC, International
Federation of Gynecology and Obstetrics stage IIIC2, pT3bN1M0.
Concurrent chemoradiotherapy (CCRT) was initiated 4
weeks after surgery. Chemotherapy was performed according to the
treatment applied for SC carcinoma of the lung: Irinotecan 60
mg/m2 on days 1, 8 and 15 and cisplatin 60
mg/m2 on day 1 every 4 weeks. External radiation
(intensity-modulated radiation therapy) was applied to the entire
pelvis over the para-aortic lymph nodes. Tumor lesions sized 2 cm
were observed in the vaginal stump 1 month after surgery and
expansions of peritoneal dissemination and lymph node metastases
were identified on CT. These lesions markedly shrank or disappeared
after treatment initiation, and tumor markers became negative
(Fig. 3). However, the tumor marker
levels gradually re-increased, whereas new lymph node metastases
were observed above the splenic hilum located outside the
irradiated region 136 days after surgery. Additional radiation of
the cervix and chest resulted in markedly decreased size of the
lesions, but cervicothoracic spinal metastases and paracardiac
lymph node metastases developed. As renal dysfunction (increased
serum creatinine level) developed after 4 cycles of chemotherapy,
one cycle of the paclitaxel (175 mg/m2) and carboplatin
(area under the curve 5) chemotherapy was administered. However,
continuation of active treatment was difficult due to the
development of severe neutropenia and thrombocytopenia: Therefore,
palliative radiation therapy of the cervicothoracic spine was
performed. Due to the lymph node metastasis in the upper abdominal
region, endoscopic biliary stenting was performed to relieve
jaundice. The patient succumbed to the disease 309 days after
surgery.
The patient provided informed consent to publication
of the details of this case and associated images.
Discussion
LCNEC originates from the lungs in the majority of
the cases. In the gynecological field, the primary region is
generally the uterine cervix and ovary (3–5). NETs of
the endometrium include low-grade carcinoid tumors and high-grade
SCNEC and LCNEC (2). Most cases of
NEC of the endometrium are SCNECs, with ~90 cases reported to date
(6). LCNEC arising from the
endometrium is rare, and was initially reported by Erhan et
al (7). Following a search
through Medline, only 15 cases were found to be reported from 2,000
onwards (3–12) (Table
I), and in almost all the cases the cancer was advanced. The
mean age of the patients was 60 years and the main complaint was
abnormal genital bleeding, similar to other endometrial carcinomas.
However, some cases were only diagnosed due to pain caused by
metastatic lesions. In the majority of the cases the tumor deeply
infiltrated the uterine muscle layer, and ~50% of the cases were
discovered at an advanced stage (6).
No diagnostic criteria of LCNEC of the endometrium have been
proposed to date. According to the WHO classification of pulmonary
tumors, LCNEC is defined as large-cell carcinoma with NET
morphology expressing the neuroendocrine markers synaptophysin,
chromogranin A and/or CD56 (13).
LCNEC is not included in the classification of the General Rules
for Clinical and Pathological Management of Uterine Corpus Cancer
in Japan (14).
Clinicopathologically, the morphology of LCNEC is similar to that
of SC carcinoma and LCNEC developing in the lungs, and in
approximately half of the cases it coexists with endometrioid
adenocarcinoma observed as part of carcinosarcoma (4). LCNEC complicated by lesions with a
different histological diagnosis was also observed. The
histological characteristics of LCNEC of the endometrium include
cells with abundant cytoplasm, microgranular chromatin, clear
nucleolus, polymorphism with various sizes, multinucleated cells,
large tumor cells, ≥10 mitoses/high-power field, map-like
hemorrhage and necrosis, island-like or columnar tumor cell
aggregates and vascular invasion (7,8). On
immunostaining, the tumor cells stained positive for synaptophysin,
chromogranin A, CD56, p53 and NSE, confirming the diagnosis of
LCNEC (15–18).
 | Table I.Large-cell neuroendocrine tumor of the
endometrium. |
Table I.
Large-cell neuroendocrine tumor of the
endometrium.
| Case | Age (years) | Surgery | Stage | Histology | Treatment | IHC profile | Outcome (months) | (Refs.) |
|---|
| 1 | 52 | ATH, BSO | IC | Pure LCNEC | RT, CDDP, VP16 | NSE, SNP | DOD3 | (7) |
| 2 | 50 | ATH, BSO, OMT,
LN | IIIC | Pure LCNEC | RT, CDDP, VP16 | NSE, SNP | AWD12 | (3) |
| 3 | 80 | ATH, BSO, LN | IC | LCNEC +
endometrioid | None | NSE, CGA | DOD5 | (3) |
| 4 | 77 | ATH, BSO | IIB | LCNEC +
endometrioid | RT | NSE, SNP, CGA,
CD56 | DOD23 | (3) |
| 5 | 79 | ATH, BSO, OM | IIIA | LCNEC +
endometrioid | RT | NSE, CGA, CD56 | AWD2 | (3) |
| 6 | 88 | ATH, BSO, LN | IIIC | L/SCNEC +
endometrioid | RT | NSE, CGA, CD56 | AWD1 | (3) |
| 7 | 42 | RH | IC | Pure LCNEC | CDDP, VP-16 | SNP, CGA, CD56 | AWD9 | (4) |
| 8 | 59 | RH, BSO, OM,
PPALND | IIIB | LCNEC + serous | RT + CT
(unknown) | NSE, SNP, CD56 | NED5 | (11) |
| 9 | 40 | ATH, BSO, PLND,
OM | IB | LCNEC +
sarcomatoid | None | SNP, CD56 | NED26 | (5) |
| 10 | 70 | ATH, BSO, OM | IB | Pure LCNEC | CDDP, VP-16 | SNP, CGA, CD56 | NED6 | (12) |
| 11 | 59 | ATH, BSO, OM,
PPALND | IIIC2 | Pure LCNEC | CBDCA, PTX, RT, PLD,
CDDP, VP-16 | NSE, SNP, CGA,
CD56 | DOD12 | (10) |
| 12 | 73 | None | IVB | Pure LCNEC | None | NSE, SNP, CGA | DOD1 | (9) |
| 13 | 73 | ATH, BSO, OM,
PPALND | IIIC1 | Pure LCNEC | CDDP/CPT-11 | SNP, CGA, CD56 | AWD13 | (9) |
| 14 | 71 | RH, BSO, OM,
PPALND | IVB | Pure LCNEC | Planned CDDP,
VP-16 | SNP, CGA | DOD1 | (8) |
| 15 | 51 | RH, BSO, OM,
PPALND | IIIA | LCNEC +
endometrioid | CDDP/CPT-11 | SNP, CGA, CD56 | NED18 | (6) |
| 16 | 52 | ATH, BSO, PLND | IIIC2 | Pure LCNEC | RT, CDDP/CPT-11 | SNP, CGA, CD56 | DOD10 | Present case |
Specific imaging findings of uterine LCNEC have not
been reported to date. Makihara et al reported that the MRI
findings of LCNEC are similar to those of other poorly
differentiated endometrial cancers and sarcoma (9). In the present case, the preoperative
diagnosis of LCNEC based on MRI and PET/CT was difficult.
LCNEC of the endometrium is treated similar to other
endometrial carcinomas, i.e., surgical resection, radiation and
chemotherapy, as no standard therapy has been established to date
due to the small number of reported cases. The regimen is selected
based on LCNEC of other organs. Cases of LCNEC of the lungs and
digestive tract that responded to irinotecan and cisplatin therapy
(19) and pulmonary SC carcinoma
patients with a significant response to cisplatin and etoposide
therapy (20) have been reported. In
addition, cases treated with a combination of chemotherapy and an
octreotide similar to somatostatin were reported (10). Inhibition of tumor growth by
somatostatin analogues has been shown in animal models and human
tumor cell lines. Its mechanism is reported to be inhibition of
secretion of insulin-like growth factor-1, other tumor growth
factors and vascularization, and specific direct tumor inhibition
through the somatostatin receptor, which is strongly expressed in
NETs (10,21). Partial response was observed in a
SCNEC of the endometrium treated with octreotide (22), but other cases exhibited tumor
enlargement and adverse effects, such as repeated
hypo/hyperglycemia (10). Thus,
despite these investigations, LCNECs of the endometrium rapidly
progress and the prognosis is very poor. In the present case, the
tumor recurred 4 weeks after surgery. CCRT was initiated, but the
disease relapsed and metastasized; thus, continuation of active
treatment was no longer feasible and the patient succumbed to the
disease 309 days after surgery.
In conclusion, LCNEC of the endometrium is a very
rare, highly malignant tumor, which is difficult to diagnose as its
imaging and pathological findings are non-specific. The prognosis
of LCNEC is poor due to its rapid progression and lack of
established therapy. Designing an effective therapy protocol
through the accumulation and investigation of reported cases is
urgently required.
References
|
1
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: Nomenclature and classification of neuroendocrine
neoplasma of the digestive systemWHO Classification of Tumours of
the Digestive System. 4th. Lyon: IARC; pp. 13–14. 2010;
|
|
2
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
Organs. 4th. Lyon: IARC; pp. 1222014
|
|
3
|
Mulvany NJ and Allen DG: Combined large
cell neuroendocrine and endometrioid carcinoma of the endometrium.
Int J Gynecol Pathol. 27:49–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albores-Saavedra J, Martinez-Benitez B and
Luevano E: Small cell carcinomas and large cell neuroendocrine
carcinomas of the endometrium and cervix: Polypoid tumors and those
arising in polyps may have a favorable prognosis. Int J Gynecol
Pathol. 27:333–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terada T: Large cell neuroendocrine
carcinoma with sarcomatous changes of the endometrium: A case
report with immunohistochemical studies and molecular genetic study
of KIT and PDGFRA. Pathol Res Pract. 206:420–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto H, Nasu K, Kai K, Nishida M,
Narahara H and Nishida H: Combined large-cell neuroendocrine
carcinoma and endometrioid adenocarcinoma of the endometrium: A
case report and survey of related literature. J Obstet Gynaecol
Res. 42:206–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erhan Y, Dikmen Y, Yucebilgin MS, Zekioglu
O, Mgoyi L and Terek MC: Large cell neuroendocrine carcinoma of the
uterine corpus metastatic to brain and lung: Case report and review
of the literature. Eur J Gynaecol Oncol. 25:109–112.
2004.PubMed/NCBI
|
|
8
|
Nguyen ML, Han L, Minors AM,
Bentley-Hibbert S, Pradhan TS, Pua TL and Tedjarati SS: Rare large
cell neuroendocrine tumor of the endometrium: A case report and
review of the literature. Int J Surg Case Rep. 4:651–655. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makihara N, Maeda T, Nishimura M, Deguchi
M, Sonoyama A, Nakabayashi K, Kawakami F, Itoh T and Yamada H:
Large cell neuroendocrine carcinoma originating from the uterine
endometrium: A report on magnetic resonance features of 2 cases
with very rare and aggressive tumor. Rare Tumors. 4:e372012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shahabi S, Pellicciotta I, Hou J, Graceffa
S, Huang GS, Samuelson RN and Goldberg GL: Clinical utility of
chromogranin A and octreotide in large cell neuro endocrine
carcinoma of the uterine corpus. Rare Tumors. 3:e412011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Posligua L, Malpica A, Liu J, Brown J and
Deavers MT: Combined large cell neuroendocrine carcinoma and
papillary serous carcinoma of the endometrium with pagetoid spread.
Arch Pathol Lab Med. 132:1821–1824. 2008.PubMed/NCBI
|
|
12
|
Deodhar KK, Kerkar RA, Suryawanshi P,
Menon H and Menon S: Large cell neuroendocrine carcinoma of the
endometrium: An extremely uncommon diagnosis, but worth the
efforts. J Cancer Res Ther. 7:211–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Large cell neuroendocrine carcinomaWHO
Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th.
Lyon: IARC; pp. 69–72. 2015
|
|
14
|
Tsukamoto N: The general rules for
clinical and pathological management of uterine corpus cancer.
Nihon Rinsho. 10:285–289. 2004.(In Japanese).
|
|
15
|
Gilks CB, Young RH, Gersell DJ and Clement
PB: Large cell neuroendocrine [corrected] carcinoma of the uterine
cervix: A clinicopathologic study of 12 cases. Am J Surg Pathol.
21:905–914. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCluggage WG, Kennedy K and Busam KJ: An
immunohistochemical study of cervical neuroendocrine carcinomas:
Neoplasms that are commonly TTF1 positive and which may express
CK20 and P63. Am J Surg Pathol. 34:525–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krivak TC, McBroom JW, Sundborg MJ,
Crothers B and Parker MF: Large cell neuroendocrine cervical
carcinoma: A report of two cases and review of the literature.
Gynecol Oncol. 82:187–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato Y, Shimamoto T, Amada S, Asada Y and
Hayashi T: Large cell neuroendocrine carcinoma of the uterine
cervix: A clinicopathological study of six cases. Int J Gynecol
Pathol. 22:226–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanimoto H, Hamasaki A, Akimoto Y, Honda
H, Takao Y, Okamoto K, Teramoto M, Teramoto H, Kaneko M and Oshita
T: A case of large cell neuroendocrine carcinoma (LCNEC) of the
uterine cervix successfully treated by postoperative CPT-11+CDDP
chemotherapy after non-curative surgery. Gan To Kagaku Ryoho.
39:1439–1441. 2012.(In Japanese). PubMed/NCBI
|
|
20
|
Noda K, Nishiwaki Y, Kawahara M, Negoro S,
Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K and Tamura T:
Irinotecan plus cisplatin compared with etoposide plus cisplatin
for extensive small-cell lung cancer. N Engl J Med. 346:85–91.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scully RE, Aguirre P and DeLellis RA:
Argyrophilia, serotonin, and peptide hormones in the female genital
tract and its tumors. Int J Gynecol Pathol. 3:51–70. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verschraegen CF, Matei C, Loyer E, Malpica
A, Tornos C, Kudelka AP and Kavanagh JJ: Octreotide induced
remission of a refractory small cell carcinoma of the endometrium.
Int J Gynecol Cancer. 9:80–85. 1999. View Article : Google Scholar : PubMed/NCBI
|