Introduction
Thyroid nodules are one of the most common thyroid
disorders. Ultrasound-guided fine needle aspiration (US-FNA) has
been accepted as the gold standard for the differential diagnosis
of thyroid nodules (1). However,
there are certain limitations of US-FNA, including indeterminate
results, which range in frequency from 10–33% (2,3). The
recommended clinical management for indeterminate aspirates is
repeated US-FNA. However, a repeated US-FNA still has 38–48%
indeterminate rate (4,5). Otherwise, diagnostic surgery may be
performed, which eventually will increase medical costs and
suffering of patients (1).
Ultrasound-guided core needle biopsy (US-CNB) presents a useful
alternative method to obtain tissue from thyroid nodules for
diagnosis. Previous studies have demonstrated that US-CNB was a
useful alternative for the management of thyroid nodules with
indeterminate FNA results (4,6).
However, US-CNB is not commonly used in the routine evaluation of
thyroid nodules as a first-line method, and the major reason may be
that US-CNB is proposed to increase the risk of bleeding and
discomfort of patients (7,8).
The purpose of the current meta-analysis was to
evaluate US-CNB in the diagnosis of the thyroid nodules with
suspicious US findings.
Data collection methods
Literature search
Literature databases consisted of the Cochrane
Library, Medline, Embase, Scopus and Google Scholar. The search
terms used were core needle biopsy/coarse needle biopsy/core
biopsy/CNB; thyroid nodules; thyroid cancer; head and neck tumors;
neck masses. Articles were selected with publication dates up to
July 29, 2015.
Eligibility
Initially, the titles and abstracts of studies were
independently reviewed for eligibility by two investigators.
Discrepancies were resolved by a third evaluator or by consensus
following a re-evaluation. There were no restrictions on study
design, language or time period. The initial screening process
produced a set of potentially eligible studies. Full reprints of
all these potential studies were obtained and subjected to a more
rigorous screen to produce a final set of eligible studies.
Prospective and retrospective observational studies were considered
eligible if they contained accuracy data for the diagnosis of
thyroid nodules.
Inclusion and exclusion criteria
Studies were finally included if they contained
extractable data on the diagnosis of thyroid nodules. The main
exclusion criteria were: i) articles not within the field of
interest in this review, ii) nodules had selection bias, iii)
diagnostic values such as sensitivity and specificity for
malignancy were not studied with respect to thyroid nodules and iv)
US-FNA has been performed but the outcomes were indeterminate or
failed.
Quality assessment
Quality appraisals of retrieved full-text articles
were graded independently by two investigators for quality and
applicability according to the Quality Assessment Tool for
Diagnostic Accuracy Studies-2 (QUADAS-2) method. This widely used
tool consisted of 11 items: Representative spectrum (item 1),
reference standard (item 2), acceptable delay between tests (item
3), partial verification bias (item 4), differential verification
bias (item 5), incorporation bias (item 6), reference standard
results blinded (item 7), index text results blinded (item 8),
relevant clinical information (item 9), uninterpretable results
reported (item 10) and withdrawals explained (item 11).
Disagreements were resolved by a third evaluator or by consensus
following a re-evaluation of the references.
Outcome analysis
The primary outcomes were the number of nodules that
were true positive (TP), true negative (TN), false positive (FP) or
false negative (FN). Subsequently, sensitivity, specificity,
positive likelihood ratio (LR), negative LR, diagnostic odds ratio
(DOR) and the area under the summery receiver operating
characteristic curve (AUC) were evaluated. The biopsy results of
US-CNB were grouped into six categories according to the Bethesda
system (9), which was originally
used for the analysis of US-FNA cytology: i) non-diagnostic, ii)
benign, iii) atypical follicular lesion of undetermined
significance (AUS/FLUS), iv) follicular neoplasm or suspected
follicular lesion (FN/SFN), v) suspicious for cancer and vi)
malignant lesions.
For malignant nodules, the final diagnoses were
based on histological findings following surgical resection. For
benign nodules, the final diagnoses were based on histological
findings following surgical resection or a stable and uneventful
follow-up. In the current meta-analysis, inconclusive results
including non-diagnostic findings, AUS/FLUS and SFN/FN were
excluded.
Statistical analysis
The results of US-CNB were assessed using values for
TP, TN, FP and FN. Diagnostic parameters were calculated as
followed: Sensitivity = TP/(TP + FN), specificity = TN/(TN + FP),
positivity LR = sensitivity/(1-specificity), negative LR =
(1-sensivivity)/specificity and DOR = (TP/FP)/(FN/TN). Pooled
estimates for sensitivity, specificity, positive LR, negative LR
and DOR with the corresponding 95% confidence intervals (CIs) were
used to examine the diagnostic ability of US-CNB for
differentiating benign from malignant thyroid nodules. The pooled
estimates were derived using the fixed-effects model
(Mantel-Haenszel method) if significant heterogeneity was not
present. In case of heterogeneity, the random-effects model
(DerSimonian-Laird method) was applied. Summary receiver operating
characteristic (SROC) curves were constructed using the
Moses-Shapiro-Lit-tenberg (inverse variance) method. Heterogeneity
was explored via the Cochran Q test, and a P-value of <0.01
indicated the presence of heterogeneity. Inconsistency was
calculated by I2 to describe the percentage of
variability due to heterogeneity, rather than sampling errors. An
I2 value >50% was considered indicative of
substantial heterogeneity (10).
Data were analyzed using Review Manager software (RevMan version
5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane
Collaboration, 2014) and Meta-Disc software (version 1.4) (11). P<0.05 was considered to indicate a
statistically significant difference.
Results
Study retrieval
Initially, 1,274 studies were identified. Following
title and abstract scrutiny, 1,245 irrelevant studies were removed.
From the 29 obtained potentially eligible articles, 8 papers were
finally included (Fig. 1) (7,8,12–17)
There was 1 prospective study and 7 retrospective studies, and the
parameters of the investigations are provided in Table I.
 | Table I.Basic characteristics of studies
included in this meta-analysis |
Table I.
Basic characteristics of studies
included in this meta-analysis
| First author
(year) | Design | No. of nodules | Inadequate samples
(%) | No. of cases
including follow-up | Finally
included | Male/female | Age, years
(range) | (Refs.) |
|---|
| Chen (2015) | Retrospective | 365 | 63 (17.3) | 89 | 89 | 286/79 | 58 (14–85) | (12) |
| Harvey (2005) | Retrospective | 79 | 10 (12.7) | 69 | 69 | NK | NK | (13) |
| Karstrup
(2001) | Retrospective | 77 | 9 (11.7) | 41 | 36 | 13/64 | 51 (33–81) | (7) |
| Paja (2015) | Retrospective | 3,517 | 364 (10.3) | 676 | 522 | NK | NK | (14) |
| Renshaw (2007) | Retrospective | 377 | 67 (17.8) | 62 | 55 | 76/301 | 52 (14–86) | (8) |
| Sung (2012) | Retrospective | 555 | 82 (14.8) | 555 | 473 | 85/453 | 44.32±11.86 | (15) |
| Trimboli
(2014) | Prospective | 31 | 1 (3.2) | 31 | 30 | NK | NK | (16) |
| Zhang (2014) | Retrospective | 369 | 22 (6.0) | 369 | 347 | 101/254 | 47.7±11.8
(14–78) | (17) |
Study quality
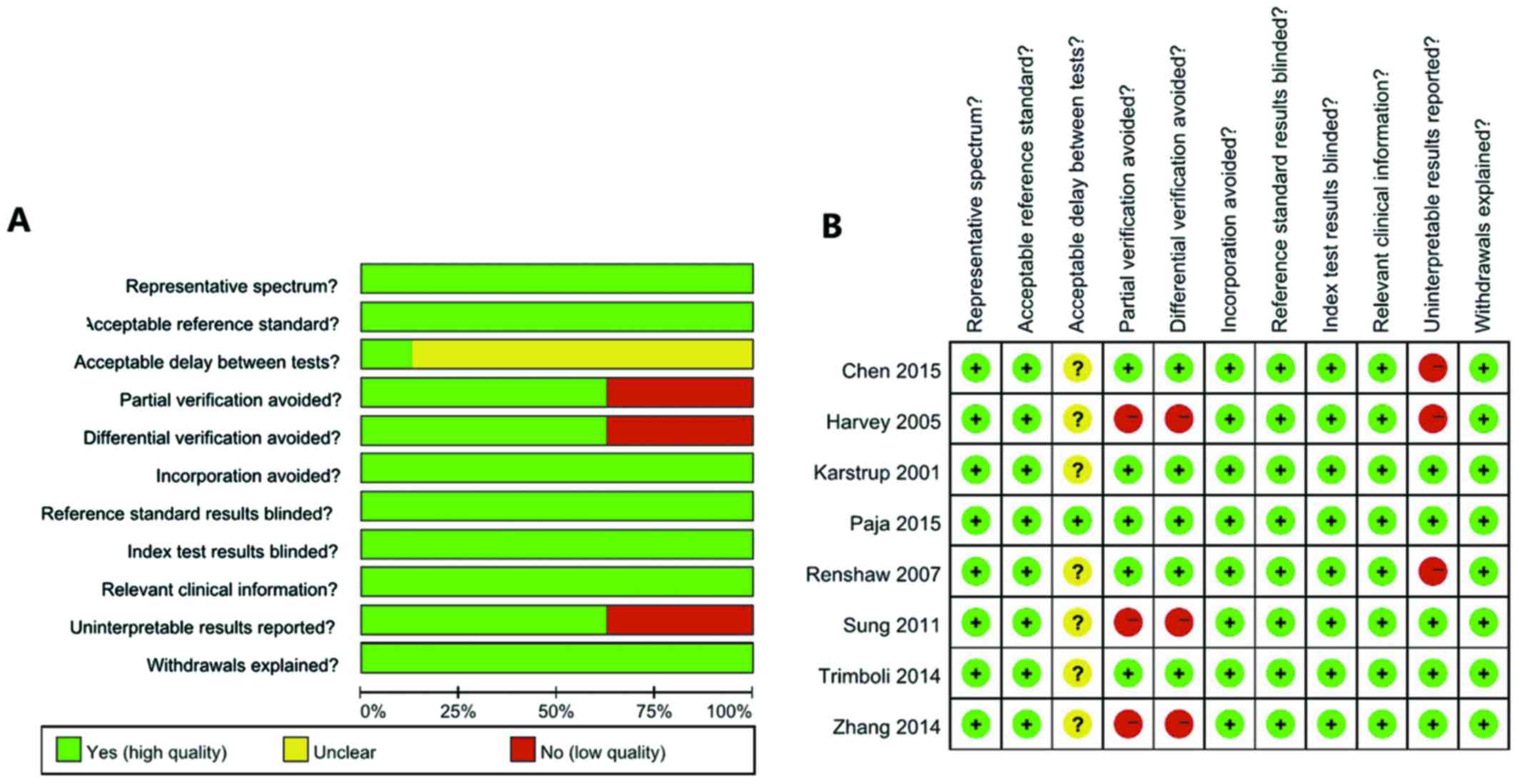
A summary of the QUADAS-2 quality assessment is
presented in Fig. 2. Nodules with at
least one of the following malignancy-suspicious US findings were
subjected to US-CNB: Calcification, hypoechogenicity, irregular
margin, vascularity, and taller than wide (item 1). The gold
standard was surgical pathology (item 2). No studies specifically
mentioned the time between US-CNB and histological evaluation,
except for that by Paja et al (14), which clearly stated that the interval
between US-CNB and surgery was less than 12 months in all cases
(item 3). Although this may lead to timing bias, in the majority of
institutions, the interval between US-CNB and histological
evaluation was likely to be short. All cases were evaluated against
a reference standard. The majority of studies were based only on
histologically verified samples, which may lead to partial
verification bias (item 4). In addition, 2 studies used
histological confirmation and clinical follow-up as reference
standards (item 5). The index test was independent of the reference
test (item 6). It was usual practice for the reference standard to
be evaluated with knowledge of the index test results (item 7), and
the index test was always interpreted without knowledge of the
reference standard (item 8), thus, there was potential for the
knowledge of the US-CNB diagnosis to influence the histological
diagnosis. The majority of the studies were retrospective, and
clinical data were available at the time of diagnosis by the index
test (item 9). Three studies included non-diagnostic findings,
AUS/FLU and SFN/FN (item 10), and the withdrawals were explained
(item 11).
Diagnostic analysis
In the 8 studies included in this meta-analysis,
there were a total of 5,370 nodules, including 618 indeterminate
nodules. The rate of indeterminate results ranged from 0–17.8%, and
the pooled rate of indeterminate results was 11.5%. In 5,370
nodules, 1,738 nodules were followed-up, but 117 of these nodules
were indeterminate, so 1,621 nodules were finally included in this
meta-analysis (Table I).
The compilations of sensitivity, specificity,
accuracy and positive predictive value of US-CNB for thyroid cancer
are displayed in Table II. The
sensitivity for malignancy ranged from 0.69–1, the specificity for
malignancy ranged from 0.35–1, and the accuracy ranged from 0.53–1.
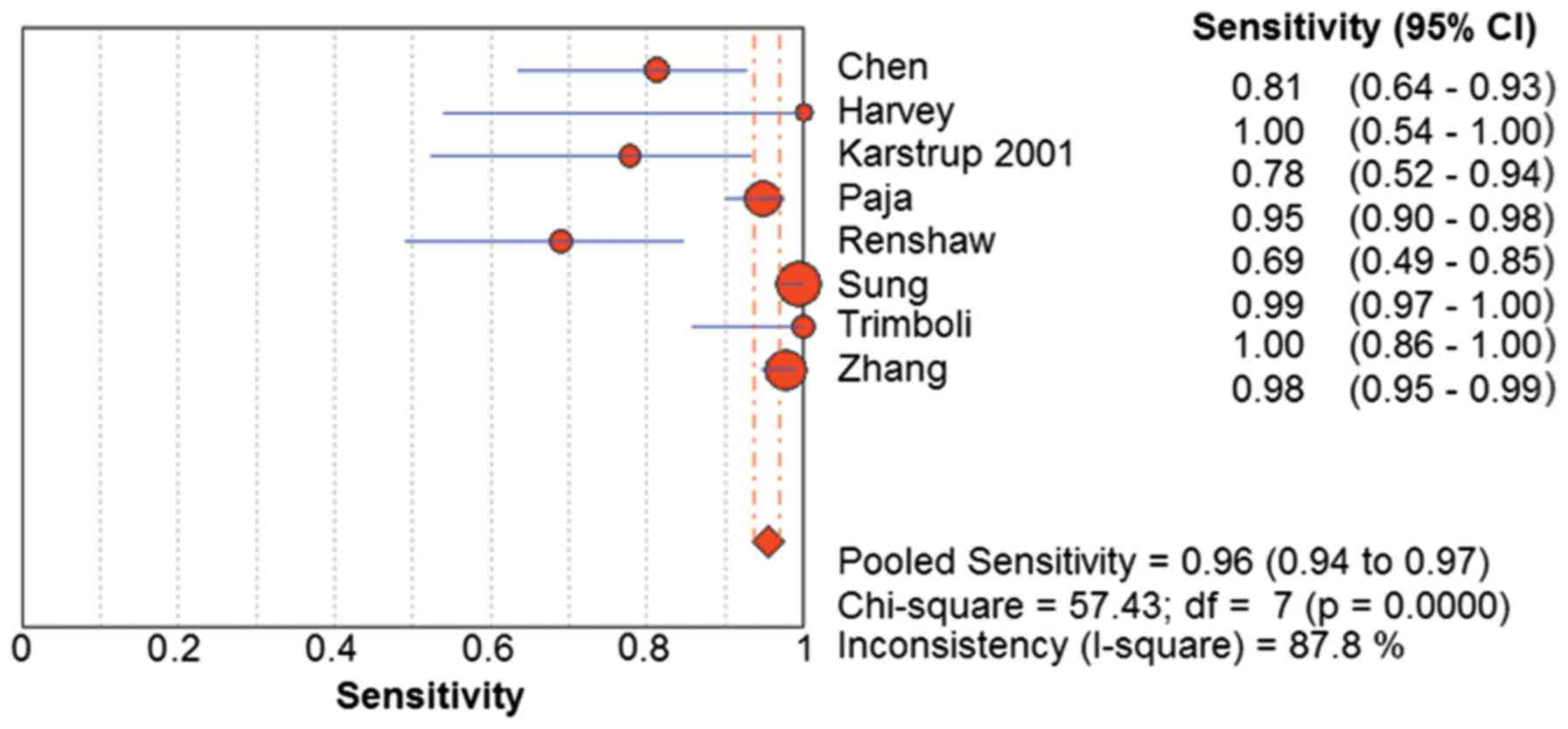
The random-effects model was used for determining the pooled
diagnostic sensitivity, as homogeneity tests of sensitivity
revealed that Q=57.43 (P<0.01) and I2=87.8%. The
pooled sensitivity for malignancy was 0.96 (95% CI=0.94–0.97)
(Fig. 3). Similarly, the
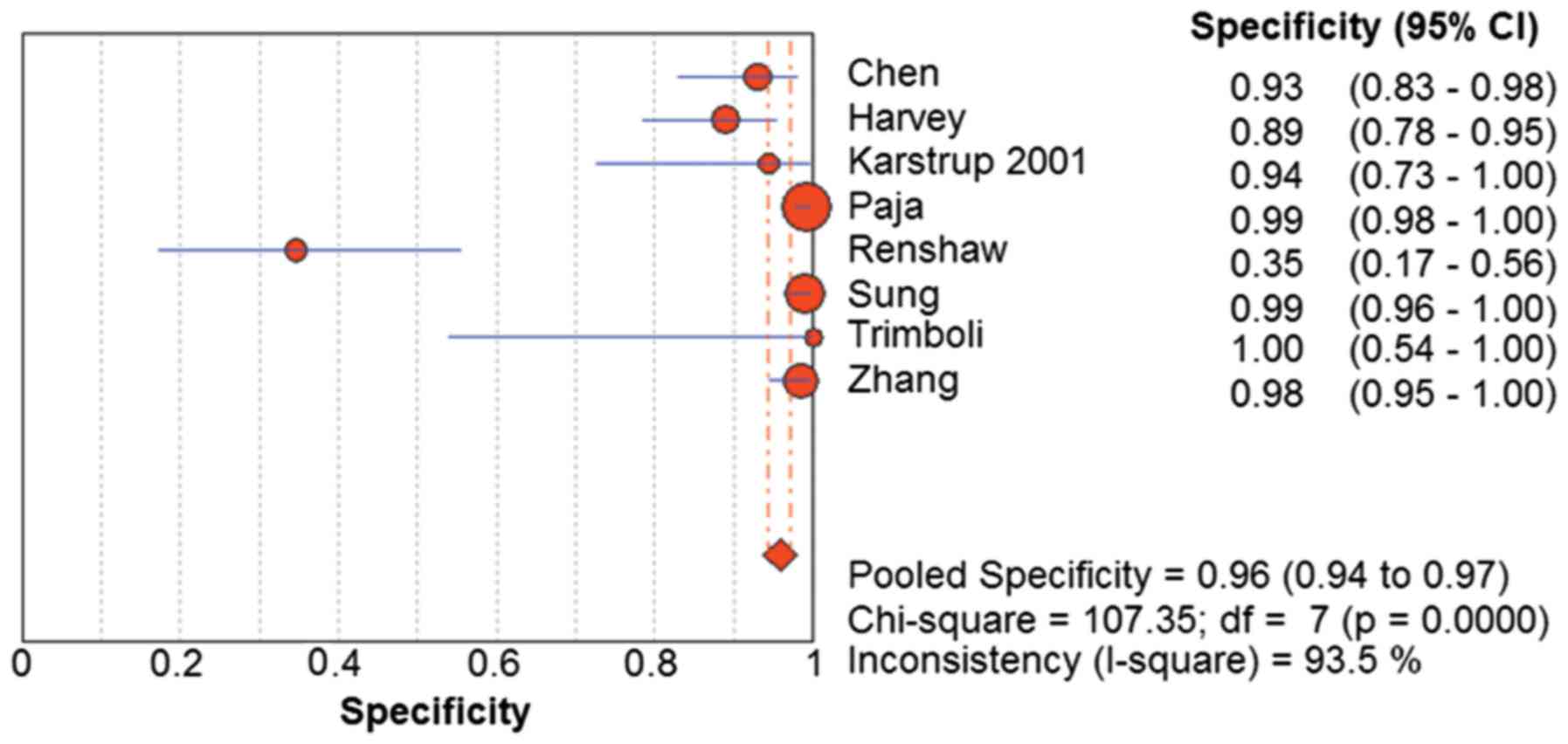
random-effects model was used for determining the pooled diagnostic
specificity, as homogeneity tests of sensitivity showed Q=107.35
(P<0.01) and I2=93.5%. The pooled specificity was
0.96 (95% CI=0.94–0.97) (Fig.
4).
 | Table II.Performance of the included
studies. |
Table II.
Performance of the included
studies.
| First author
(year) | TP | FP | FN | TN | Total | Sensitivity
(%) | Specificity
(%) | Accuracy (%) | Positive predictive
value (%) | Final
diagnosis | (Refs.) |
|---|
| Chen (2015) | 26 | 4 | 6 | 53 | 89 | 81.3 | 93 | 88.8 | 86.7 | Surgical
resection | (12) |
| Harvey (2005) | 6 | 7 | 0 | 56 | 69 | 100 | 88.9 | 89.9 | 46.2 | Surgical resection
and clinical follow-up | (13) |
| Karstrup
(2001) | 14 | 1 | 4 | 17 | 36 | 77.8 | 94.4 | 86.1 | 93.3 | Surgical
resection | (7) |
| Paja (2015) | 145 | 3 | 8 | 366 | 522 | 94.8 | 99.2 | 97.9 | 98 | Surgical
resection | (14) |
| Renshaw (2007) | 20 | 17 | 9 | 9 | 55 | 69.0 | 34.6 | 52.7 | 54.1 | Surgical
resection | (8) |
| Sung (2012) | 276 | 2 | 2 | 193 | 473 | 99.3 | 99.0 | 99.2 | 99.3 | Surgical resection
and clinical follow-up | (15) |
| Trimboli
(2014) | 24 | 0 | 0 | 6 | 30 | 100.0 | 100.0 | 100.0 | 100.0 | Surgical
resection | (16) |
| Zhang (2014) | 211 | 2 | 5 | 129 | 347 | 97.7 | 98.5 | 98.0 | 99.1 | Surgical resection
and clinical follow-up | (17) |
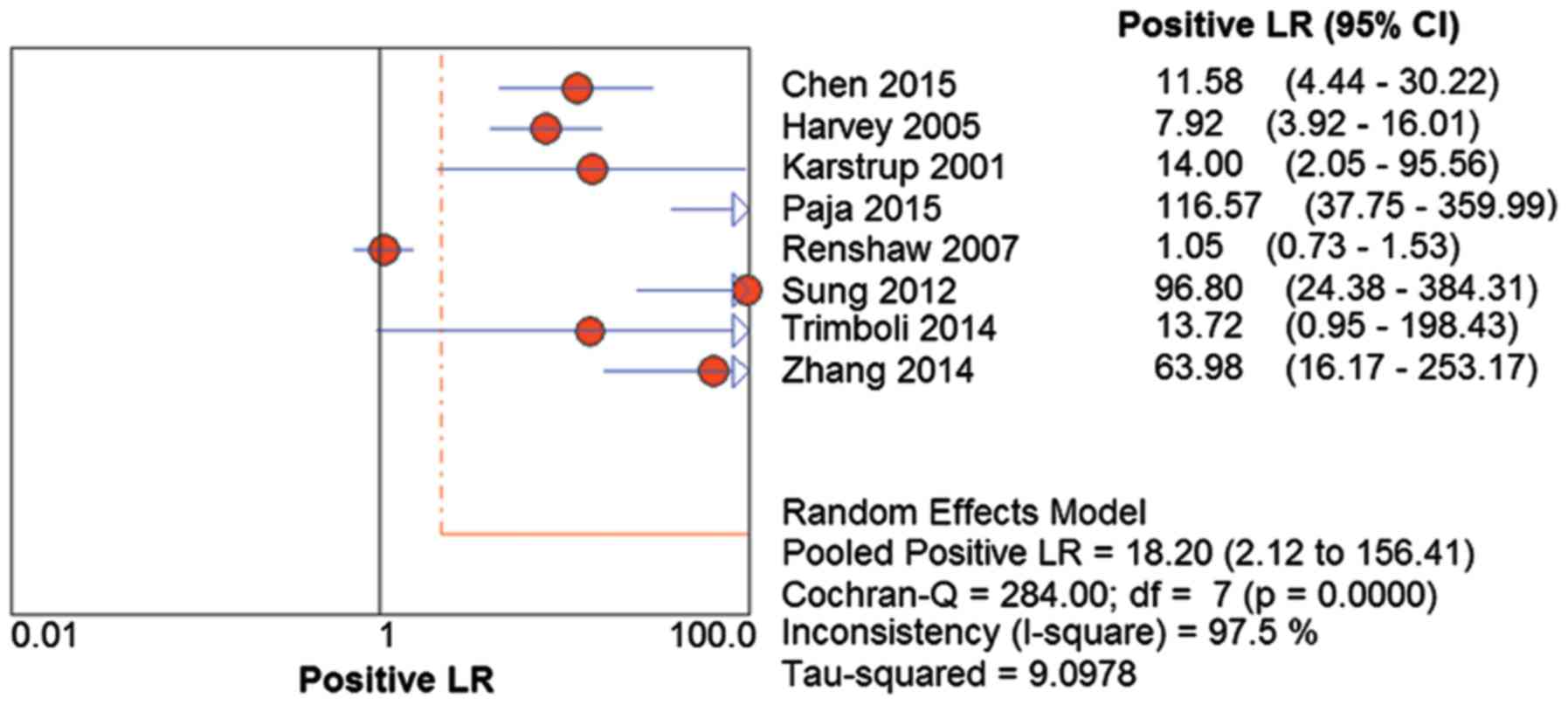
The positive LR was 18.20 (95% CI=2.21–156.41;
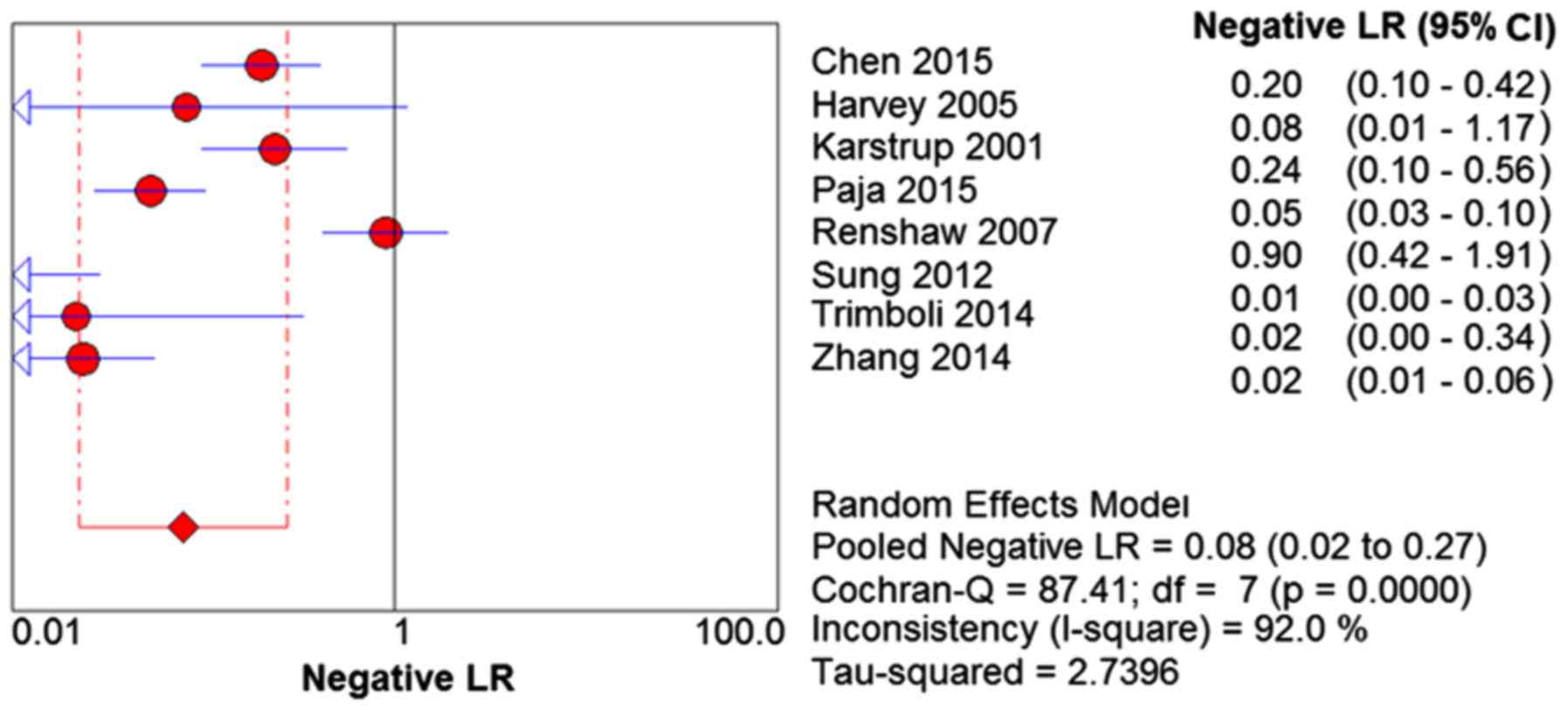
Q=284.00; P<0.01) and I2=97.5% (Fig. 5). The negative LR was 0.08 (95%
CI=0.02–0.27; Q=87.41; P<0.01) and I2=92.0% (Fig. 6). The DOR was 250.60 (95%
CI=19.11–3286.76) (Q=120.83; P<0.01) and I2=94.2%
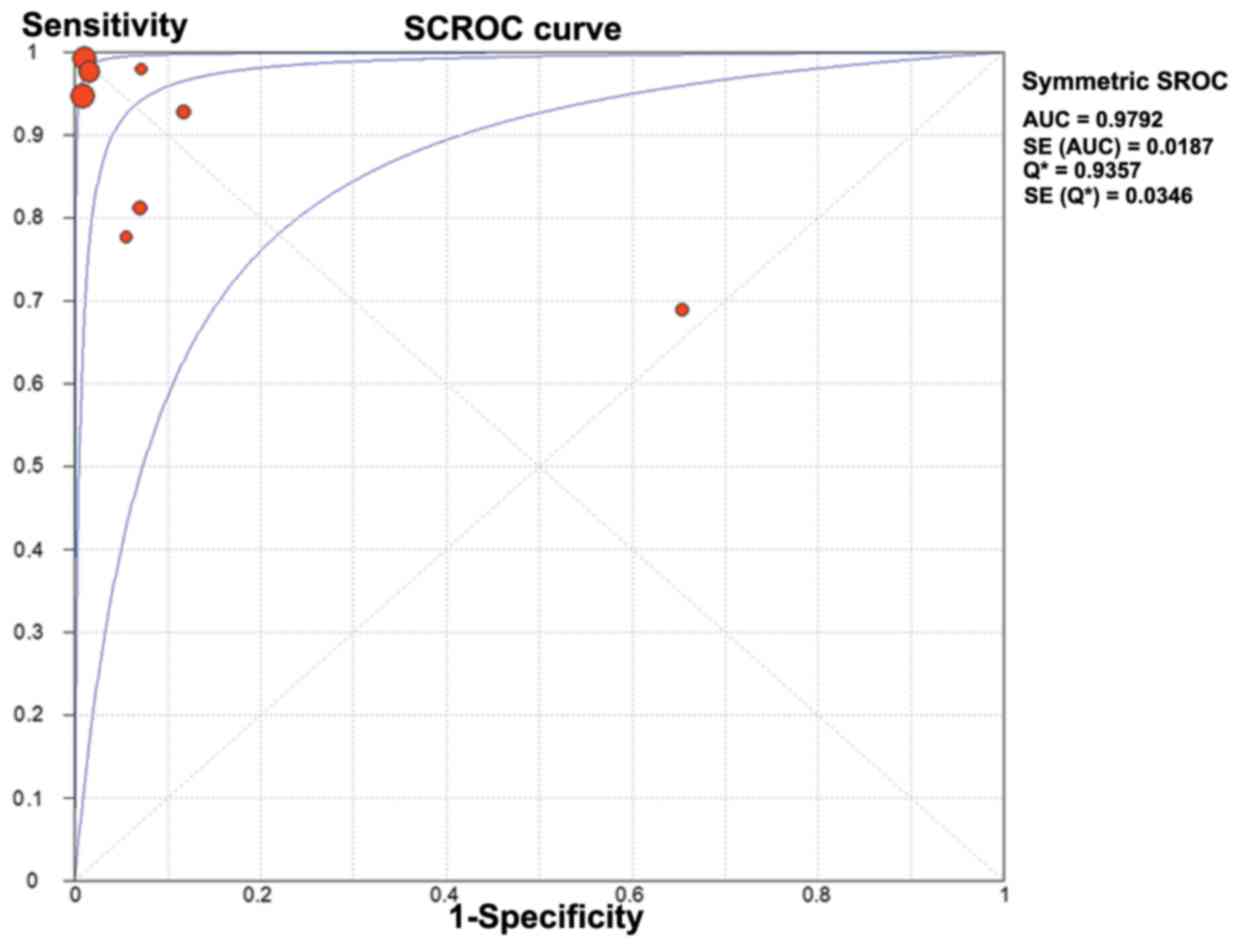
(Fig. 7). The AUC was 0.979
(standard error=0.018) (Fig. 8).
Complications of US-CNB
In total, 73 hematomas and 1 recurrent nerve lesion
were reported from a total of 5,370 nodules (Table III). The overall risk of the
hematoma was 1.3%.
 | Table III.Complications in the included
studies |
Table III.
Complications in the included
studies
| First author
(year) | Needle gauge
passes | Total | Complications | (Refs.) |
|---|
| Chen (2015) | NK | 365 | 1 hematoma | (12) |
| Harvey (2005) | 18 | 79 | 1 hematoma | (13) |
| Karstrup
(2001) | 18 | 77 | 3 hematomas | (7) |
| Paja (2015) | 18 | 3,517 | 56 hematomas, 1
recurrent nerve lesion following a direct puncture of the
nerve | (14) |
| Renshaw (2007) | 18, 20, 21 | 377 | 1 hematoma | (8) |
| Sung (2012) | 18 | 555 | 11
hematomasa | (15) |
| Trimboli
(2014) | 21 | 31 | None | (16) |
| Zhang (2014) | 18 | 369 | None | (17) |
Discussion
Thyroid nodules are very common, and their
prevalence has dramatically increased in recent years (18,19).
Although the risk of malignancy is fairly low, it must be
considered (1,20,21). The
early detection and distinction between benign or malignant thyroid
nodules is particularly important to guide clinical treatment and
select operative methods. At present, ultrasound is widely used in
the initial differentiation. Several ultrasound features, such as
irregular, taller than wide dimensions, microcalcifications and
hypoechogenicity have been considered to be malignant signs
(22). However, none of these
characteristics appears sufficient to diagnose malignancy
individually, and in numerous cases ambiguous features result in
uncertain ultrasonic diagnosis. Therefore, US-FNA and cytopathology
is now considered as the gold standard to identify thyroid nodules
with suspiciously concerning clinical and/or sonographic features.
Nevertheless, US-FNA has been limited by the rate of indeterminate
results (including non-diagnostic AUS/FLUS and FN/SFN findings) to
a certain extent. It has been reported that indeterminate rate of
the US-FNA was between 10.9–33% (2,3,23–25). The
malignancy rate of the surgical follow-up in AUS/FLUS ranged from
6–48% (26) and in FN/SFN between 10
and 30% (27,28).
US-CNB has been revealed to be useful in combination
with US-FNA or following insufficient, non-diagnostic or atypia of
uncertain significance results in US-FNA (5,6,29,30).
However, few reports have been conducted to evaluate the usefulness
of US-CNB as a routine diagnostic procedure for thyroid nodules. In
a prior meta-analysis, Li et al (31) evaluated US-FNA and US-CNB in
diagnosing thyroid nodule malignancy. This recently published
review included 5 studies and elucidated a US-FNA sensitivity of
68% and a specificity of 93% for detecting thyroid cancer, and
US-CNB was able to detect thyroid malignancy with a sensitivity of
83% and a specificity of 94%. The number of studies included in the
above meta-analysis was smaller than that in the current study. To
the best of our knowledge, the present study is the only dedicated
meta-analysis and literature review of US-CNB for thyroid nodules.
The present meta-analysis included 1,621 nodules from 8 studies.
The results revealed that US-CNB for thyroid nodules was a useful
technique with high pooled sensitivity (96%) and high pooled
specificity (96%), confirming that US-CNB was an effective method
for identifying malignancy in thyroid nodules. With increased
evidence inclusion in the current analysis, markedly higher
diagnostic capabilities of US-CNB than the findings from Li et
al (31) were identified.
Higher economical cost, concerns about potential
complications, technical requirements and time consumption may
limit the use of US-CNB. It may be considered that US-CNB has the
potential to increase patient discomfort and enhance complications.
However, prior investigations revealed that compared with US-FNA,
US-CNB did not increase the patient discomfort significantly
(5–8,15,29,30,32–36).
In the present meta-analysis, the reported complications are
compiled in Table III. The most
common was post-biopsy hematoma (7,8,12–15), but
the majority of patients did not require treatment for this
complication. Only one patient suffered from recurrent nerve damage
following a direct puncture of the nerve, which finally caused
permanent dysphonia. However, this type of complication was also
reported with US-FNA (37).
Furthermore, Bergeron et al (38) reported an iatrogenic arteriovenous
fistula formation following US-CNB, eventually causing tinnitus. In
order to minimize such critical complications, US-CNB must be
performed with experienced radiologists with dedicated training,
who are familiar with the radiological features of important
anatomical structures in the cervical region. Compared with US-FNA,
US-CNB appears to depend more on the experience and skill of the
operator and cytology interpretation. In addition, the cost of
US-CNB requires consideration. Trimboli et al (39) reported that US-CNB cost 1,000 Euros
per patient, and this price was much higher than the cost of US-FNA
(about 150 Euros). Although US-CNB is more expensive, considering
the collective cost of repeat US-FNA and diagnostic surgery, and
the associated patient suffering due to surgery, the cost of US-CNB
is reasonable.
The current meta-analysis had several potential
sources of bias and limitations. Firstly, 7 of the 8 included
studies were retrospective. Retrospective studies frequently tend
to include post-surgical cases with positive results, and in this
way positive rate will be much higher than the negative rate. Three
retrospective and one prospective study included in this
meta-analysis avoided this problem in that they included all cases
within a specified period of time and used clinical follow-up to
verify cases with a negative US-CNB. Second, the heterogeneity of
the included studies was also a matter of concern. The degree of
heterogeneity in the present study was relatively high. It was
considered that variance across the included studies was attributed
to heterogeneity. The potential confounding variances included, for
example: The choice of the nodules, the shape and size of the
nodules and the experience of the operator. In addition, baseline
differences among the patients in the included studies and in the
study qualities may also contribute to heterogeneity. Third,
publication bias was another concern, as studies that reported
significance were more likely to be published than those reporting
non-significant results. Fourth, although the current study
provided evidence suggesting that US-CNB had high sensitivity and
specificity for the detection of malignancy nodules in thyroid, it
was also based on a relatively small number of studies.
In conclusion, the present meta-analysis suggests
that US-CNB is a safe, reliable, and accurate method to assess
thyroid nodules. It has high sensitivity and specificity, and has
low risk of complications for the diagnosis of malignant thyroid
nodules, which may avoid repeat US-FNA and diagnostic surgery.
Acknowledgements
The present study was supported by the National Key
Clinical Specialty Project (awarded to the Departments of Nuclear
Medicine and Radiology); The Tianjin Medical University General
Hospital New Century Excellent Talent Program; Young and
Middle-aged Innovative Talent Training Program from Tianjin
Education Committee; and Talent Fostering Program (the 131 Project)
from Tianjin Education Committee, Tianjin Human Resources and
Social Security Bureau (awarded to Dr Zhaowei Meng); The China
National Natural Science Foundation (grant no. 81571709) Key
Project of Tianjin Science and Technology Committee Foundation
grant (awarded to Dr Zhaowei Meng). The funders had no role in
study design, data collection and analysis, decision to publish, or
preparation of the manuscript.
References
|
1
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, ; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American thyroid association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nayar R and Ivanovic M: The indeterminate
thyroid fine-needle aspiration: Experience from an academic center
using terminology similar to that proposed in the 2007 national
cancer institute thyroid fine needle aspiration state of the
science conference. Cancer. 117:195–202. 2009.PubMed/NCBI
|
|
3
|
Degirmenci B, Haktanir A, Albayrak R, Acar
M, Sahin DA, Sahin O, Yucel A and Caliskan G: Sonographically
guided fine-needle biopsy of thyroid nodules: The effects of nodule
characteristics, sampling technique, and needle size on the
adequacy of cytological material. Clin Radiol. 62:798–803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SH, Kim MH, Bae JS, Lim DJ, Jung SL
and Jung CK: Clinical outcomes in patients with non-diagnostic
thyroid fine needle aspiration cytology: Usefulness of the thyroid
core needle biopsy. Ann Surg Oncol. 21:1870–1877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park KT, Ahn SH, Mo JH, Park YJ, Park DJ,
Choi SI and Park SY: Role of core needle biopsy and
ultrasonographic finding in management of indeterminate thyroid
nodules. Head Neck. 33:160–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Na DG, Kim JH, Sung JY, Baek JH, Jung KC,
Lee H and Yoo H: Core-needle biopsy is more useful than repeat
fine-needle aspiration in thyroid nodules read as nondiagnostic or
atypia of undetermined significance by the Bethesda system for
reporting thyroid cytopathology. Thyroid. 22:468–475. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karstrup S, Balslev E, Juul N, Eskildsen
PC and Baumbach L: US-guided fine needle aspiration versus coarse
needle biopsy of thyroid nodules. Eur J Ultrasound. 13:1–5. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renshaw AA and Pinnar N: Comparison of
thyroid fine-needle aspiration and core needle biopsy. Am J Clin
Pathol. 128:370–374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cibas ES and Ali SZ: NCI Thyroid FNA State
of the Science Conference: The Bethesda system for reporting
thyroid cytopathology. Am J Clin Pathol. 132:658–665. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: A software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen BT, Jain AB, Dagis A, Chu P, Vora L,
Maghami E and Salehian B: Comparison of the efficacy and safety of
ultrasound-guided core needle biopsy versus fine-needle aspiration
for evaluating thyroid nodules. Endocr Pract. 21:128–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harvey JN, Parker D, De P, Shrimali RK and
Otter M: Sonographically guided core biopsy in the assessment of
thyroid nodules. J Clin Ultrasound. 33:57–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paja M, Del Cura JL, Zabala R, Corta I,
Lizarraga A, Oleaga A, Expósito A, Gutiérrez MT, Ugalde A and López
JI: Ultrasound-guided core-needle biopsy in thyroid nodules. A
study of 676 consecutive cases with surgical correlation. Eur
Radiol. 26:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung JY, Na DG, Kim KS, Yoo H, Lee H, Kim
JH and Baek JH: Diagnostic accuracy of fine-needle aspiration
versus core-needle biopsy for the diagnosis of thyroid malignancy
in a clinical cohort. Eur Radiol. 22:1564–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trimboli P, Nasrollah N, Guidobaldi L,
Taccogna S, Modica DD Cicciarella, Amendola S, Romanelli F, Lenzi
A, Nigri G, Centanni M, et al: The use of core needle biopsy as
first-line in diagnosis of thyroid nodules reduces false negative
and inconclusive data reported by fine-needle aspiration. World J
Surg Oncol. 12:612014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang M, Zhang Y, Fu S, Lv F and Tang J:
Thyroid nodules with suspicious ultrasound findings: The role of
ultrasound-guided core needle biopsy. Clin Imaging. 38:434–438.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tunbridge WM, Evered DC, Hall R, Appleton
D, Brewis M, Clark F, Evans JG, Young E, Bird T and Smith PA: The
spectrum of thyroid disease in a community: The Whickham survey.
Clin Endocrinol (Oxf). 7:481–493. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacini F, Schlumberger M, Dralle H, Elisei
R, Smit JW and Wiersinga W: European Thyroid Cancer Taskforce:
European consensus for the management of patients with
differentiated thyroid carcinoma of the follicular epithelium. Eur
J Endocrinol. 154:787–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rago T, Santini F, Scutari M, Pinchera A
and Vitti P: Elastography: New developments in ultrasound for
predicting malignancy in thyroid nodules. J Clin Endocrinol Metab.
92:2917–2922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frates MC, Benson CB, Charboneau JW, Cibas
ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB,
Goellner JR, et al: Management of thyroid nodules detected at US:
Society of Radiologists in Ultrasound consensus conference
statement. Radiology. 237:794–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yassa L, Cibas ES, Benson CB, Frates MC,
Doubilet PM, Gawande AA, Moore FD Jr, Kim BW, Nosé V, Marqusee E,
et al: Long-term assessment of a multidisciplinary approach to
thyroid nodule diagnostic evaluation. Cancer. 111:508–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Schnadig V, Logrono R and
Wasserman PG: Fine-needle aspiration of thyroid nodules: A study of
4703 patients with histologic and clinical correlations. Cancer.
111:306–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gharib H, Papini E, Paschke R, Duick DS,
Valcavi R, Hegedüs L and Vitti P: AACE/AME/ETA Task Force on
Thyroid Nodules: American Association of Clinical Endocrinologists,
Associazione Medici Endocrinologi, and European Thyroid Association
medical guidelines for clinical practice for the diagnosis and
management of thyroid nodules. J Endocrinol Invest Invest
Endocrinol Invest. 33 5 Suppl:S1–S50. 2010.
|
|
26
|
Ohori NP and Schoedel KE: Variability in
the atypia of undetermined significance/follicular lesion of
undetermined significance diagnosis in the Bethesda System for
Reporting Thyroid Cytopathology: Sources and recommendations. Acta
Cytol. 55:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cibas ES and Ali SZ: The bethesda system
for reporting thyroid cytopathology. Thyroid. 19:1159–1165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deandrea M, Ragazzoni F, Motta M, Torchio
B, Mormile A, Garino F, Magliona G, Gamarra E, Ramunni MJ,
Garberoglio R and Limone PP: Diagnostic value of a
cytomorphological subclassification of follicular patterned thyroid
lesions: A study of 927 consecutive cases with histological
correlation. Thyroid. 20:1077–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK,
Song DE, Kim TY and Lee JH: Thyroid nodules with initially
nondiagnostic cytologic results: The role of core-needle biopsy.
Radiology. 268:274–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi SH, Baek JH, Lee JH, Choi YJ, Hong
MJ, Song DE, Kim JK, Yoon JH and Kim WB: Thyroid nodules with
initially non-diagnostic, fine-needle aspiration results:
Comparison of core-needle biopsy and repeated fine-needle
aspiration. Eur Radiol. 24:2819–2826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Chen BD, Zhu HF, Wu S, Wei D, Zhang
JQ and Yu L: Comparison of pre-operation diagnosis of thyroid
cancer with fine needle aspiration and core-needle biopsy: A
meta-analysis. Asian Pac J Cancer Prev. 15:7187–7193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khoo TK, Baker CH, Hallanger-Johnson J,
Tom AM, Grant CS, Reading CC, Sebo TJ and Morris JC III: Comparison
of ultrasound-guided fine-needle aspiration biopsy with core-needle
biopsy in the evaluation of thyroid nodules. Endocr Pract.
14:426–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strauss EB, Iovino A and Upender S:
Simultaneous fine-needle aspiration and core biopsy of thyroid
nodules and other superficial head and neck masses using
sonographic guidance. AJR Am J Roentgenol. 190:1697–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hahn SY, Shin JH, Han BK, Ko EY and Ko ES:
Ultrasonography-guided core needle biopsy for the thyroid nodule:
Does the procedure hold any benefit for the diagnosis when
fine-needle aspiration cytology analysis shows inconclusive
results? Br J Radiol. 86:201300072013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ha EJ, Baek JH, Lee JH, Kim JK, Kim JK,
Lim HK, Song DE, Sung TY, Kim TY, Kim WB and Shong YK: Core needle
biopsy can minimise the non-diagnostic results and need for
diagnostic surgery in patients with calcified thyroid nodules. Eur
Radiol. 24:1403–1409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nasrollah N, Trimboli P, Rossi F, Amendola
S, Guidobaldi L, Ventura C, Maglio R, Nigri G, Romanelli F,
Valabrega S and Crescenzi A: Patient's comfort with and
tolerability of thyroid core needle biopsy. Endocrine. 45:79–83.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Polyzos SA and Anastasilakis AD: Clinical
complications following thyroid fine-needle biopsy: A systematic
review. Clin Endocrinol (Oxf). 71:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bergeron M and Beaudoin D: Simple
core-needle biopsy for thyroid nodule, complicated tinnitus. Eur
Thyroid J. 3:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trimboli P and Crescenzi A: Thyroid core
needle biopsy: Taking stock of the situation. Endocrine.
48:779–785. 2015. View Article : Google Scholar : PubMed/NCBI
|