Introduction
Malignant melanoma (MM) is a malignant skin tumor
derived from melanocytes. MM is characterized as highly malignant
due to its rapid growth and early metastasis. Approximately 160,000
new cases of MM are diagnosed annually worldwide (1). MM most frequently occurs on the skin,
but may also arise in other organs and tissues, including the oral
cavity, paranasal sinuses, esophagus, larynx, vagina and anorectal
region, whereas the majority of the respiratory system cases are
metastatic. Noncutaneous MM is relatively uncommon, whereas primary
MM of the lung (PMML) is extremely rare. PMML accounts for 0.01% of
all lung tumors, and its incidence is ~0.4% of all MMs (2), with only ~40 cases published in the
English literature to date (3). We
herein present the case of a 61-year-old female patient with PMML
who displayed a small solid nodule during an annual computed
tomography (CT) screening of the lung and discuss the
clinicopathological characteristics.
Case report
A 61-year-old Japanese female non-smoker was
referred to the Yao Municipal Hospital (Yao, Japan) with an
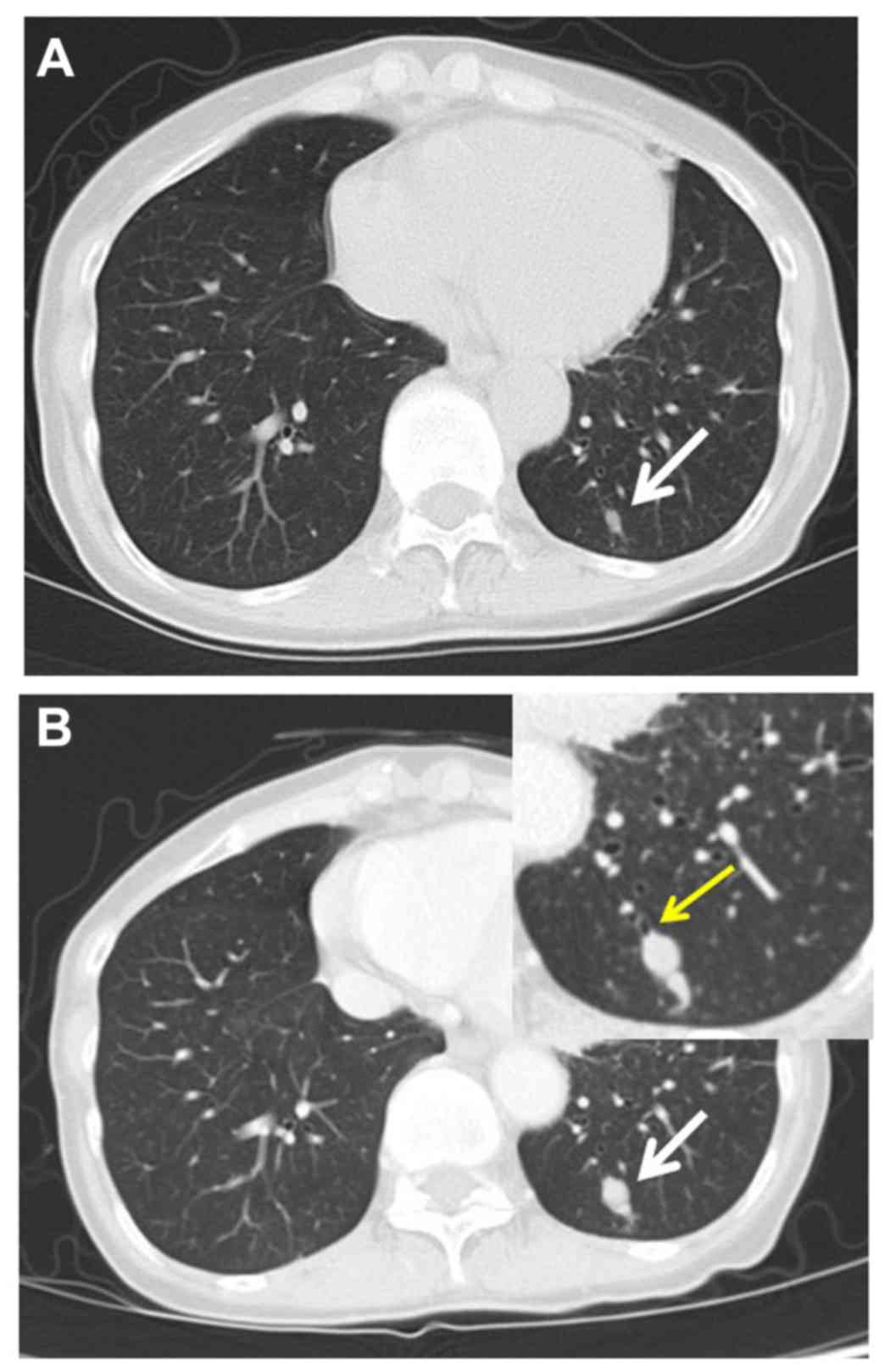
asymptomatic pulmonary nodule (diameter, 6 mm) discovered on annual
CT screening (Fig. 1A). The
patient's medical history was unremarkable. Clinical examinations
and routine laboratory tests, including tumor markers, were within
normal limits. A chest CT taken after a 2-month observation
revealed a homogeneous and well-defined 13mm nodule in the left
basal segment (S10), without mediastinal or hilar lymphadenopathy
(Fig. 1B). The nodule had rapidly
increased in diameter from 6 to 13 mm over 2 months and the CT
revealed disruption of the branch of the B10 bronchus by the nodule
(Fig. 1B, inset).
As lung cancer or an atypical carcinoid tumor was
suspected, thoracotomy was performed under the assistance of
videoscopy. A wedge resection of S10 with a safe margin was
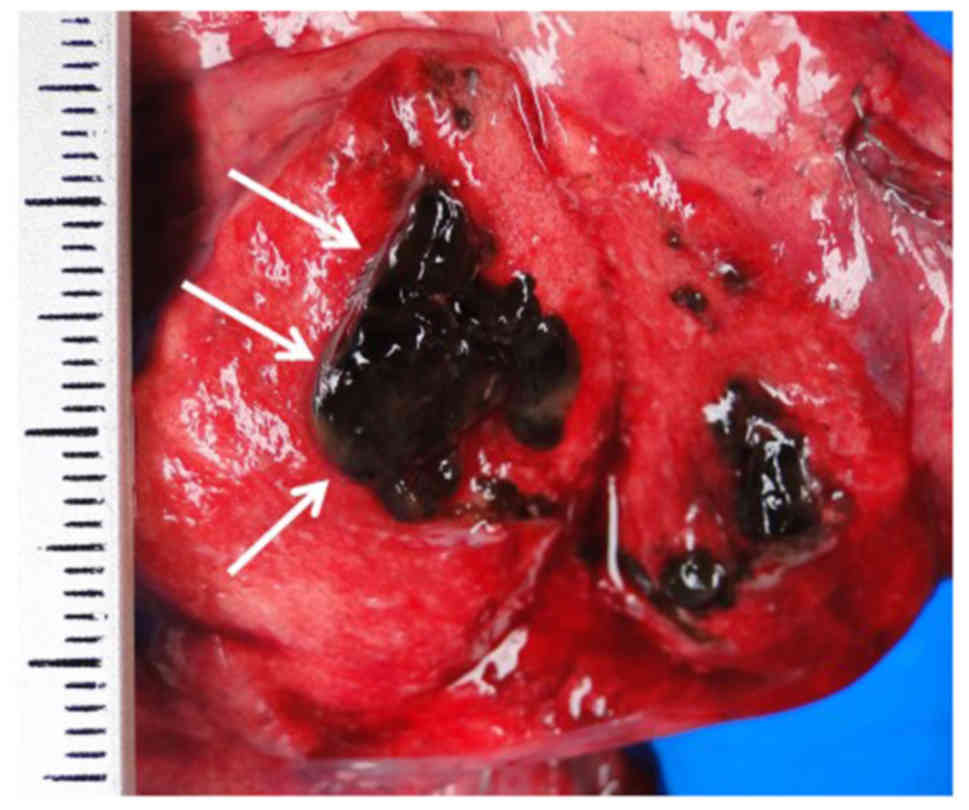
performed through the left fifth intercostal space. The tumor was
of soft consistency, and homogenously black and fleshy on the cut
surface (Fig. 2). Macroscopically,
MM was suspected, and it was confirmed by frozen-section histology.
Subsequently, left lower lobectomy with systematic lymph node
dissection were performed.
Grossly, the tumor was darkly pigmented, with a
solid growth pattern and measured 19×14 mm. The tumor borders were
well-circumscribed by the dilated bronchial wall. Histological
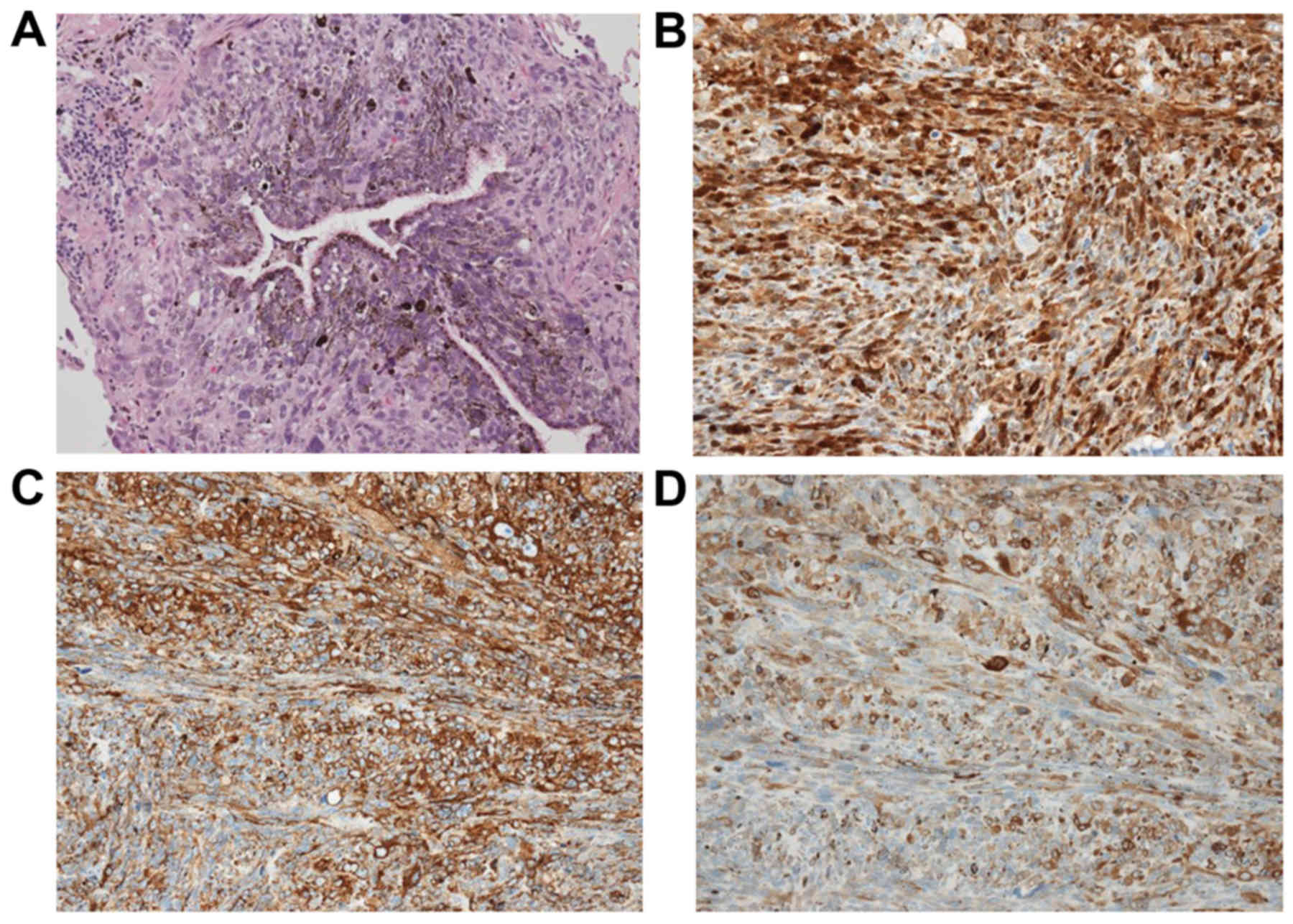
examination of permanent sections (hematoxylin and eosin staining)
revealed multiplying spindle cells with large nuclei including
clear nucleoli, and extensive brown pigmentation in the cytoplasm.
The tumor extended to the bronchial epithelium (Fig. 3A) and lung parenchyma
bidirectionally. However, vascular or lymphatic invasion was not
detected. Immunohistochemical staining was positive for S100
protein (Fig. 3B), human melanoma
black-45 (Fig. 3C) and melan-A
(Fig. 3D). Fontana-Masson stain was
also positive. There was no lymph node involvement. The patient was
diagnosed with MM.
Postoperatively, to distinguish between primary and
metastatic pulmonary melanoma, the skin, mucosae, scalp, anogenital
region and eyes were thoroughly examined, but no melanocytic lesion
was identified. Magnetic resonance imaging of the brain, upper
gastrointenstinal endoscopy and colonoscopy also did not identify
any extrapulmonary disease. Finally, a full-body positron emission
tomography scan (PET/CT) showed no evidence of malignancy.
Therefore, the patient was diagnosed with PMML.
The postoperative course was uneventful and the
patient was discharged with no complications on postoperative day
14. At a regular work-up performed 1 year after the operation,
multiple lung, liver and bone metastases were identified. As
BRAFV600E mutation was not detected, high-dose dacarbazine (DTIC;
1,000 mg/m2) was administered. However, a clinically
meaning full response was not achieved, the patient exhibited
elevation of the serum lactate dehydrogenase level and her
performance status deteriorated. After one course of DTIC, the
patient received immunotherapy 3 times using programmed cell death
protein 1 (PD-1) antibody (2 mg/kg) at 3-week intervals, but she
succumbed to the disease 15 months after the first surgery for the
primary tumor.
Discussion
Non-cutaneous MM is relatively uncommon, whereas
PMML is extremely rare. PMML accounts for 0.01% of all lung tumors,
and the incidence of PMML is ~0.4% of all MMs (1). MM involving the respiratory tract is
nearly always metastatic. As previously reported, skin melanomas
may spontaneously disappear after they have already metastasized
(4). Distinguishing PMML from
metastatic melanoma to the lung may be difficult. The definitive
diagnosis of PMML is based on clinical, radiological and
pathological findings. Criteria for the diagnosis of PMML were
previously proposed by Allen and Drash (5), as follows: i) Junctional change with
dropping off or nesting of melanoma cells just beneath the
bronchial epithelium; ii) invasion of the bronchial epithelium by
the melanoma cells in an area where the bronchial epithelium is not
ulcerated; and iii) an obvious melanoma beneath the abovementioned
changes.
The clinical criteria proposed by Jensen and Egedorf
are widely accepted. The diagnosis requires four clinical criteria
(6): i) A solitary lung mass or
nodule; ii) typical histopathology confirmed by
immunohistochemistry and/or electron microscopy; iii) no prior
history of excision/fulguration of a cutaneous, mucous membrane, or
ocular lesion, unless the pathological examination explicitly ruled
out a melanoma; and iv) no demonstrable melanoma outside the chest
at the time of diagnosis.
On the basis of these criteria, the case presented
herein was compatible with a diagnosis of PMML.
The pathogenesis of PMML remains unclear.
Melanocytes have been identified in the larynx and esophagus,
which, along with the lower respiratory tract, are derived from the
sixth bronchial arch. Thus, it plausible that PMML is derived from
benign melanocytes that have migrated with the respiratory tract
during embryogenesis. As shown in the inset of Fig. 1B, PMML was considered to have
originated from the bronchial wall proximal to the sixth bronchial
arch in the present case.
According to previous reports (7), a surgical approach with adjuvant
chemotherapy/immunochemotherapy may enable long-term survival. The
therapeutic effect of conventional chemotherapy or radiation
therapy is currently considered to be poor. Novel immunotherapy,
with a combination of check-point inhibitors, such as ipilimumab
(anti-cytotoxic T-lymphocyte antigen-4, CTLA-4) and nivolumab
(anti-PD1), has been tested in patients with advanced melanoma in
several trials, with promising results (8).
The volume-doubling time (VDT) of melanoma is
shorter (mean, 48 days) than in any other malignant tumors, apart
from testicular and anaplastic thyroid cancer (9). However, there are no data on VDT in
PMML. In the present case, the VDT of PMML was calculated to be 40
days.
Early detection and complete removal with regional
lymph node dissection are crucial for long-term survival of PMML
patients. As there was no lymph node metastasis or
vascular/lymphatic invasion in the present case, postoperative
adjuvant therapy was not performed. However, diligent follow-up
taking the short VDT into consideration is mandatory for such an
aggressive malignant tumor.
This rare case should raise awareness that early
diagnosis prior to hematogenous spread is the most important
survival factor for such a metabolically active tumor with a
shorter VDT. The PET scan performed immediately after removal of
the primary lesion failed to identify micro-metastases in the
present case. When a small solid nodule is identified affecting the
bronchus proximal to the sixth bronchial arch on high-resolution
CT, particularly in the lower lobe, open thoracotomy and excisional
biopsy should be considered, to offer a chance for cure.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson RW and Moran CA: Primary melanoma
of the lung: A clinicopathologic and immunohistochemical study of
eight cases. Am J Surg Pathol. 21:1196–1202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kyriakopoulos C, Zarkavelis G,
Andrianopoulou A, Papoudou-Bai A, Stefanou D, Boussios S and
Pentheroudakis G: Primary pulmonary malignant melanoma: Report of
an important entity and literature review. Case Rep Oncol Med.
2017:86543262017.PubMed/NCBI
|
|
4
|
Emanuel PO, Mannion M and Phelps RG:
Complete regression of primary malignant melanoma. Am J
Dermatopathol. 30:178–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allen MS Jr and Drash EC: Primary melanoma
of the lung. Cancer. 21:154–159. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jensen OA and Egedorf J: Primary malignant
melanoma of the lung. Scand J Respir Dis. 48:127–35. 135.
1967.PubMed/NCBI
|
|
7
|
Maeda R, Isowa N, Onuma H, Miura H,
Tokuyasu H and Kawasaki Y: Primary malignant melanoma of the lung
with rapid progression. Gen Thorac Cardiovasc Surg. 57:671–674.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henschke CI, Yankelevitz DF, Yip R, Reeves
AP, Farooqi A, Xu D, Smith JP, Libby DM, Pasmantier MW and
Miettinen OS: Writing Committee for the I-ELCAP Investigators: Lung
cancers diagnosed at annual CT screening: Volume doubling times.
Radiology. 263:578–583. 2012. View Article : Google Scholar : PubMed/NCBI
|