Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in men, and the seventh most common cancer in women
worldwide, and it is the most common cause of cancer-associated
mortality (1). Radiofrequency
ablation (RFA) was first described by Rossi et al (2) as a local thermal ablation therapy for
HCC. It has been endorsed as a curative treatment modality by
several clinical management guidelines for early-stage,
unresectable HCC (3–9). However, to further improve the
treatment outcomes of RFA for HCC, it is important to accurately
identify the position of tumors on performing ultrasonography. Even
in cases for which the visualization of tumors is difficult with
the conventional B-mode ultrasound imaging, performing RFA using
treatment support such as artificial pleural effusion, fusion
imaging, and contrast-enhanced ultrasound with Sonazoid™
(Perflubutane; Daiichi Sankyo Co., Ltd., Tokyo, Japan), is expected
to improve treatment outcomes in clinical settings (10).
The aim of the present study was to evaluate which
treatment support most effectively contributed to reducing the rate
of local recurrences of HCC directly abutting the diaphragm, which
are difficult to visualize using B-mode ultrasound imaging, and the
factors that affect local recurrences.
Patients and methods
A total of 103 HCC tumors, measuring <5 cm
located immediately abutting the diaphragm and difficult to
visualize using B-mode ultrasound imaging, were evaluated at the
Saiseikai Niigata Daini Hospital, Niigata, Japan, between January
2009 and December 2015, including 33 HCC tumors treated using RFA
without treatment support and 70 HCC tumors treated using RFA with
artificial pleural effusion, contrast ultrasound, and fusion
imaging, either individually or in combination for an improved
visualization of the tumors.
HCC was diagnosed using dynamic contrast computed
tomography (CT) or dynamic contrast magnetic resonance imaging
(MRI). Exclusion criteria included: i) A tumor diameter of ≥5 cm;
ii) infiltrating type with intentionally insufficient RFA; iii) a
≥4-month interval between RFA and the first follow-up CT or MRI;
iv) no follow-up CT or MRI; and v) an observation period <6
months.
The ultrasound device used was equipped with a
fusion imaging system (the Volume Navigation System; GE Healthcare,
Milwaukee, WI, USA) and contrast-enhanced ultrasonography (CEUS)
installed in LOGIQ E9 (GE Healthcare, Milwaukee, WI, USA).
Treatment was performed in all patients through the percutaneous
approach under ultrasound guidance, using the Angiodynamics (RITA
Medical Systems, Inc., Mountain View, CA, USA) StarBurst XL (nine
arrays; 5 cm) thermal ablation catheter, and the model 1500
(150–200 W power) generator, as previously described (11). This device has the capability to
produce scalable, spherical ablations (of at least 5 cm). RFA was
performed percutaneously in the present study by two hepatologists
(T.I. and M.I.) with more than 10 years' of clinical experience in
performing liver tumor ablation. No differences existed in the
results for success rates of RFA between the two operators.
Fusion imaging
Fusion imaging was performed using volume data from
the Volume Navigation System (V-Navi) installed in the ultrasound
device. After the volume data of the CT or MRI images for reference
had been imported in the DICOM (Digital Imaging and Communication
in Medicine) format, the cross-section of ultrasound images
approximately parallel to the reference was registered.
Subsequently, one characteristic corresponding landmark between the
ultrasound images and the reference was marked for each image as CT
or MRI, and the position was aligned. In order to improve the
registration accuracy, particularly in the vicinity of the tumor,
the positional registration was repeatedly performed as close to
the tumor as possible. After the positional registration, the
multiplanar reconstruction images of CT or MRI were synchronously
displayed side by side with the real-time ultrasound images.
CEUS using Sonazoid™
Sonazoid™ (Perflubutane; Daiichi Sankyo Co., Ltd.)
was used as the CEUS agent (0.5 ml/body) in all of the
examinations. The target lesions were scanned following injection
in the arterial and Kupffer phases. The arterial phase of CEUS
imaging was defined as that which occurred from 10–60 sec following
the injection of Sonazoid™, and the Kupffer phase as that occurring
10 min following injection (10,12).
Method of inducing artificial pleural
effusion
To infuse 5% glucose solution into the right pleural
cavity without injuring the lung, a small skin incision was made on
the chest wall under local anesthesia. The procedure was performed
on patients under conscious sedation using a combination of 25 mg
intramuscular hydroxyzine (Atarax P; Pfizer Japan Inc., Tokyo,
Japan) and 15 mg pentazocine (Pentagin; Daiichi Sankyo Co., Ltd.,
Tokyo, Japan) administered 15 min prior to treatment. Following the
injection of 10 ml 1% lidocaine (Xylocaine; AstraZeneca K.K.,
Osaka, Japan) into the peritoneum along the puncture line,
percutaneous puncture was performed using a 21-gauge needle under
sonographic guidance. Subsequently a drainage catheter kit (Hanaco
Medical Co., Ltd., Saitama, Japan) was intrathoracically inserted
through the chest wall. Once the needle entered the pleural cavity
and no tissue resistance was encountered, the inner stylet extracts
and guidewire (Teromo Clinical Supply Co., Ltd., Tokyo, Japan) were
inserted into the pleural area. The outer catheter was inserted
through the guidewire. Glucose solution (5%) was infused
intrathoracically to separate the lung and liver; hence, it was
possible to obtain an image of the hepatic dome (13,14).
During follow-up observation following treatment,
local recurrence was evaluated by subsequent CT scans or MRI. All
the patients were followed up every 3 months with measurement of
the levels of serum α-fetoprotein (AFP) and des-gamma-carboxy
prothrombin (DCP), and enhanced CT or enhanced MRI. The detection
of local tumor progression was defined as a recurrent tumor within,
or adjacent to, the treated tumor.
The rate of local recurrence, and factors affecting
local recurrence, were subsequently determined.
Ethics statement
Data are available from the Saiseikai Niigata Daini
Hospital Data Access/Ethics Committee for researchers who meet the
criteria for access to confidential data. The present study was
approved by the Institutional Review Board of Saiseikai Niigata
Daini Hospital (Niigata, Japan), and the study was conducted in
accordance with the principles of the Declaration of Helsinki. All
the patients provided their written informed consent.
Statistical analysis
The primary endpoint of the current clinical study
was local recurrence. Local recurrence rates were calculated using
the Kaplan-Meier method and log-rank tests, and the generalized
Wilcoxon test was used for statistical analysis. Regarding the
patient characteristics, statistical analyses were performed using
the Fisher exact test and the Wilcoxon rank-sum test. A Cox
proportional hazards model was used to identify independent factors
of local recurrence. Statistical processing was performed using
StatView version 5.0 software (SAS Institute, Cary, NC, USA). All
reported P-values are two-sided, and P<0.05 was considered to
indicate a statistically significant difference.
Results
The patients' baseline characteristics are
summarized in Table I. The mean age
was 71.56±10.33 years and the male-to-female ratio was 80:23. Among
the 103 enrolled patients, 18 patients were seropositive for
hepatitis B surface antigen, 54 patients were seropositive for
hepatitis C virus antibody, and 31 patients were seronegative for
both the hepatitis B surface antigen and the hepatitis C virus
antibody. The mean AFP value was 122.53±396.92 ng/ml, the mean DCP
value was 643.51±2,256.19 mAU/ml, the total bilirubin value was
0.67±0.34 mg/dl, the serum albumin value was 3.67±0.46 g/dl, and
prothrombin activity was 96.32±14.73%. The mean observation period
was 12.660±10.312 (range, 6–56) months. Artificial pleural effusion
was used in 35 cases, CEUS with Sonazoid™ in 35 cases, and fusion
imaging in 25 cases. The backgrounds of the patients with or
without a supportive method were, for the most part, not
significantly different (as shown in Tables II–IV for the patients with or without
Sonazoid™, fusion analysis and artificial effusion as supportive
methods, respectively), with the exception of the prothrombin
activity data in the backgrounds of patients with or without fusion
imaging, or artificial effusion, as supportive methods (P=0.02 and
0.032, respectively). Furthermore, there were no complications
resulting from the supportive methods.
 | Table I.Baseline characteristics of the study
population. |
Table I.
Baseline characteristics of the study
population.
| Demographic
variable | Mean ± S.D. | Range |
|---|
| Age (years) | 71.56±10.33 | 31–86 |
| Sex (male:female
ratio) | 80:23 |
|
| Etiology
(HBV/HCV/non-HBV, non-HCV) | 18/54/31 |
|
| Size (mm) | 25.78±9.76 | 10–60 |
| AFP (ng/ml) | 122.53±396.92 | 1.5–2,578.7 |
| DCP (mAU/ml) | 643.51±2,256.19 | 10.0–14,874.0 |
| Serum albumin
(g/dl) | 3.67±0.46 | 2.5–4.7 |
| Prothrombin activity
(%) | 96.32±14.73 | 54.9–127.1 |
| Total bilirubin
(mg/dl) | 0.67±0.34 | 0.21–2.1 |
| Artificial effusion?
(yes/no) | 35:68 |
|
| Sonazoid™?
(yes/no) | 35:68 |
|
| Fusion imaging?
(yes/no) | 25:78 |
|
| Child-Pugh Score
(5/6/7/8) | 67/23/8/5 |
|
| Class (A/B) | 90/13 |
| With/without prior
TACE | 76/27 |
|
 | Table II.Comparison of the backgrounds of
patients with or without Sonazoid™ as a supportive method. |
Table II.
Comparison of the backgrounds of
patients with or without Sonazoid™ as a supportive method.
| Demographic
variable | With Sonazoid™
(n=35) | Without Sonazoid™
(n=68) | P-value |
|---|
| Age (years) | 73.14±9.11 | 70.75±10.88 | 0.267 |
| Gender (male:female
ratio) | 27:8 | 53:15 | 0.927 |
| Size (mm) | 24.14±8.86 | 26.64±9.88 | 0.213 |
| AFP (ng/ml) | 227.04±611.45 | 68.74±203.67 | 0.054 |
| DCP (mAU/ml) | 558.14±1,607.47 | 687.44±2536.24 | 0.785 |
| Serum albumin
(g/dl) | 3.66±0.39 | 3.68±0.51 | 0.889 |
| Prothrombin activity
(%) | 94.58±14.61 | 97.22±14.82 | 0.392 |
| Total bilirubin
(mg/dl) | 0.68±0.35 | 0.67±0.33 | 0.797 |
| With/without prior
TACE | 25:10 | 51:17 | 0.696 |
| Child-Pugh class
(A/B) | 32/3 | 58/10 | 0.375 |
 | Table IV.Comparison of the backgrounds of
patients with or without artificial effusion as a supportive
method. |
Table IV.
Comparison of the backgrounds of
patients with or without artificial effusion as a supportive
method.
| Demographic
variable | With artificial
effusion (n=35) | Without artificial
effusion (n=68) | P-value |
|---|
| Age (years) | 69.839.09 | 72.45±10.87 | 0.223 |
| Gender (male:female
ratio) | 27:8 | 53:15 | 0.927 |
| Size (mm) | 26.23±9.19 | 25.47±8.93 | 0.695 |
| AFP (ng/ml) | 114.77±439.82 | 126.52±76.35 | 0.887 |
| DCP (mAU/ml) | 205.57±401.60 |
868.91±2,141.41 | 0.158 |
| Serum albumin
(g/dl) | 3.66±0.56 | 3.67±0.41 | 0.959 |
| Prothrombin
activity (%) | 91.98±18.02 | 98.56±12.27 | 0.031 |
| Total bilirubin
(mg/dl) | 0.73±0.42 | 0.64±0.22 | 0.223 |
| With/without prior
TACE | 23:12 | 53:15 | 0.181 |
| Child-Pugh class
(A/B) | 29/6 | 61/7 | 0.322 |
Of the total 103 nodules, local recurrences were
confirmed in 17 nodules (16.50%). The overall rate of local
recurrence was 13.1% at 6 months, and 20.2% at 12 months. Of the 33
nodules that were not provided with treatment support, local
recurrences were confirmed in 12 nodules (36.3%), with a rate of
local recurrence of 32.4% at 6 months, and 58.5% at 12 months. Only
five of the 70 nodules given treatment support revealed local
recurrences (7.1%). The rate of local recurrence was 6.2, 6.2, and
8.7% over 6, 12, and 24 months, respectively. Therefore, these
results indicated that the treatment support significantly lowered
the rate of local recurrences (P<0.05). The rate of local
recurrence when fusion imaging was used was 8.7% for all the
subjects at 6, 12, and 24 months; however, the rate of local
recurrence for RFA without fusion imaging was 14.7, 25.1, and 28.4%
over 6, 12, and 24 months, respectively.
A significant difference in the rate of local
recurrence was identified with the use of artificial pleural
effusion. The rate of local recurrence in RFA with artificial
pleural effusion was 6.0% at 6 and 12 months. The rate of local
recurrence in RFA without artificial pleural effusion was 18.0%
over 6 months, and 30.4% over 12 months, showing a significant
increase (P=0.008).
A significant difference in the rate of local
recurrence of RFA was also identified, depending on whether or not
CEUS with Sonazoid™ was performed. The rate of local recurrence in
CEUS RFA with Sonazoid™ was 0.0, 0.0, and 5.9% at 6, 12, and 24
months, respectively. The rate of local recurrence in RFA CEUS
without Sonazoid™ was 20.6, 31.0, and 31.0% over 6, 12, and 24
months, respectively (P=0.0052) (Fig.
1), indicating a significant increase.
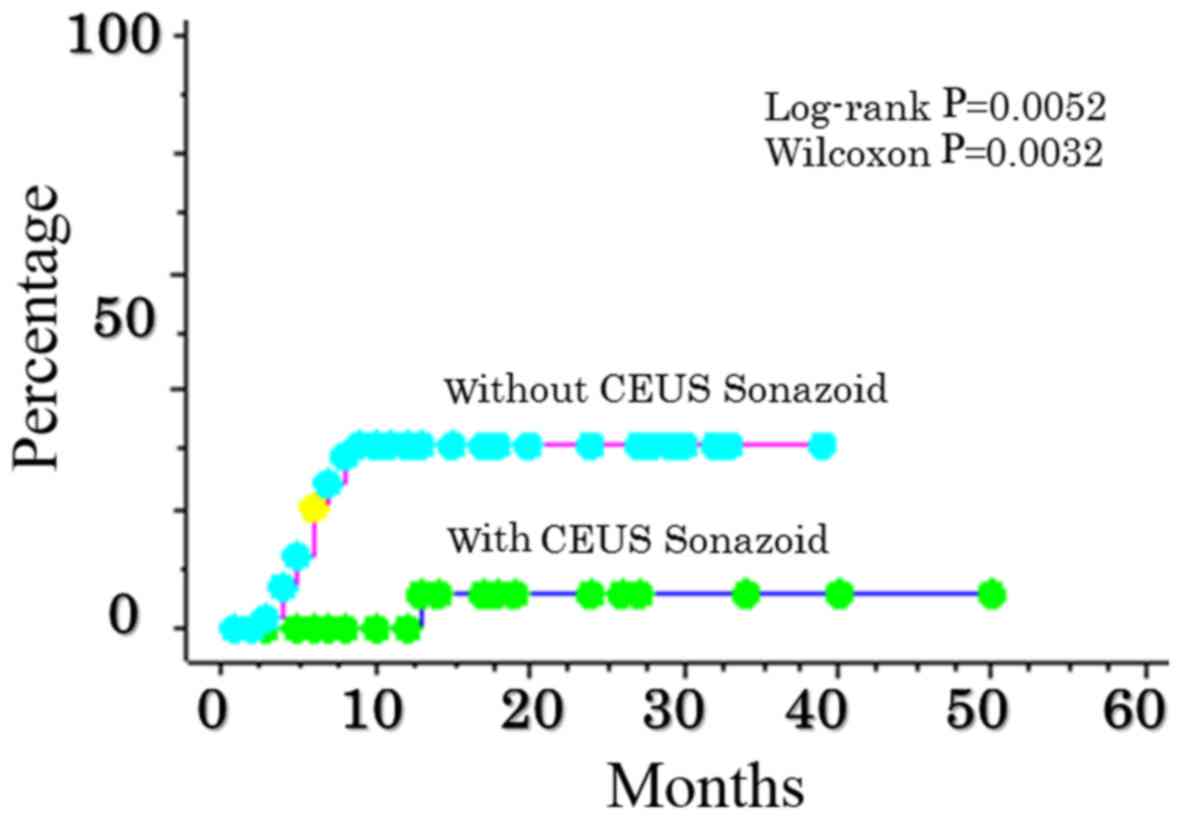 | Figure 1.Comparison of local recurrence after
RFA abutting the sub-diaphragm as determined from the use, or not,
of CEUS ultrasonography with Sonazoid™. The rate of local
recurrence in contrast ultrasonographic RFA with Sonazoid™ was 0.0,
0.0, and 5.9% at 6, 12, and 24 months, respectively. The rate of
contrast ultrasonography without Sonazoid™ was 20.6, 31.0 and 31.0%
over 6, 12 and 24 months, respectively (P=0.0052). CEUS,
contrast-enhanced ultrasonography. |
No significant differences in the rate of local
recurrence according to the size of the tumors or measurements of
hepatic function reserve, such as the Child-Pugh score, were
identified. There was no local recurrence in 21 cases: Four of
those cases were treated with artificial pleural effusion, fusion
imaging, and Sonazoid™ CEUS; three cases were treated with fusion
imaging and Sonazoid™ CEUS; seven cases were treated with
artificial pleural effusion and Sonazoid™ CEUS; and seven cases
were treated with artificial pleural effusion and fusion
imaging.
In addition, in a multivariate analysis of these
treatment factors, the use of CEUS with Sonazoid™ and artificial
pleural effusion contributed to a significantly lower rate of local
recurrences, with hazard ratios of 0.075 and 0.143, respectively
(Table V).
 | Table V.Prognostic factors related to local
recurrence determined by multivariate analysis using the Cox
proportional hazard model. |
Table V.
Prognostic factors related to local
recurrence determined by multivariate analysis using the Cox
proportional hazard model.
| Variable | HR | 95% CI | P-value |
|---|
| Child-Pugh
class |
|
|
|
| A | 0.948 | 0.210–4.281 | 0.944 |
| B | 1 |
|
|
| Size |
|
|
|
| ≥30
mm | 1.680 | 0.460–6.141 | 0.433 |
| <30
mm | 1 |
|
|
| Artificial
effusion |
|
|
|
|
With | 0.143 | 0.031–0.6.61 | 0.013 |
|
Without | 1 |
|
|
| Fusion imaging |
|
|
|
|
With | 0.223 | 0.047–1.052 | 0.058 |
|
Without | 1 |
|
|
| Sonazoid™ |
|
|
|
|
With | 0.075 | 0.010–0.571 | 0.012 |
|
Without | 1 |
|
|
Discussion
HCC is the most frequent primary hepatic malignancy,
and has been recognized as the predominant cause of mortality in
patients with cirrhosis (1). Various
types of nonsurgical treatments have been developed. RFA is able
achieve complete tumor necrosis (2–9,15,16), and
has become a principal treatment in numerous institutions for
patients with small HCC tumors.
RFA was first used for the treatment of HCC in
humans in 1993 (2) following
extensive animal studies (17). RFA
has since emerged as a popular local ablative therapy for
unresectable HCC due to its efficacy and safety.
RFA is currently recognized as an effective local
treatment (18) in patients with
Barcelona Clinic Liver Cancer (BCLC) early stage HCC who are not
eligible for surgical treatments. RFA has also been established as
a safe, effective percutaneous technique for patients with HCC. RFA
with a percutaneously inserted electrode is suitable as a method
for a more complete ablation of tumors compared with other
locoregional treatments, thereby leading to a reduction in the rate
of local recurrence (19).
Local recurrence following the successful ablation
of HCC using RFA is therefore an important issue. However,
incomplete tumor ablation (20) and
local tumor recurrence or progression remain problematic (21). In order to prevent local recurrence,
Hirooka et al (22) reported
that the safety margin for RFA should be defined as the blood
drainage area, and ablation should aim at acquiring adequate safety
margins. In the present study, CT during arterial portography
(CTAP) and CT during arteriography (CTA) were performed on the
patients with TACE. Furthermore, to assess the ablated area and
complications, dynamic CT was performed within 3 days of the
ablation. The goal of the treatment was to achieve complete
ablation of the hypoattenuating areas visualized during the portal
venous phases, and extending beyond the tumor itself. Additional
ablation sessions were scheduled if the presence of residual
lesions was confirmed. The diagnostic and treatment procedures were
repeated until complete ablation was achieved during a single
hospitalization.
Since it is usually performed under ultrasound
guidance, cases in which the whole tumor could not be visualized
due to being located immediately under the subdiaphragm, or in
contact with adjacent organs, were considered difficult to treat
using RFA. If complete necrosis of the tumor is attempted for these
difficult cases using RFA, assisting techniques might be required
in order to prevent local recurrence. When performing RFA with
ultrasound guidance, it is important from the viewpoint of
operative techniques, indications, and implementation criteria that
the whole tumor is visualized. In recent years, RFA has become more
precise and safer, with advances being made in ultrasound
technologies, such as CEUS and fusion imaging. Treatment support
can also be administered with artificial pleural effusion. It has
already been reported that RFA with artificial pleural effusion is
effective in treating difficult cases; however, there has been
insufficient examination of RFA compared with other modalities.
In the present study, HCC tumors in which the whole
tumor could not be visualized due to being located abutting the
diaphragm were examined using RFA with CT/MRI fusion imaging,
artificial pleural effusion, and CEUS with Sonazoid™. In January
2007, Sonazoid™ was approved as a new CEUS agent in Japan (10). With the development of Sonazoid™, a
second-generation ultrasound contrast agent, low sound pressure
irradiation is used to inhibit the collapse of bubbles, and the
contrast effects may be observed continuously in real time. In the
vascular phase, tumor hemodynamics may be visualized. In the
post-vascular phase 10 min after administration, by taking
advantage of its tendency to be taken up by Kupffer cells, tumors
without Kupffer cells may also be clearly visualized.
Sonazoid™ CEUS is thought to offer visualization of
tumors that is equivalent to that offered by CT angiography
(23,24). Even nodules that were not able to be
visualized using the B-mode could be clearly visualized using
Sonazoid™ CEUS, and precise localized treatment was therefore
possible. Even lesions that are clearly identified by contrast CT
and MRI may not be clearly identified by B-mode ultrasound due to
the background hepatic conditions or artifacts from previous
cauterization. In such cases, observation with Sonazoid™ CEUS may
be effective. In addition, compared with the historical controls
that were in place prior to the availability of Sonazoid™, the
number of RFA sessions per treatment is reported to have decreased
(25). Therefore, the effectiveness
of Sonazoid™ CEUS in RFA has been confirmed. When using Sonazoid™
with RFA, a puncture may be made while observing early staining on
vascular imaging immediately following an injection of Sonazoid™.
Alternatively, the puncture may be made for a Kupffer defect via
Kupffer imaging 10–15 min after the injection. If it is already
known that a Kupffer defect would not be clear, the puncture may be
made using vascular imaging. However, as there is only a short
period of time in which early staining is clear (a few sec),
puncture with vascular imaging requires a high level of skill. In
the present study, the effectiveness of RFA puncture with Sonazoid™
CEUS was examined using Kupffer imaging.
Furthermore, recently, another type of treatment
support called ‘fusion imaging’ as V-Navi, a volume navigation
system that allows for display and observation of real-time
ultrasound imaging in combination with other modalities, has been
developed (26). This system makes
it possible to perform real-time observation of ultrasound images
fused with three-dimensional ultrasound images that were previously
collected. Although ultrasonography has numerous advantages over
other modalities, it is considered inferior to CT in terms of its
dependence on the examiner and objectivity. To compensate for these
disadvantages, the V-Navi system was developed so that images
obtained using other modalities could be added to the
ultrasonography images, and fused for display. Although this may be
extremely effective in certain cases, if the hepatic condition
during treatment differs from that during CT imaging due to
respiration, body position, and the condition of the surrounding
organs, the CT and ultrasound images may not correctly overlap.
In the present study, images obtained following
treatment deviated from those obtained prior to the operation in
certain of the cases in which fusion imaging was used with
artificial pleural effusion. The artificial pleural effusion
technique, which is easier to perform than the artificial ascites
technique, is used in numerous institutions to prevent lung
obstruction and avoid complications (27,28).
Although no significant differences were observed in
the suppression of local recurrence with fusion imaging only, the
rate of local recurrence was sufficiently decreased in all the
groups that received support treatment compared with the groups
that did not. Therefore, a further examination of the indications
pertaining to fusion imaging is required.
The limitations of the present study were its
retrospective nature and the inclusion of only a small number of
patients. Furthermore, CEUS with Sonazoid™ suffers from a weakness
in being less suitable for imaging in deep positions. However, the
present study identified that CEUS with Sonazoid™ was the most
effective of the three types of treatment support in reducing the
rate of local recurrences of lesions near the diaphragm that were
difficult to visualize using the B-mode. In the future, further
examinations with a larger number of sample sizes will be required
to be performed. However, the results of the present study have
suggested that the combined use of various types of treatment
support for lesions adjacent to the diaphragm that are difficult to
visualize using the B-mode is effective, as it makes achieving safe
and favorable treatment outcomes possible.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi S, Fornari F and Buscarini L:
Percutaneous ultrasound-guided radiofrequency electrocautery for
the treatment of small hepatocellular carcinoma. J Interv Radiol.
8:97–103. 1993.
|
|
3
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of liver diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M: HCC Expert Panel
of Japan Society of Hepatology: Management of hepatocellular
carcinoma in Japan: Consensus-Based Clinical Practice Guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Livraghi T: Treatment of hepatocellular
carcinoma by interventional methods. Eur Radiol. 11:2207–2219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhim H, Lim HK and Choi D: Current status
of radiofrequency ablation of hepatocellular carcinoma. World J
Gastrointest Surg. 2:128–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossi S, Di Stasi M, Buscarini E, Quaretti
P, Garbagnati F, Squassante L, Paties CT, Silverman DE and
Buscarini L: Percutaneous RF interstitial thermal ablation in the
treatment of hepatic cancer. AJR Am J Roentgenol. 167:759–768.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livraghi T, Goldberg SN, Lazzaroni S,
Meloni F, Solbiati L and Gazelle GS: Small hepatocellular
carcinoma: Treatment with radio-frequency ablation versus ethanol
injection. Radiology. 210:655–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kindberg GM, Tolleshaug H, Roos N and
Skotland T: Hepatic clearance of Sonazoid perfluorobutane
microbubbles by Kupffer cells does not reduce the ability of liver
to phagocytose or degrade albumin microspheres. Cell Tissue Res.
312:49–54. 2003.PubMed/NCBI
|
|
11
|
Guglielmi A, Ruzzenente A, Battocchia A,
Tonon A, Fracastoro G and Cordiano C: Radiofrequency ablation of
hepatocellular carcinoma in cirrhotic patients.
Hepatogastroenterology. 50:480–484. 2003.PubMed/NCBI
|
|
12
|
Watanabe R, Matsumura M, Chen CJ, Kaneda Y
and Fujimaki M: Characterization of tumor imaging with
microbubble-based ultrasound contrast agent, sonazoid, in rabbit
liver. Biol Pharm Bull. 28:972–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minami Y, Kudo M, Kawasaki T, Chung H,
Ogawa C, Inoue T, Sakaguchi Y, Sakamoto H and Shiozaki H:
Percutaneous ultrasound-guided radiofrequency ablation with
artificial pleural effusion for hepatocellular carcinoma in the
hepatic dome. J Gastroenterol. 38:1066–1070. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minami Y, Kudo M, Kawasaki T, Chang H,
Ogawa C and Shiozaki H: Percutaneous radiofrequency ablation guided
by contrast enhanced harmonic sonography with artificial pleural
effusion for hepatocellular carcinoma in the hepatic dome. AJR Am J
Roentgenol. 182:1224–1226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tateishi R, Shiina S, Teratani T, Obi S,
Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T and Omata M:
Percutaneous radiofrequency ablation for hepatocellular carcinoma.
An analysis of 1000 cases. Cancer. 103:1201–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lencioni RA, Allgaier HP, Cioni D,
Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J,
Zuber I, Blum HE and Bartolozzi C: Small hepatocellular carcinoma
in cirrhosis: Randomized comparison of radio-frequency thermal
ablation versus percutaneous ethanol injection. Radiology.
228:235–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossi S, Fornari F, Paties C and Buscarini
L: Thermal lesions induced by 480 KHz localized current field in
guinea pig and pig livers. Tumori. 76:54–57. 1990.PubMed/NCBI
|
|
18
|
European Association For The Study Of The
Liver1, ; European Organisation For Research And Treatment Of
Cancer, . EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiina S, Teratani T, Obi S, Sato S,
Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T
and Omata M: A randomized controlled trial of radiofrequency
ablation with ethanol injection for small hepatocellular carcinoma.
Gastroenterology. 129:122–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pulvirenti A, Garbagnati F, Regalia E,
Coppa J, Marchiano A, Romito R, Schiavo M, Fabbri A, Burgoa L and
Mazzaferro V: Experience with radiofrequency ablation of small
hepatocellular carcinomas before liver transplantation. Transplant
Proc. 33:pp. 1516–1517. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrison LE, Koneru B, Baramipour P,
Fisher A, Barone A, Wilson D, Torre A Dela, Cho KC, Contractor D
and Korogodsky M: Locoregional recurrences are frequent after
radiofrequency ablation for hepatocellular carcinoma. J Am Coll
Surg. 197:759–764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirooka M, Ochi H, Koizumi Y, Tokumoto Y,
Hiraoka A, Kumagi T, Abe M, Tanaka H and Hiasa Y: Local recurrence
of hepatocellular carcinoma in the tumor blood drainage area
following radiofrequency ablation. Mol Clin Oncol. 2:182–186.
2014.PubMed/NCBI
|
|
23
|
Kudo M: Diagnostic imaging of
hepatocellular carcinoma: Recent progress. Oncology. 81 Suppl
1:S73–S85. 2011. View Article : Google Scholar
|
|
24
|
Mita K, Kim SR, Kudo M, Imoto S, Nakajima
T, Ando K, Fukuda K, Matsuoka T, Maekawa Y and Hayashi Y:
Diagnostic sensitivity of imaging modalities for hepatocellular
carcinoma smaller than 2 cm. World J Gastroenterol. 16:4187–4192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masuzaki R, Shiina S, Tateishi R, Yoshida
H, Goto E, Sugioka Y, Kondo Y, Goto T, Ikeda H, Omata M and Koike
K: Utility of contrast-enhanced ultrasonography with Sonazoid in
radiofrequency ablation for hepatocellular carcinoma. J
Gastroenterol Hepatol. 26:759–764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makino Y, Imai Y, Igura T, Kogita S, Sawai
Y, Fukuda K, Hori M, Kudo M and Murakami T: Usefulness of the
extracted-overlay function in CT/MR-ultrasonography fusion imaging
for radiofrequency ablation of hepatocellular carcinoma. Dig Dis.
31:485–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koda M, Ueki M, Maeda Y, Mimura K, Okamoto
K, Matsunaga Y, Kawakami M, Hosho K and Murawaki Y: Percutaneous
sonographicially guided radiofrequency ablation with artificial
pleural effusion for hepatocellular carcinoma located under the
diaphragm. AJR Am J Roentgenol. 183:583–588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukuno H, Tamaki K, Urata M, Kohno N,
Shimizu I, Nomura M, Ito S and Saito K: Influence of an artificial
pleural effusion technique on cardio-pulmonary function and
autonomic activity. J Med Invest. 54:48–53. 2007. View Article : Google Scholar : PubMed/NCBI
|















