Introduction
The life expectancy of young cancer patients has
significantly increased due to advances in the treatment of
malignant diseases. Thus, the focus of medical attention has
expanded to include improvements in the quality of life of patients
who have undergone cancer treatment. However, cytotoxic damage to
ovarian stromal cells and germ cells appears to be progressive and
irreversible (1–3) and chemotherapy occasionally exerts
detrimental and often unavoidable effects on ovarian function,
resulting in female sterility (1–3).
Compared with male patients, female patients are more susceptible
to gonadal toxicants due to the fact that, unlike men, women are
born with an irreplaceable supply of germ cells in their
ovaries.
Newly developed molecularly targeted agents, such as
monoclonal antibodies, have been utilized as adjuvant or
single-agent chemotherapy for over a decade. Bevacizumab, a
monoclonal antibody that targets vascular endothelial growth factor
(VEGF), is expected to become the first targeted agent to be
approved for the treatment of a wide array of malignancies,
including breast, ovarian, lung, colorectal and cervical cancer
(4–13). Targeted therapies are considered to
obtain a good antitumor response, without causing any major damage
to healthy angiogenic tissues. However, introduction of targeted
agents with anti-angiogenic properties may negatively affect
ovarian function in patients of reproductive age. The effects of
bevacizumab therapy on reproductive function have not been clearly
determined; however, it is crucial to elucidate this toxicity by
investigating the effects of anti-angiogenic agents at the
molecular level and what physiologically important roles these
processes play in healthy tissues (14). The aim of the present review was to
discuss the unintended effects of an anti-angiogenic agent,
bevacizumab, on ovarian function, and suggest strategies for the
treatment of women of reproductive age.
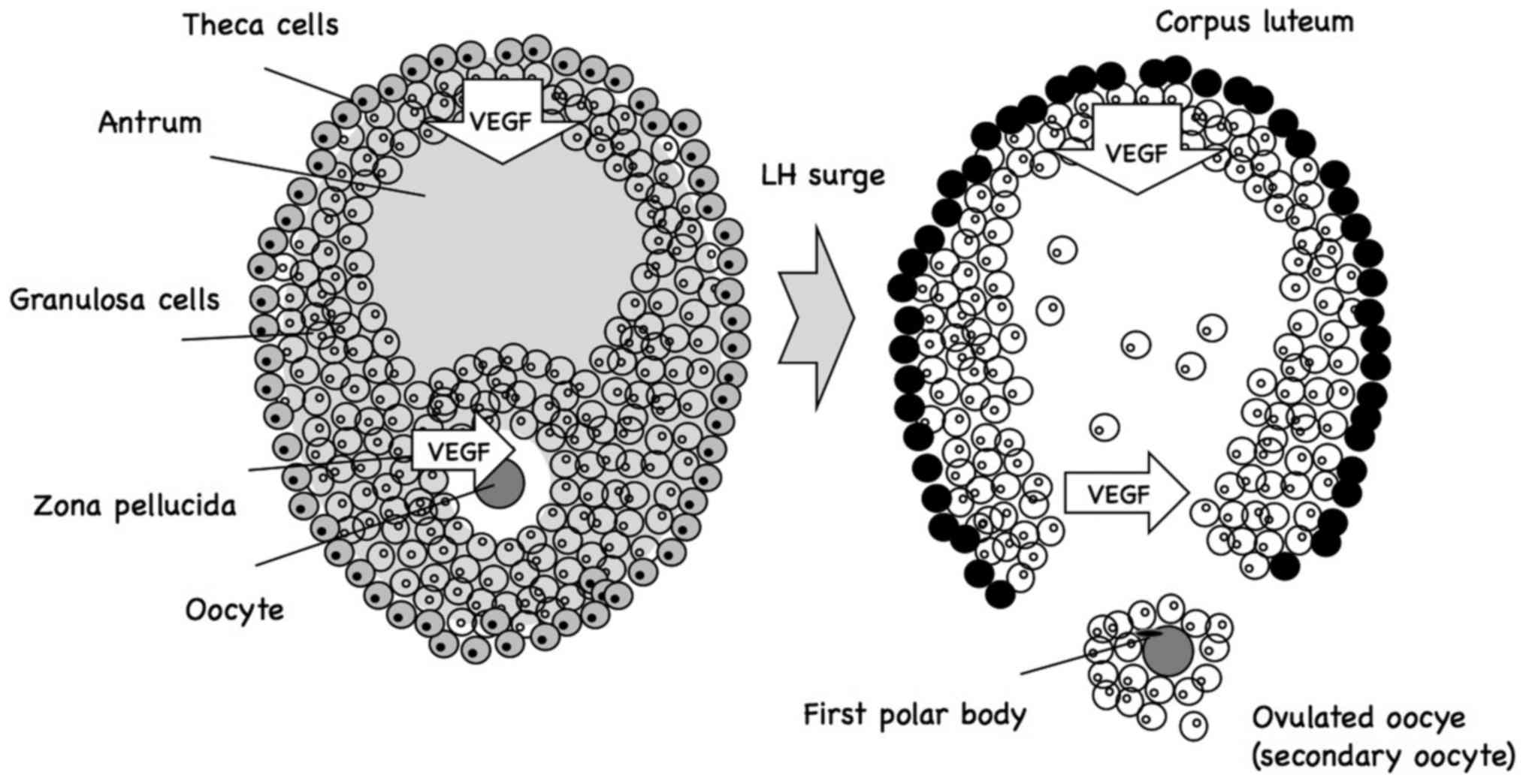
Follicular growth
Invertebrates as well as vertebrates have accessory
cells in the ovary surrounding the oocytes that help nourish
developing oocytes. These are ordinary somatic cells referred to as
follicle cells (Fig. 1), which form
an epithelial layer surrounding the oocyte, and are connected to
each other and to the oocyte via gap junctions, which allow
exchange of only small molecules, but not macromolecules (15,16).
Although follicle cells are prevented from providing the oocyte
with preformed macromolecules through these gap junctions, they are
able to supply smaller precursor molecules from which
macromolecules may be synthesized. Of note, the investigation of
gap junction communication in mammalian ovaries demonstrated that
the gap junction proteins involved in connecting follicle cells to
each other differ from those involved in connecting the follicle
cells to the oocytes (15,16). By disrupting the genes that encode
either of these proteins in mammals, both the follicle cells and
oocytes are prevented from normal development, causing female
sterility. Follicle cells secrete macromolecules that either
contribute to the oocyte coat, are taken up by receptor-mediated
endocytosis into the growing oocyte, or act on oocyte surface
receptors to control the spatial patterning and axial asymmetries
of the oocyte (17–19).
The communication between oocytes and their follicle
cells is bidirectional. Coordination of timing is crucial for the
developmental processes in the two sets of cells, which appear to
be dependent on signals from the oocyte to the follicle cells.
3. Oocyte maturation
The majority of the primary oocytes in female
newborns are surrounded by a single layer of follicle cells. Those
oocytes and their surrounding follicle cells are referred to as
primordial follicles (20–22). Before birth, a small portion of
primordial follicles begin to develop multiple layers of follicle
cells (granulosa cells) surrounding the growing oocyte. It remains
unknown what triggers certain primordial follicles to start growing
in this manner. Furthermore, some of these developing follicles
develop a fluid-filled cavity, or antrum, and are referred to as
antral follicles.
After puberty, approximately once a month, the
pituitary gland secretes a surge of follicle-stimulating hormone
(FSH), accelerating the development of ~10–12 antral follicles. One
of these antral follicles becomes dominant, and a surge in FSH and
luteinizing hormone induces ovulation towards the middle of the
menstrual cycle: The dominant primary oocyte completes meiosis I,
and the resulting secondary oocyte arrests at metaphase II; the
rapidly grown follicle then ruptures at the surface of the ovary,
releasing the secondary oocyte, which remains surrounded by a shell
of granulosa cells embedded in a hyaluronan-rich gel-like matrix
(Fig. 1) (20–23). The
released oocyte is triggered to complete meiosis II if fertilized
by a sperm within ~1 day.
VEGF and follicular growth
The reproductive system has a process of vascular
development termed angiogenesis. This development of new blood
vessels in the ovary is required for delivering necessary nutrients
and hormones to ensure follicular growth and formation of the
corpus luteum. Since preantral follicles have no vascular supply
system of their own, they have to depend on vessels in the
surrounding stroma (24,25). However, a vascular sheath consisting
of two capillary networks in the theca interna and externa is
developed within the thecal layer during antral development. These
newly formed ovarian blood vessels are used to supply an increased
level of gonadotropins, growth factors, oxygen, steroid precursors
and other substances to the growing follicle. The adequate increase
of vascular supply may be a rate-limiting step in the selection and
maturation process of the dominant follicle to be ovulated
(24,25). However, follicular atresia may be
caused by degeneration of the capillary bed in follicles that are
prevented from developing. Although the ovarian follicle as well as
the corpus luteum have been shown to produce some angiogenic
factors, VEGFA is considered to play an essential role in
regulating angiogenesis in the ovary (24,25).
Expression of VEGFA in ovarian follicles is determined by
follicular size, such that in bovine and porcine follicles, the
expression of VEGFA is weak during early ovarian follicular
development, becoming stronger in granulosa and theca cells as the
dominant follicle develops (26–28). The
findings were similar in the rat ovary, which also exhibited some
secondary follicles with extremely strong VEGFA immunoreactivity in
the zona pellucida (28).
VEGFA, a cytokine and homodimeric glycoprotein, has
been found in several preantral mammalian follicles, including
human (29,30). VEGFA functions as a regulator of
angiogenesis in the ovary through the action of its kinase insert
domain receptor (KDR; also referred to as fetal liver kinase 1 or
VEGFR2) (31,32). A study on mice demonstrated that
administering a KDR antibody acts as an inhibitor of
gonadotropin-dependent follicular angiogenesis, which in turn
impedes development of mature antral follicles (33). It was also observed that inhibition
of VEGFA with a VEGFA trap antagonist caused a reduction in
follicular angiogenesis and development, as well as a reduction in
VEGFR1 (also referred to as FLT1) and KDR expression in monkeys
(34). Therefore, ovulation and the
subsequent development and functional capacity of the corpus luteum
may be inhibited by intrafollicular injection of a VEGFA antagonist
(35).
Chemotherapy-induced ovarian damage
Several factors affect the rates of permanent
infertility and compromised fertility following cancer treatment.
Those factors include the drug or size/location of the radiation
field, dose, dose-intensity, method of administration (oral vs.
intravenous), disease, age and gender of the patient, combination
chemotherapy and pretreatment fertility status of the patient
(36–40). Older patients are at higher risk of
developing ovarian failure. Conversely, younger patients may expect
recovery of ovarian function in 30% of the cases at 6–48 months
after therapy (37–40). The reason why older patients are
clinically observed to be more affected by chemotherapy, is
possibly that older women naturally have a smaller ovarian reserve.
Therefore, it may be hypothesized that recovery of ovarian function
following cancer therapy may be associated with a significant
reduction in ovarian reserve.
Effect of bevacizumab on ovarian
function
Cancer treatment may temporarily or permanently
affect female fertility. It may also become apparent later as
premature ovarian failure (POF) (2,3,37). It should be noted that female
fertility may be compromised, even when there is maintenance or
resumption of cyclic menses. The presence of regular menstruation
is not a guarantee for normal fertility, as any reduction in
ovulatory reserve may reduce the likelihood of subsequent
conception and increase the risk of early menopause. Preserved
fertility following cancer treatment may be shortened by premature
menopause. Due to the risk of POF, these patients should not delay
childbearing (41–46). Even if regular menses and a normal
reproductive outcome are observed following chemotherapy, it should
not be taken as a certain indicator of survival of the ovarian
follicular reserve from treatment. Patients who have been exposed
to high-dose chemotherapy or radiotherapy are advised not to delay
childbearing for long once they recover from ovarian failure
(3,37). These patients are encouraged to
conceive within a few years of a disease-free period, but to avoid
pregnancy <6–12 months after treatment on growing oocytes
(38). Histological studies
demonstrated that chemotherapy on human ovarian tissue may result
in ovarian atrophy, with marked loss of primordial follicles
(23).
In view of the functions of anti-angiogenic agents
on normal physiological processes (14), it has become more evident that female
fertility may be temporarily or permanently affected. The half-life
of bevacizumab is estimated to be ~20 days (range, 10–50 days).
With doses of 1–20 mg/kg, either weekly or triweekly, drug
clearance is estimated to be 100 days (5 half-lives). Time analysis
of onset and resolution of the adverse effects of bevacizumab may
be found in the literature to a limited extent (47,48).
Even if bevacizumab induces ovarian damage, it is likely that this
damage is transient and disappears within the expected drug
clearance timeframe in the majority of the cases. However, frequent
and/or prolonged drug administration may further complicate the
anticipated toxicity.
Bevacizumab during pregnancy
The availability of data on the use of bevacizumab
on pregnant women is currently limited; however, due to its
anti-angiogenic and potentially damaging effects on fetal
development, its use on pregnant women should be avoided (49). Based on studies on pregnant women
exposed to chemotherapy prior to conception, no increase of
miscarriages or congenital abnormalities has been reported compared
with the general population. It is hypothesized that corrective
mechanisms within the oocyte may have taken place, or that
undetected miscarriages at a very early stage may have occurred due
to dominant lethal mutations, since these pregnancies occurred long
after treatment was completed (3,38,39).
Conclusion
Considering the importance of VEGF for
folliculogenesis and maturation of the oocyte, ovarian dysfunction
appears to be a plausible side effect of the angiogenic treatment.
The aim of using bevacizumab is obtaining a favorable antitumor
response, without damage to healthy tissues. The majority of
bevacizumab-induced side effects are expected to be transient and
eliminated within the anticipated drug clearance time frame;
however, fundamental investigations on these effects are required
to generate more evidence-based practice guidelines.
References
|
1
|
Imai A, Furui T and Yamamoto A:
Preservation of female fertility during cancer treatment. Reprod
Med Biol. 7:17–27. 2008. View Article : Google Scholar
|
|
2
|
Yap JK and Davies M: Fertility
preservation in female cancer survivors. J Obstet Gynaecol.
27:390–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maltaris T, Seufert R, Fischl F,
Schaffrath M, Pollow K, Koelbl H and Dittrich R: The effect of
cancer treatment on female fertility and strategies for preserving
fertility. Eur J Obstet Gynecol Reprod Biol. 130:148–155. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pham E, Yin M, Peters CG, Lee CR, Brown D,
Xu P, Man S, Jayaraman L, Rohde E, Chow A, et al: Preclinical
efficacy of bevacizumab with CRLX101, an investigational
nanoparticle-drug conjugate, in treatment of metastatic
triple-negative breast cancer. Cancer Res. 76:4493–4503. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneeweiss A, Förster F, Tesch H, Aktas
B, Gluz O, Geberth M, Hertz-Eichenrode MM, Schönegg W, Schumacher
C, Kutscheidt A, et al: First-line bevacizumab-containing therapy
for HER2-negative metastatic breast cancer: final results from a
prospective german study. Anticancer Res. 36:967–974.
2016.PubMed/NCBI
|
|
6
|
McClung EC and Wenham RM: Profile of
bevacizumab in the treatment of platinum-resistant ovarian cancer:
current perspectives. Int J Womens Health. 8:59–75. 2016.PubMed/NCBI
|
|
7
|
Marchetti C, de Felice F, Palaia I,
Musella A, Di Donato V, Gasparri ML, Musio D, Muzii L, Tombolini V
and Panici PB: Efficacy and toxicity of bevacizumab in recurrent
ovarian disease: an update meta-analysis on phase III trials.
Oncotarget. 7:13221–13227. 2016.PubMed/NCBI
|
|
8
|
Stratigos M, Matikas A, Voutsina A,
Mavroudis D and Georgoulias V: Targeting angiogenesis in small cell
lung cancer. Transl Lung Cancer Res. 5:389–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stinchcombe TE: Targeted therapies for
lung cancer. Cancer Treat Res. 170:165–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saltz LB: Bevacizumab in colorectal
cancer: it should have worked. Lancet Oncol. 17:1469–1470. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ilic I, Jankovic S and Ilic M: Bevacizumab
combined with chemotherapy improves survival for patients with
metastatic colorectal cancer: evidence from meta analysis. PLoS
One. 11:e01619122016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bizzarri N, Ghirardi V, Alessandri F,
Venturini PL, Menada M Valenzano, Rundle S, Maggiore U Leone
Roberti and Ferrero S: Bevacizumab for the treatment of cervical
cancer. Expert Opin Biol Ther. 16:407–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oaknin A, Rubio MJ, Redondo A, de Juan A,
Bañuelos JF Cueva, Gil-Martin M, Ortega E, Garcia-Arias A,
Gonzalez-Martin A and Bover I: SEOM guidelines for cervical cancer.
Clin Transl Oncol. 17:1036–1042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stone RL, Sood AK and Coleman RL:
Collateral damage: toxic effects of targeted antiangiogenic
therapies in ovarian cancer. Lancet Oncol. 11:465–475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunlop CE and Anderson RA: The regulation
and assessment of follicular growth. Scand J Clin Lab Invest Suppl.
244:13–17, discussion 17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsueh AJ, Kawamura K, Cheng Y and Fauser
BC: Intraovarian control of early folliculogenesis. Endocr Rev.
36:1–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Araújo VR, Duarte AB, Bruno JB, Lopes CA
Pinho and de Figueiredo JR: Importance of vascular endothelial
growth factor (VEGF) in ovarian physiology of mammals. Zygote.
21:295–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Byrne AM, Bouchier-Hayes DJ and Harmey JH:
Angiogenic and cell survival functions of vascular endothelial
growth factor (VEGF). J Cell Mol Med. 9:777–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dvorak HF: VPF/VEGF and the angiogenic
response. Semin Perinatol. 24:75–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Emori C and Sugiura K: Role of
oocyte-derived paracrine factors in follicular development. Anim
Sci J. 85:627–633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Field SL, Dasgupta T, Cummings M and Orsi
NM: Cytokines in ovarian folliculogenesis, oocyte maturation and
luteinisation. Mol Reprod Dev. 81:284–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hennet ML and Combelles CM: The antral
follicle: a microenvironment for oocyte differentiation. Int J Dev
Biol. 56:819–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Familiari G, Caggiati A, Nottola SA,
Ermini M, Di Benedetto MR and Motta PM: Ultrastructure of human
ovarian primordial follicles after combination chemotherapy for
Hodgkins disease. Hum Reprod. 8:2080–2087. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stouffer RL, Martínez-Chequer JC,
Molskness TA, Xu F and Hazzard TM: Regulation and action of
angiogenic factors in the primate ovary. Arch Med Res. 32:567–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamanini C and De Ambrogi M: Angiogenesis
in developing follicle and corpus luteum. Reprod Domest Anim.
39:206–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barboni B, Turriani M, Galeati G, Spinaci
M, Bacci ML, Forni M and Mattioli M: Vascular endothelial growth
factor production in growing pig antral follicles. Biol Reprod.
63:858–864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greenaway J, Connor K, Pedersen HG,
Coomber BL, LaMarre J and Petrik J: Vascular endothelial growth
factor and its receptor, Flk-1/KDR, are cytoprotective in the
extravascular compartment of the ovarian follicle. Endocrinology.
145:2896–2905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Celik-Ozenci C, Akkoyunlu G, Kayisli UA,
Arici A and Demir R: Localization of vascular endothelial growth
factor in the zona pellucida of developing ovarian follicles in the
rat: a possible role in destiny of follicles. Histochem Cell Biol.
120:383–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harata T, Ando H, Iwase A, Nagasaka T,
Mizutani S and Kikkawa F: Localization of angiotensin II, the AT1
receptor, angiotensin-converting enzyme, aminopeptidase A,
adipocyte-derived leucine aminopeptidase, and vascular endothelial
growth factor in the human ovary throughout the menstrual cycle.
Fertil Steril. 86:433–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Otani N, Minami S, Yamoto M, Shikone T,
Otani H, Nishiyama R, Otani T and Nakano R: The vascular
endothelial growth factor/fms-like tyrosine kinase system in human
ovary during the menstrual cycle and early pregnancy. J Clin
Endocrinol Metab. 84:3845–3851. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geva E and Jaffe RB: Role of vascular
endothelial growth factor in ovarian physiology and pathology.
Fertil Steril. 74:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geva E and Jaffe RB: Role of angiopoietins
in reproductive tract angiogenesis. Obstet Gynecol Surv.
55:511–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wulff C, Wilson H, Wiegand SJ, Rudge JS
and Fraser HM: Prevention of thecal angiogenesis, antral follicular
growth, and ovulation in the primate by treatment with vascular
endothelial growth factor Trap R1R2. Endocrinology. 143:2797–2807.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zimmermann RC, Hartman T, Kavic S, Pauli
SA, Bohlen P, Sauer MV and Kitajewski J: Vascular endothelial
growth factor receptor 2-mediated angiogenesis is essential for
gonadotropin-dependent follicle development. J Clin Invest.
112:659–669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hazzard TM, Xu F and Stouffer RL:
Injection of soluble vascular endothelial growth factor receptor 1
into the preovulatory follicle disrupts ovulation and subsequent
luteal function in rhesus monkeys. Biol Reprod. 67:1305–1312. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heath JA and Stern CJ: Fertility
preservation in children newly diagnosed with cancer: existing
standards of practice in Australia and New Zealand. Med J Aust.
185:538–541. 2006.PubMed/NCBI
|
|
37
|
Lee SJ, Schover LR, Partridge AH, Patrizio
P, Wallace WH, Hagerty K, Beck LN, Brennan LV and Oktay K: American
Society of Clinical Oncology: American Society of Clinical Oncology
recommendations on fertility preservation in cancer patients. J
Clin Oncol. 24:2917–2931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meirow D: Ovarian injury and modern
options to preserve fertility in female cancer patients treated
with high dose radio-chemotherapy for hemato-oncological neoplasias
and other cancers. Leuk Lymphoma. 33:65–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meirow D, Levron J, Eldar-Geva T, Hardan
I, Fridman E, Zalel Y, Schiff E and Dor J: Pregnancy after
transplantation of cryopreserved ovarian tissue in a patient with
ovarian failure after chemotherapy. N Engl J Med. 353:318–321.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schimmer AD, Quatermain M, Imrie K, Ali V,
McCrae J, Stewart AK, Crump M, Derzko C and Keating A: Ovarian
function after autologous bone marrow transplantation. J Clin
Oncol. 16:2359–2363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blumenfeld Z: Ovarian rescue/protection
from chemotherapeutic agents. J Soc Gynecol Investig. 8 Suppl
Proceedings:60–64. 2001. View Article : Google Scholar
|
|
42
|
Blumenfeld Z: Preservation of fertility
and ovarian function and minimalization of chemotherapy associated
gonadotoxicity and premature ovarian failure: the role of inhibin-A
and -B as markers. Mol Cell Endocrinol. 187:93–105. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blumenfeld Z, Dann E, Avivi I, Epelbaum R
and Rowe JM: Fertility after treatment for Hodgkins disease. Ann
Oncol. 13 Suppl 1:138–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Blumenfeld Z, Shapiro D, Shteinberg M,
Avivi I and Nahir M: Preservation of fertility and ovarian function
and minimizing gonadotoxicity in young women with systemic lupus
erythematosus treated by chemotherapy. Lupus. 9:401–405. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brydøy M, Fosså SD, Dahl O and Bjøro T:
Gonadal dysfunction and fertility problems in cancer survivors.
Acta Oncol. 46:480–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Larsen EC, Müller J, Schmiegelow K,
Rechnitzer C and Andersen AN: Reduced ovarian function in long-term
survivors of radiation- and chemotherapy-treated childhood cancer.
J Clin Endocrinol Metab. 88:5307–5314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Syrigos KN, Karapanagiotou E, Boura P,
Manegold C and Harrington K: Bevacizumab-induced hypertension:
pathogenesis and management. BioDrugs. 25:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Corr BR, Breed C, Sheeder J, Weisdack S
and Behbakht K: Bevacizumab induced hypertension in gynecologic
cancer: does it resolve after completion of therapy? Gynecol Oncol
Rep. 17:65–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sarno MA, Mancari R, Colombo HA Jr, Azim N
and Peccatori FA: Are monoclonal antibodies a safe treatment for
cancer during pregnancy? Immunotherapy. 5:733–741. 2013. View Article : Google Scholar : PubMed/NCBI
|