Introduction
Mucosa-associated lymphoid tissue lymphoma (MALT)
was first described by Isaacson et al (1) as a subtype of non-Hodgkin lymphoma and
was subsequently classified as marginal zone lymphoma of MALT by
the Word Health Organization (2)
based on the Revised European-American Classification of lymphoid
neoplasms (3). A large national
database study reported that marginal zone lymphoma accounted for
~10% of non-Hodgkin lymphomas (4,5) and had
a better prognosis compared with diffuse large B-cell lymphoma
(4), which has the characteristics
of an indolent lymphoma. Extranodal marginal zone lymphoma, which
accounted for the majority of marginal zone lymphomas, mainly
included the stomach and ocular adnexa. The etiology of marginal
zone lymphoma was chronic inflammation, which occurred due to
Chlamydia psittaci infection (6).
Non-Hodgkin lymphoma involved the ocular adnexa in
45–75% of cases of extranodal marginal zone lymphoma, 15–30% of
cases of follicular lymphoma and 10% of cases of diffuse large
B-cell lymphoma (7–11). In ocular adnexal marginal zone
lymphoma, the primary sites were the conjunctiva (30–80%), the
retrobulbar soft tissue (10–50%) and the lacrimal gland (10–55%)
(12–16). Radiotherapy is currently the standard
treatment for marginal zone lymphoma of the ocular adnexa. A number
of studies have reported that a radiotherapy dose of 24–46 Gy to
the entire orbit for primary marginal zone lymphoma of the ocular
adnexa was highly effective in terms of local control and survival
(12–15). A paucity of reports and the various
types of ocular adnexa have made it difficult to undertake an
optimal dose volume analysis. Accordingly, a dose volume analysis
was conducted herein, based on the ocular adnexa region, to
determine the optimal dose-volume association for marginal zone
lymphoma of MALT.
Patients and methods
Patients
This retrospective study was approved by the Ethics
Committee of the Tokyo Medical University Hospital (no. 3199).
Between January, 2008 and December, 2013, 46 patients underwent
radiotherapy for primary non-Hodgkin lymphoma of the ocular adnexa
at the Tokyo Medical University Hospital (Tokyo, Japan). A total of
40 patients had marginal zone lymphoma of MALT, 4 had diffuse large
B-cell lymphoma, whereas follicular lymphoma and T-cell lymphoma
were present in 1 patient each. A total of 40 patients with
histologically proven primary marginal zone lymphoma of the ocular
adnexa were evaluated. Ophthalmic examination, whole blood count,
biochemical examination and body computed tomography (CT) scans
were performed prior to radiotherapy initiation. A total of 38
(95%) patients were proven to have immunoglobulin heavy chain
rearrangement, while the remaining 2 (5%) were not examined. The
patient characteristics and radiotherapy method used are summarized
in Table I. The median age of the
patients was 66.7 years (range, 89.0–26.2 years) and the
male/female ratio was 18/22. The most commonly affected sites were
the conjunctiva in 19 (47.5%), the retrobulbar soft tissue in 19
(47.5%) and the lacrimal gland in 2 (5%) patients. A total of 33
(82.5%) and 7 (17.5%) patients were stage IAE (unilateral) and IIAE
(bilateral), respectively, according to the Ann Arbor criteria
(17).
 | Table I.Patient characteristics and treatment
methods (n=40). |
Table I.
Patient characteristics and treatment
methods (n=40).
| Characteristics | N |
|---|
| Median age, years
(range) | 66.7 (26.2–89.0) |
| Gender |
|
|
Male:female | 18:22 |
| Main involvement
site |
|
|
Conjunctiva:retrobulbar soft
tissue:lacrimal gland | 19:19:2 |
| Laterality |
|
|
Unilateral:bilateral | 33:7 |
| Pathology test |
|
|
Proven:unproven | 40:0 |
| Gene
rearrangement |
|
|
Positive:negative | 38:2 |
| Treatment
methods |
|
| Radiotherapy |
|
|
Photon:electron | 30:10 |
| Number of ports |
|
|
1:2:3:4:5 | 11:12:8:8:1 |
| Photon energy |
|
|
4MV:6MV | 11:19 |
| Number of photon
ports (n=30) |
|
|
1:2:3:4:5 | 1:12:8:8:1 |
All the patients received a radiation dose of 30 Gy
in 15 fractions to the iso-center following immobilization with a
custom-made thermoplastic mask. A total of 30 and 10 patients
underwent photon and electron therapy, respectively. The 10 (53%)
patients with tumors located in the conjunctiva were irradiated by
electrons. The radiotherapy method was individualized based on the
involvement site of the ocular adnexa. The clinical target volume
(CTV) was determined as the entire orbit, apart from tumors located
in the conjunctiva alone. Among the 30 patients irradiated by
photons, 19 and 11 patients exhibited primary involvement of the
retrobulbar space and conjunctiva, respectively. The number of
ports ranged from one to five, which was primarily determined by
doctor preference. No patients underwent chemotherapy or antibiotic
therapy.
When reviewing the dosimetry, the association
between dose volume and clinical outcome was analyzed in 30
patients based on the date treated by three-dimensional (3D)
radiotherapy. Dosimetry was analyzed using a treatment planning
system (Xio v. 4.6; Elekta AB, Stockholm, Sweden). The growth of
the gross tumor volume (GTV) was measured based on the extent of
involvement of lymphoma on the CT image, with reference to
information from the ophthalmic examination and/or magnetic
resonance imaging. Doses to the organs at risk, the conjunctiva,
bulbus oculi, retina and retrobulbar space were also estimated. The
retrobulbar space was divided into anterior and posterior spaces to
evaluate differences in the irradiation doses. When lymphoma was
detected bilaterally, both sides of the GTV and the organs at risk
were delineated.
Statistical analysis
The overall survival time was calculated from the
first day of radiotherapy to the date of death from any cause. The
progression-free survival time was calculated from the first day of
radiotherapy to the date of the first relapse or the date of death.
The time to cataract surgery was calculated from the first day of
radiotherapy to the date of the cataract surgery. Continuous
variables were analyzed by the Wilcoxon rank-sum test. Paired
variables were analyzed by the Wilcoxon signed rank-sum test.
Survival time was calculated using the Kaplan-Meier method and the
difference was compared using the log-rank test. Stata v.12
software (Stata Co., College Station, TX, USA) was used as the
statistical software package. A P-value of <0.05 was considered
to indicate statistically significant differences. The adverse
events were assessed by the Common Terminology Criteria for Adverse
Events, version 4.0 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
Results
Patient survival
No patients experienced local relapse and there were
no reported deaths. Thus, the overall survival and local
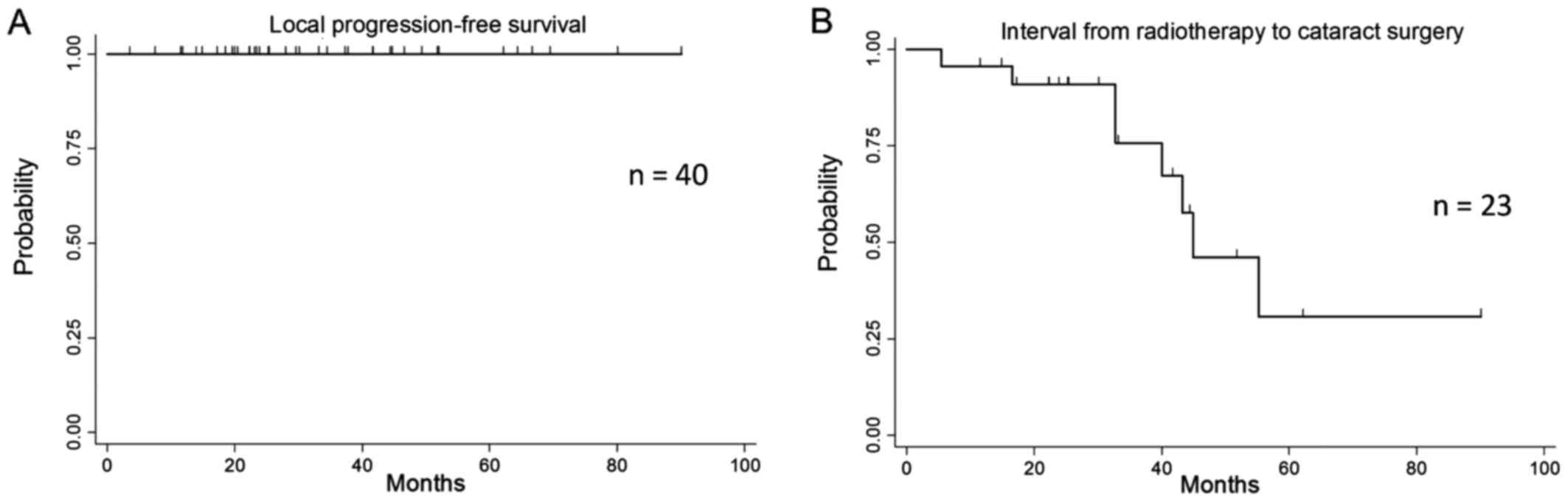
progression-free survival rates at 3 and 5 years were both 100%
during a median observation period of 32 months (Fig. 1A). Two patients developed recurrence
at the contralateral ocular adnexa at 40 and 89 months after
radiotherapy. Accordingly, the disease progression-free survival
rates at 3 and 5 years were 100 and 93.3% (95% confidence interval:
61.3–99%), respectively. All the patients experienced faint
erythema or dry desquamation, which did not progress. Of the 40
patients, 7 (18%) had already undergone cataract surgery prior to
radiotherapy, while 10 (30%) of 33 underwent cataract surgery at a
median time of 52 months after radiotherapy (Fig. 1B). The time to cataract surgery did
not differ significantly between electron therapy and photon
therapy (P=0.22).
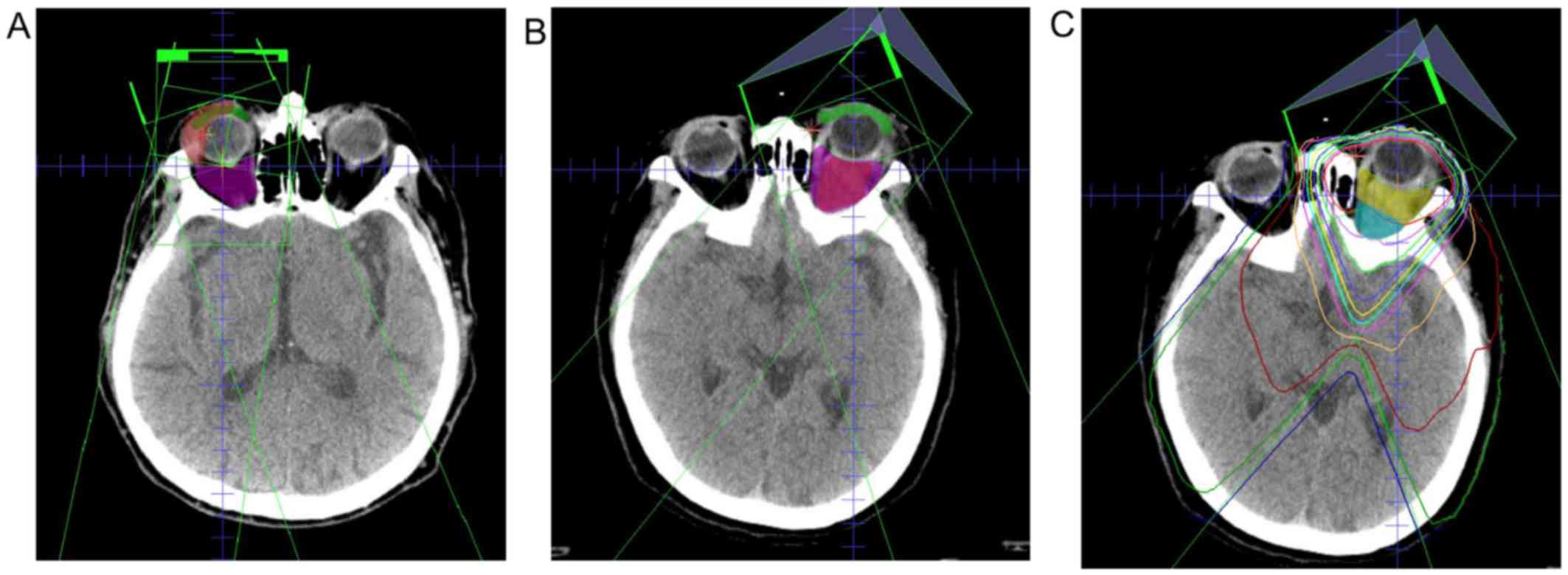
The typical dosimetry for lymphoma involving the
primary conjunctiva and the retrobulbar space are shown in Fig. 2A and B. The estimated dose
differences to the GTV are shown in Fig.
3A. The mean dose and 95% received dose (D95) to the GTV were
2.0 and 1.9 Gy, respectively. The minimum dose for the GTV ranged
from 0.9 to 2.0 Gy (median, 1.8 Gy). The D95 to the GTV was ≤1.8 Gy
in 25% of the patients. The dose to the GTV compared with the dose
to the other orbital sites is shown in Table II. The mean dose and D95 to the
conjunctiva were statistically significantly lower compared with
those administered to the GTV in conjunctival lymphoma (P<0.05).
The mean dose and D95 to the retrobulbar space was statistically
significantly lower compared with those to the GTV in cases of
retrobulbar lymphoma (P<0.05). The mean dose and D95 to the
retina were 2.0 Gy (range, 1.8–2.1 Gy) and 2.0 (range, 1.9–2.1 Gy),
respectively.
 | Table II.GTV dose comparison for the primary
site of involvement across various orbital sites. |
Table II.
GTV dose comparison for the primary
site of involvement across various orbital sites.
|
|
| Conjunctiva | Bulbus oculi | Retrobulbar
space |
|---|
|
|
|
|
|
|
|---|
| Primary involvement
site of lymphoma | GTV, median
(range) | Median (range) | P-value | Median (range) | P-value | Median (range) | P-value |
|---|
| Mean dose (cGy) |
|
Conjunctivaa (n=11) | 199 (180–204) | 190 (150–205) | <0.05 | 202 (196–207) | N.S. | 198 (149–200) | N.S. |
|
Retrobulbar (n=19) | 200 (171–210) | 191 (142–212) | <0.001 | 205 (192–214) | <0.05 | 196 (175–203) | <0.05 |
| Total
(n=30) | 200 (171210) | 191 (142–212) | <0.001 | 204 (192–214) | <0.05 | 197 (149–203) | <0.001 |
| D95 (cGy) |
|
Conjunctivaa (n=11) | 189 (155–201) | 155 (116–202) | <0.05 | 191 (182–201) | N.S. | 190 (98–195) | N.S. |
|
Retrobulbar (n=19) | 190 (127–203) | 171 (109–207) | <0.001 | 195 (148–208) | N.S. | 185 (127–197) | N.S. |
| Total
(n=30) | 190 (127–203) | 167 (109–207) | <0.001 | 195 (148–208) | N.S. | 187 (148–208) | N.S. |
An estimated dose comparison between the anterior
and posterior retrobulbar spaces is shown in Fig. 2C. The mean dose and D95 to the
posterior retrobulbar space (1.9 and 1.8 Gy, respectively) were
statistically significantly lower compared with those to the
anterior retrobulbar space (2.0 and 1.9 Gy, respectively,
P<0.001; Fig. 3B). When
restricted only to retrobulbar lymphoma, the mean dose (P<0.05)
and D95 (P<0.001) to the posterior retrobulbar space were also
statistically significantly lower compared with the anterior
space.
Discussion
Radiotherapy for patients with marginal zone
lymphoma of the ocular adnexa achieved good local control with
acceptable toxicity (18). Recently,
a Japanese study reported that a median dose of 30 Gy in 15
fractions achieved excellent long-term local control for primary
marginal zone lymphoma of the ocular adnexa (12). The contralateral orbit is known as
the predominant site of distant relapse, although rare (12,16,18).
These results indicated that achieving local control was important
for patients with primary marginal zone lymphoma of the ocular
adnexa and that an investigation of the optimal dose was
required.
Currently, the recommended treatment for ocular
adnexal lymphoma is to irradiate the entire bony orbit with a dose
of 24–25 Gy in 1.5–2.0-Gy fractions, except for cases in which the
disease is limited to the conjunctiva (19). This dose is lower than previously
published doses (7,11,13). Two
studies reported outcomes and a dose analysis for local control of
marginal zone lymphoma of ocular adnexa. Fung et al reported
that a dose of ≥30 Gy was superior to a dose of <30 Gy in terms
of local control for 53 patients with a median observation period
of 82 months. Accordingly, they indicated that the optimal dose for
ocular adnexal lymphoma was 30.6–32.4 Gy in 1.8 Gy per fraction
(7). Bayraktar et al reported
that 3/18 patients who received a dose of <30.6 Gy relapsed,
while 3/58 patients who received a dose of 30.6 Gy relapsed during
a median observation period of 5 years. As the former group had a
significantly higher relapse rate, they recommended a dose of at
least 30.6 Gy for ocular adnexal lymphoma (14).
In our study, all patients were irradiated at a dose
of 30 Gy in 2.0 per fraction at the iso-center, and all patients
attained local control. However, 25% of the patients were
irradiated at 1.8 Gy or lower in the GTV, and doses to the
posterior retrobulbar space were 10% lower compared with the
anterior space. These results suggested that a dose of 27 Gy in 1.8
Gy per fraction provided favorable local control. Goda et al
reported that a radiation dose of 25 Gy in 2.5 Gy per fraction
achieved 97% local control after 7 years (13). A dose of 25 Gy in 2.5 Gy per fraction
was found to be equivalent to a dose of 27 Gy in 1.8 Gy per
fraction, calculated by the linear quadrant model (20).
Several studies have been performed to evaluate low
doses for marginal zone lymphoma of the ocular adnexa. Tran et
al reported that 24 tumors receiving a dose of 24–25 Gy in
1.5–1.8 Gy per fraction were controlled during a median observation
period of 41 months, except for 1 patient who had a local relapse
due to a marginal miss (15); they
estimated that the irradiated dose in the relapse area was 5–23
Gy.
Regarding low-dose radiotherapy of 4 Gy in 2
fractions, both follicular and marginal zone lymphoma have been
studied. Fasola et al reported that 20 patients with a total
of 27 sites of ocular adnexal lymphoma were treated with a
radiation dose of 4 Gy in 2 fractions (21). The complete response rate was 85%
during a median observation period of 26 months. Patient histology
showed follicular lymphoma in 55% and marginal zone lymphoma in 40%
of the patients. This response rate was lower compared with that
observed in the present study, although the majority had follicular
lymphoma. Hoskin et al reported a randomized non-inferiority
study comparing a dose of 4 Gy in 2 fractions with 24 Gy in 12
fractions (22). Of the 614 sites
treated in 548 patients, 86% were follicular lymphomas and 14% were
marginal zone lymphomas. A dose of 4 Gy in 2 fractions did not
achieve non-inferiority, compared with a dose of 24 Gy in 12
fractions. Local progression was observed in two groups: One which
was administered a dose of 24 Gy to 21 sites, and a second which
was administered 4 Gy to 70 sites. The results of Fasola et
al compared favorably with those of Hoskin et al,
although the latter study included more cases of follicular
lymphoma. Fasola et al treated ocular adnexal lymphoma via
3D conformal radiotherapy, except for cases involving conjunctival
tumors. In the study of Hoskin et al, a proportion of the
patients were treated by anteroposterior-posteroanterior photon
fields.
In Peffer et al, partial orbit treatment was
compared with entire orbit treatment in 23 patients (23). A total of 33% of patients who
underwent partial orbit radiotherapy had a relapse in previously
uninvolved areas not included in the initial target volume. By
contrast, no patients who underwent entire orbit treatment
experienced a relapse. Accordingly, it was asserted that
irradiation encompassing the entire orbit was necessary and that 25
Gy was effective for local control. As it was difficult to identify
the extension of lymphoma to the retrobulbar area, a CTV
encompassing the entire orbit is considered to be appropriate.
Kaushik et al reported a risk of retinopathy
in 67 patients who received a radiotherapy dose of 18.9–54.0 Gy
(24). Eight patients who were
irradiated at a dose of 24–40 Gy developed retinopathy at a median
of 27 months. The daily fractionated dose of ≥2.0 Gy was a
significant risk factor for retinopathy, compared to a dose of 1.8
Gy. A total dose of 30 Gy or more was not a significant risk,
compared to <30 Gy. As the retina was unavoidable in several
cases, a daily dose of 1.8 Gy may be favorable in preventing the
onset of retinopathy.
In the present study, no difference in outcome was
observed between the conjunctiva and the retrobulbar space as the
primary location site. Similarly, Harada et al also reported
the absence of prognostic differences between the conjunctiva and
other anatomical subsites (12). The
same dose was adequate among ocular adnexal anatomical subsites.
Goda et al reported that the lacrimal gland and retrobulbar
space were associated with high distant relapse rates compared with
the conjunctiva (13). The distant
relapse rates in Asian studies (12,16),
including ours, were relatively lower compared with those reported
in Western studies (7,13). The relapse rates in lacrimal gland
lymphoma in Western studies (7,13) were
higher compared with Asian studies (12,16), and
our study may elucidate the reason for this difference. Attention
must be focused on the possibility of a distant relapse in lacrimal
gland lymphoma.
It was previously demonstrated that electron
radiotherapy provides favorable local control (7,11,13) and
reduces cataract formation (12)
when the lymphoma is located only in the conjunctiva. The
association between dose and cataract has been difficult to
elucidate, as several patients had cataract due to advanced age. In
the present study, the primary endpoint for cataract formation was
set to the date of cataract surgery. Electron therapy was more
likely to prolong the interval to cataract surgery compared with
photon therapy, but the difference was not statistically
significant.
The use of an antibiotic therapy targeting
Chlamydia psittaci in marginal zone lymphoma of the ocular
adnexa has been attempted. According to the majority of the
studies, complete response was obtained in only 20% of the cases
(25). From the data, radiotherapy
remains the standard treatment, and the addition of antibiotics
during radiotherapy may reduce the necessary dose of
radiotherapy.
Based on these findings, the optimal dose for the
D95 to the GTV currently appears to be 27 Gy in 15 fractions when
the lymphoma is located in the retrobulbar space. An entire orbit
CTV is most effectively irradiated at a D95 of 25 Gy in 15
fractions. When the lymphoma is limited to the conjunctiva, the GTV
is most effectively irradiated at the same dose using electron
therapy with a contact lens, whereas irradiation of the retrobulbar
space is omitted.
Acknowledgements
The authors would like to thank RT Kazunari Nakada
for his help in retrieving radiation dose volume data.
References
|
1
|
Isaacson P and Wright DH: Malignant
lymphoma of mucosa-associated lymphoid tissue. A distinctive type
of B-cell lymphoma. Cancer. 52:1410–1416. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaffe ES, Harris NL, Stein H, et al: World
Health Organization Classification of Tumors: Pathology and
Genetics of Tumorus of Haematopoietic and Lymphoid Tissues. IARC
Press; Lyon: 2001
|
|
3
|
Harris NL, Jaffe ES, Stein H, Banks PM,
Chan JK, Cleary ML, Delsol G, de Wolf-Peeters C, Falini B, Gatter
KC, et al: A revised European-American classification of lymphoid
neoplasms: A proposal from the International Lymphoma study group.
Blood. 84:1361–1392. 1994.PubMed/NCBI
|
|
4
|
Al-Hamadani M, Habermann TM, Cerhan JR,
Macon WR, Maurer MJ and Go RS: Non-Hodgkin lymphoma subtype
distribution, geodemographic patterns, and survival in the US: A
longitudinal analysis of the National Cancer Data Base from
1998–2011. Am J Hematol. 90:790–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith A, Howell D, Patmore R, Jack A and
Roman E: Incidence of haematological malignancy by sub-type: A
report from the Haematological Malignancy research network. Br J
Cancer. 105:1684–1692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferreri AJ, Guidoboni M, Ponzoni M, de
Conciliis C, Dell'Oro S, Fleischhauer K, Caggiari L, Lettini AA,
Dal Cin E, Ieri R, et al: Evidence for an association between
Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer
Inst. 96:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fung CY, Tarbell NJ, Lucarelli MJ,
Goldberg SI, Linggood RM, Harris NL and Ferry JA: Ocular adnexal
lymphoma: Clinical behavior of distinct World Health Organization
classification subtypes. Int J Radiat Oncol Biol Phys.
57:1382–1391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graue GF, Finger PT, Maher E, Rocca D
Della, Rocca R Della, Lelli GJ Jr and Milman T: Ocular adnexal
lymphoma staging and treatment: American joint committee on cancer
versus Ann Arbor. Eur J Ophthalmol. 23:344–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jenkins C, Rose GE, Bunce C, Wright JE,
Cree IA, Plowman N, Lightman S, Moseley I and Norton A:
Histological features of ocular adnexal lymphoma (REAL
classification) and their association with patient morbidity and
survival. Br J Ophthalmol. 84:907–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parikh RR, Moskowitz BK, Maher E, Rocca D
Della, Rocca R Della, Culliney B, Shapira I, Grossbard ML, Harrison
LB and Hu K: Long-term outcomes and patterns of failure in orbital
lymphoma treated with primary radiotherapy. Leuk Lymphoma.
56:1266–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou P, Ng AK, Silver B, Li S, Hua L and
Mauch PM: Radiation therapy for orbital lymphoma. Int J Radiat
Oncol Biol Phys. 63:866–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harada K, Murakami N, Kitaguchi M, Sekii
S, Takahashi K, Yoshio K, Inaba K, Morota M, Ito Y, Sumi M, et al:
Localized ocular adnexal mucosa-associated lymphoid tissue lymphoma
treated with radiation therapy: A long-term outcome in 86 patients
with 104 treated eyes. Int J Radiat Oncol Biol Phys. 88:650–654.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goda JS, Le LW, Lapperriere NJ, Millar BA,
Payne D, Gospodarowicz MK, Wells W, Hodgson DC, Sun A, Simpson R
and Tsang RW: Localized orbital mucosa-associated lymphoma tissue
lymphoma managed with primary radiation therapy: Efficacy and
toxicity. Int J Radiat Oncol Biol Phys. 81:e659–e666. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bayraktar S, Bayraktar UD, Stefanovic A
and Lossos IS: Primary ocular adnexal mucosa-associated lymphoid
tissue lymphoma (MALT): Single institution experience in a large
cohort of patients. Br J Haematol. 152:72–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tran KH, Campbell BA, Fua T, MacManus M,
Ryan G, Chesson B and Wirth A: Efficacy of low dose radiotherapy
for primary orbital marginal zone lymphoma. Leuk Lymphoma.
54:491–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suh CO, Shim SJ, Lee SW, Yang WI, Lee SY
and Hahn JS: Orbital marginal zone B-cell lymphoma of MALT:
Radiotherapy results and clinical behavior. Int J Radiat Oncol Biol
Phys. 65:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
18
|
Stefanovic A and Lossos IS: Extranodal
marginal zone lymphoma of the ocular adnexa. Blood. 114:501–510.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yahalom J, Illidge T, Specht L, Hoppe RT,
Li YX, Tsang R and Wirth A; International Lymphoma Radiation
Oncology Group, : Modern radiation therapy for extranodal
lymphomas: Field and dose guidelines from the international
lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys.
92:11–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fowler JF: The linear-quadratic formula
and progress in fractionated radiotherapy. Br J Radiol. 62:679–694.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fasola CE, Jones JC, Huang DD, Le QT,
Hoppe RT and Donaldson SS: Low-dose radiation therapy (2 Gy × 2) in
the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys.
86:930–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoskin PJ, Kirkwood AA, Popova B, Smith P,
Robinson M, Gallop-Evans E, Coltart S, Illidge T, Madhavan K,
Brammer C, et al: 4 Gy versus 24 Gy radiotherapy for patients with
indolent lymphoma (FORT): A randomised phase 3 non-inferiority
trial. Lancet Oncol. 15:457–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfeffer MR, Rabin T, Tsvang L, Goffman J,
Rosen N and Symon Z: Orbital lymphoma: Is it necessary to treat the
entire orbit? Int J Radiat Oncol Biol Phys. 60:527–530. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaushik M, Pulido JS, Schild SE and
Stafford S: Risk of radiation retinopathy in patients with orbital
and ocular lymphoma. Int J Radiat Oncol Biol Phys. 84:1145–1150.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kiesewetter B and Raderer M: Antibiotic
therapy in nongastrointestinal MALT lymphoma: A review of the
literature. Blood. 122:1350–1357. 2013. View Article : Google Scholar : PubMed/NCBI
|