Introduction
Platinum-based agents have been key drugs for
epithelial ovarian cancer during the past three decades (1). The standard treatment for ovarian
cancer is debulking surgery followed by a combination of taxanes
and carboplatin (TC), but more than half of cases with advanced
disease relapse (2). Relapse within
6 months after the last platinum-based therapy is defined as
platinum-resistant recurrence (Pt-R) (3), and non-platinum single agents such as
pegylated liposomal doxorubicin, paclitaxel, topotecan and
gemcitabine are commonly used for patients with Pt-R recurrent
ovarian cancer (ROC) (4). Among
these drugs, there appear to be no differences in response rate
(10–15%), progression-free survival (PFS) (3–4 months) and overall
survival (OS) (approximately 12 months) (5–10). Due
to the poor prognosis of patients with Pt-R ROC by these
treatments, novel agents including molecular-targeting drugs have
been investigated to overcome platinum-resistance.
The efficacy of administration of platinum
re-treatment for Pt-R ROC has not yet been established. Weekly
paclitaxel with carboplatin for Pt-R ROC had no advantage in terms
of PFS compared with weekly paclitaxel alone (11). By contrast, dose-dense weekly
administration of platinum agents has been reported to extend PFS
for Pt-R ROC in recent years (12,13).
Additionally, patients with Pt-R ROC may benefit again from
platinum-based chemotherapy following the longer interval until
platinum re-treatment with non-platinum single agents (14). Therefore, by modified method or
timing of platinum administration, platinum re-treatment is
potentially effective for Pt-R ROC.
Irinotecan (CPT), a topoisomerase-1 inhibitor, has
modest activity for patients with Pt-R ROC (15,16), and
has synergic effects in combination with cisplatin (CPT-P) in
vitro (17). Furthermore, the
combination of CPT and CPT-P was reported to be effective for
patients with Pt-R ROC (18,19). The present study was performed to
retrospectively evaluate the efficacy and adverse events (AE) in 28
patients treated with irinotecan and platinum (CPT-Pt) for Pt-R
ROC.
Patients and methods
Patients
Platinum-resistance recurrence (Pt-R) was defined as
a relapse within 6 months from the last platinum-based
chemotherapy. After approval by the Institutional Review Board of
the institution, a total of 28 patients with Pt-R ROC treated with
CPT-Pt at the National Defense Medical College Hospital
(Tokorowaza, Japan) between January, 2002 and December, 2012 were
identified from a retrospective review of medical charts. All
patients received combination therapy with TC as the first-line
chemotherapy, and received CPT-Pt for the treatment of recurrent
tumors with Pt-R. Platinum-resistance was sub-classified as follows
(14,20): i) Platinum-refractory, patients who
relapsed during TC therapy; ii) primary platinum-resistance,
patients who relapsed within 6 months after the primary TC therapy;
and iii) secondary platinum-resistance, patients who relapsed
within 6 months after the second-line therapy with platinum-based
regimen for the first relapse following primary TC therapy.
Treatment
Four CPT-Pt regimens were used for the patients with
Pt-R ROC: i) Five-day CPT-P, irinotecan 22.5 mg/m2 and
cisplatin 10 mg/m2 on days 1–5, once every 4 weeks; ii)
weekly (w)CPT-P, irinotecan 40–60 mg/m2 on days 1, 8,
and 15 and cisplatin 50–60 mg/m2 on day 1, once every 4
weeks; iii) weekly CPT-nedaplatin (wCPT-N), irinotecan 40–60
mg/m2 on days 1, 8 and 15 and nedaplatin 60
mg/m2 on day 1, once every 4 weeks; and iv) weekly
CPT-carboplatin (wCPT-C), irinotecan 40–60 mg/m2 on days
1, 8, 15 and carboplatin target area under the concentration vs.
time curve = 5 on day 1, once every 4 weeks. Five-day CPT-P was
used between 2002 and 2007. From 2007, other regimens aside from
five-day CPT-P were selected by clinicians according to kidney
function and history of allergic reactions. CPT-Pt regimens were
administrated until progression of the disease or development of
unacceptable AE. Approximately 80% of the initial dose of CPT-Pt
was delivered in subsequent cycles when the patients developed
severe AE (grade ≥3) in prior cycles. Uridine diphosphate
glucuronosyltransferase 1A1 (UGT1A1) genotyping was available in
approximately half of the cases, and the incidences of severe AE
grade >2 were analyzed according to UGT1A1 genotype.
Assessments
Performance status (PS) was evaluated according to
the Eastern Cooperative Oncology Group criteria (21). Response to treatment was assessed by
computed tomography (CT) imaging using Response Evaluation Criteria
in Solid Tumors (RECIST) guidelines (version 1.1) (22), or cancer antigen (CA)-125 criteria
defined by Rustin et al (23). CA-125 levels were checked before
every cycle. CT was performed every 2–3 cycles, or when disease
progression was clinically suspected. Clinical benefit rate (CBR)
was defined as the percentage of the patients who achieved complete
response (CR), partial response (PR) and stable disease (SD) to all
enrolled patients (24). AE were
assessed using Common Terminology Criteria for AE version 4.0
(25). PFS was defined as the period
of time between the date of the first cycle of CPT-Pt and the date
of progression or mortality. OS was defined as the period of time
between the date of the first cycle of CPT-Pt and mortality.
Statistical analysis
The χ2 test was used to evaluate
difference in patients characteristics and the incidence of AE.
Survival curves were generated using the Kaplan-Meier method, and a
95% two-sided confidence interval (CI) was also estimated. Survival
variation among the regimens was analyzed using log-rank test. The
data was analyzed using StatView software version 5.0 (SAS
Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
The patient characteristics are presented in
Table I. The median age of the
patients was 59 years (range, 16–78) and their PS was 0. The median
number of administrated cycles was 5.5 (range, 2–16). Thirteen
patients received the 5-day CPT-P regimen, 9 patients received
wCPT-P, 5 patients received wCPT-N and 1 patient received wCPT-C.
Twelve patients (43%) were platinum refractory, 10 patients (36%)
had primary resistance and 6 patients (21%) had secondary
resistance. In total, 25 patients were assessed for progression and
response using CT imaging using RECIST guidelines (version 1.1),
and other 3 patients were assessed using CA-125 criteria due to
lack of measurable disease. The overall response rate was 14%: 2
patients with a CR and 2 patients with a PR. Additionally, 15
patients (54%) achieved SD, resulting in a CBR of 68%. There were
no differences in CBR among platinum refractory disease, primary
resistance cases and secondary resistance cases (67 vs. 70 vs. 67%,
respectively; P=0.93). In addition, there was no difference in CBR
between 5-day CPT-P and other regimens (69 vs. 67%, P=0.99). The
median PFS of 5-day CPT-P and other regimens were 8 and 6 months,
respectively (P=0.27). A total of 21 patients (75%) succumbed to
disease, and the median follow-up time of these patients was 10
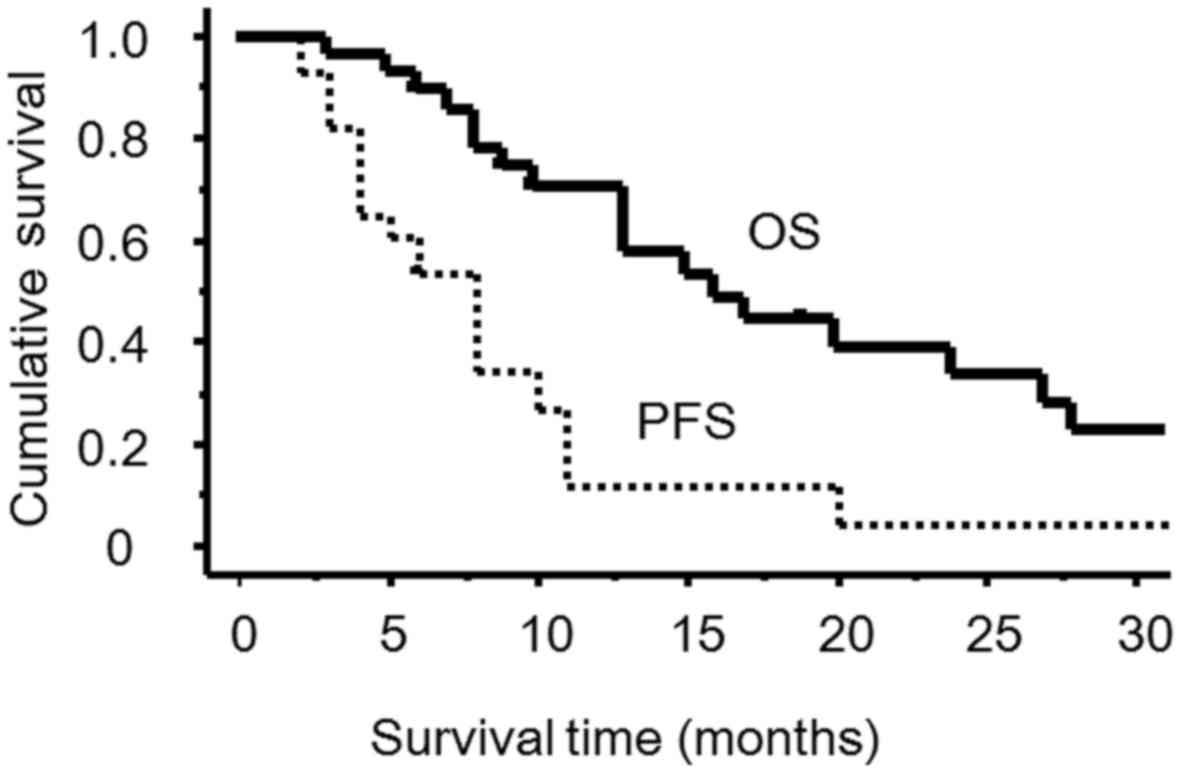
months (range, 6–94 months). The median PFS was 8 months (95% CI =
4–10 months), and the median OS was 15 months (95% CI = 10–27
months) (Fig. 1).
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
| Characteristic | Number of patients
(%) |
|---|
| Total | 28 cases |
| Age, years | 57.5 |
| Median (range) | (16–78) |
| PS |
|
| 0 | 28
(100) |
| FIGO stage |
|
| I/II | 3
(11) |
|
II/IV | 25 (89) |
| Histology |
|
| Serous
AC | 17 (60) |
|
Endometrioid AC | 1 (4) |
| Clear
cell AC | 4
(14) |
| Mucinous
AC | 1 (4) |
| AC,
NOS | 5
(18) |
| Residual disease at
primary surgery, cm |
|
|
<1 | 12 (43) |
| ≥1 | 16 (57) |
| Status of platinum
resistance |
|
|
Platinum-refractory | 12 (43) |
| Primary
platinum resistance | 10 (36) |
| Secondary
platinum resistance | 6
(21) |
| Number of prior
chemotherapy regimens |
|
| 1 | 22 (78) |
| 2 | 3
(11) |
| 3 | 3
(11) |
| CPT-Pt regimens |
|
| 5-day
CPT-P | 13 (46) |
|
wCPT-P | 9
(32) |
|
wCPT-N | 5
(18) |
|
wCPT-C | 1 (4) |
| UGT1A1 |
|
|
Wild-type | 9
(32) |
|
Hetero-type (*6) | 3
(11) |
|
Hetero-type (*28) | 2 (7) |
| Not
available | 14 (50) |
There were no cases that developed treatment-related
mortality, or that discontinued CPT-Pt therapy due to unmanageable
AEs. AE are summarized in Table II.
Neutropenia was the most common AE, and neutropenia of grade >2
was observed in 68% of the patients. Neutropenia was resolved by
treatment delay, dose reduction of CPT-Pt, or administration of
granulocyte-colony stimulating factor. Diarrhea and nausea/vomiting
were also frequently observed. These non-hematological AEs were
resolved by conservative management or dose reduction. Among all 28
patients, 15 patients required dose reduction of CPT-Pt in the
subsequent cycle dose due to AE. Grade 1–3 diarrhea was observed
more frequently in 5-day CPT-P compared with other regimens (85 vs.
20%; P<0.01). The incidence of severe AE of grade >2
according to UGT1A1 genotyping are presented in Table III. UGT1A1 genotypes were available
in 14 cases: 9 cases with UGT1A1 wild-type and 5 cases with UGT1A1
hetero-type. There were no significant differences in the incidence
of grade 3/4 AE between these groups.
 | Table II.Adverse events of CPT-Pt regimens
according to CTCAE version 4.0. |
Table II.
Adverse events of CPT-Pt regimens
according to CTCAE version 4.0.
|
| CTCAE grade of
adverse event (n) |
|---|
|
|
|
|---|
| Adverse event | 2 | 3 | 4 | 3/4 (%) |
|---|
| Hematologic
toxicities |
|
|
|
|
|
Anemia | 16 | 6 | 0 | 6 (21) |
|
Neutropenia | 5 | 11 | 8 | 19 (68) |
|
Thrombocytopenia | 3 | 3 | 3 | 6 (21) |
| Febrile
neutropenia | 0 | 3 | 0 | 3 (11) |
| Non-hematologic
toxicities |
|
|
|
|
|
Diarrhea | 7 | 3 | 0 | 3 (11) |
|
Nausea/vomiting | 2 | 1 | 0 | 1 (4) |
|
Anorexia | 2 | 1 | 0 | 1 (4) |
|
Allergic reaction | 2 | 1 | 0 | 1 (4) |
 | Table III.Grade 3/4 adverse events of CPT-Pt
regimens according to UGT1A1 genotype. |
Table III.
Grade 3/4 adverse events of CPT-Pt
regimens according to UGT1A1 genotype.
| Adverse event | UGT1A1 wild-type, n
(%) (n=9) | UGT1A1 hetero-type,
n (%) (n=5) | UGT1A1 unknown, n
(%) (n=14) | P-value |
|---|
| Hematologic
toxicities |
|
|
|
|
|
Anemia | 1 (3) | 1
(20) | 4
(29) | 0.61 |
|
Neutropenia | 6
(67) | 2
(40) | 10 (71) | 0.45 |
|
Thrombocytopenia | 1 (3) | 1
(20) | 4
(29) | 0.61 |
| Febrile
neutropenia | 0 (0) | 0 (0) | 3
(21) | 0.06 |
| Non-hematologic
toxicities |
|
|
|
|
|
Diarrhea | 1 (3) | 1
(20) | 1 (7) | 0.73 |
|
Nausea/vomiting | 1 (3) | 0 (0) | 0 (0) | 0.33 |
|
Anorexia | 0 (0) | 0 (0) | 1 (7) | 0.60 |
|
Allergic reaction | 0 (0) | 1
(20) | 0 (0) | 0.09 |
Discussion
Our preliminary data revealed that CPT-Pt for
patients with Pt-R ROC achieved a CBR of 68%, median PFS of 8
months and a median OS of 15 months. Although direct comparison was
challenging due to patients heterogeneity and the four treatment
regimens, CPT-Pt had the potential to produce longer PFS compared
with other non-platinum single agents such as gemcitabine and
pegylated liposomal doxorubicin, which achieved a median PFS of 3–4
months (5–10). On the other hand, combination therapy
with conventional chemotherapy with bevacizumab produced a PFS of
6.7 months (26). CPT-Pt regimens
had a similar PFS compared with cisplatin/etoposide and
paclitaxel/carboplatin (median PFS, ~8 months) (12,13).
A previous study suggested that CPT combined with
cisplatin had a CBR of 72% and the median PFS of 6 months in a case
series of 25 patients including 21 patients with Pt-R ROC (7). By contrast, CPT combined with
carboplatin had a CBR of 53% and the median PFS of 3.7 months in 17
patients with Pt-R ROC (27). In the
present study, 22 patients (79%) received cisplatin in combination
and only one patient received carboplatin in combination. At
present, almost all patients with ovarian cancer receive the TC
regimen as the first-line chemotherapy in general clinical
practice. Although carboplatin was reported to show
cross-resistance with cisplatin in vitro (26), the data from the present study
suggested that patients with Pt-R ROC benefited from the
combination of CPT with cisplatin, and not from CPT with
carboplatin.
CPT-Pt for patients with Pt-R disease develop higher
frequencies of grade 3/4 hematological AE, compared with single
non-platinum single agents (5). In
cancer patients treated with CPT, genotyping of UGT1A1 may be a
useful predictive marker for severe AEs such as neutropenia
(28,29). UGT1A1 genotyping may allow the
avoidance of severe AEs occurring as a result of the use of CPT-Pt
for the treatment of Pt-R ROC, although there were no significant
differences in the incidence of severe AE according to UGT1A1
genotype in the present case series. For the schedule of
administration, grade 1–3 diarrhea was observed more frequently in
the 5-day CPT-P group compared with other regimens in the present
study. A weekly schedule of irinotecan, such as wCPT-P and wCPT-N
regimens, may be safer for patients with Pt-R ROC.
Bevacizumab has been widely used for the treatment
of ovarian cancer. However, it is inevitable that severe AEs, such
as gastrointestinal perforation, may be observed in patients with
ROC, particularly in the patients that had peritoneal dissemination
(24,30). CPT-Pt regimens might be an
alternative treatment for patients with Pt-R ROC who may suffer
from bevacizumab-associated AEs.
In conclusion, CPT-Pt achieved a longer PFS in
patients with Pt-R ROC, although a slight elevation of AE frequency
was observed compared with reported incidence by non-platinum
single agents. Weekly schedules of irinotecan, such as wCPT-P and
wCPT-N, may be safer regimens compared with 5-day CPT-P regimens.
CPT-Pt may be a candidate regimen for the treatment of Pt-R ROC;
however, further investigation is needed.
References
|
1
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong DK: Relapsed ovarian cancer:
challenges and management strategies for a chronic disease.
Oncologist. 7 Suppl 5:20–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutch DG, Orlando M, Goss T, Teneriello
MG, Gordon AN, McMeekin SD, Wang Y, Scribner DR Jr, Marciniack M,
Naumann RW and Secord AA: Randomized phase III trial of gemcitabine
compared with pegylated liposomal doxorubicin in patients with
platinum-resistant ovarian cancer. J Clin Oncol. 25:2811–2818.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monk BJ, Herzog TJ, Kaye SB, Krasner CN,
Vermorken JB, Muggia FM, Pujade-Lauraine E, Lisyanskaya AS, Makhson
AN, Rolski J, et al: Trabectedin plus pegylated liposomal
Doxorubicin in recurrent ovarian cancer. J Clin Oncol.
28:3107–3114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meier W, du Bois A, Reuss A, Kuhn W,
Olbricht S, Gropp M, Richter B, Lück HJ, Kimmig R and Pfisterer J:
Topotecan versus treosulfan, an alkylating agent, in patients with
epithelial ovarian cancer and relapse within 12 months following
1st-line platinum/paclitaxel chemotherapy. A prospectively
randomized phase III trial by the Arbeitsgemeinschaft
Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR).
Gynecol Oncol. 114:199–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buda A, Floriani I, Rossi R, Colombo N,
Torri V, Conte PF, Fossati R, Ravaioli A and Mangioni C: Randomised
controlled trial comparing single agent paclitaxel vs
epidoxorubicin plus paclitaxel in patients with advanced ovarian
cancer in early progression after platinum-based chemotherapy: an
Italian Collaborative Study from the Mario Negri Institute, Milan,
G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto
Oncologico Romagnolo) group. Br J Cancer. 90:2112–2117.
2004.PubMed/NCBI
|
|
9
|
Gordon AN, Fleagle JT, Guthrie D, Parkin
DE, Gore ME and Lacave AJ: Recurrent epithelial ovarian carcinoma:
a randomized phase III study of pegylated liposomal doxorubicin
versus topotecan. J Clin Oncol. 19:3312–3322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
ten Bokkel Huinink W, Gore M, Carmichael
J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson
N, Spanczynski M, et al: Topotecan versus paclitaxel for the
treatment of recurrent epithelial ovarian cancer. J Clin Oncol.
15:2183–2193. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lortholary A, Largillier R, Weber B,
Gladieff L, Alexandre J, Durando X, Slama B, Dauba J, Paraiso D and
Pujade-Lauraine E: GINECO group France: Weekly paclitaxel as a
single agent or in combination with carboplatin or weekly topotecan
in patients with resistant ovarian cancer: the CARTAXHY randomized
phase II trial from Groupe dInvestigateurs Nationaux pour lEtude
des Cancers Ovariens (GINECO). Ann Oncol. 23:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Burg ME, De Wit R, van Putten WL,
Logmans A, Kruit WH, Stoter G and Verweij J: Weekly cisplatin and
daily oral etoposide is highly effective in platinum pretreated
ovarian cancer. Br J Cancer. 86:19–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma R, Graham J, Mitchell H, Brooks A,
Blagden S and Gabra H: Extended weekly dose-dense
paclitaxel/carboplatin is feasible and active in heavily
pre-treated platinum-resistant recurrent ovarian cancer. Br J
Cancer. 100:707–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
See HT, Freedman RS, Kudelka AP, Burke TW,
Gershenson DM, Tangjitgamol S and Kavanagh JJ: Retrospective
review: re-treatment of patients with ovarian cancer with
carboplatin after platinum resistance. Int J Gynecol Cancer.
15:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto K, Katsumata N, Yamanaka Y,
Yonemori K, Kohno T, Shimizu C, Andoh M and Fujiwara Y: The safety
and efficacy of the weekly dosing of irinotecan for platinum- and
taxanes-resistant epithelial ovarian cancer. Gynecol Oncol.
100:412–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bodurka DC, Levenback C, Wolf JK, Gano J,
Wharton JT, Kavanagh JJ and Gershenson DM: Phase II trial of
irinotecan in patients with metastatic epithelial ovarian cancer or
peritoneal cancer. J Clin Oncol. 21:291–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukuda M, Nishio K, Kanzawa F, Ogasawara
H, Ishida T, Arioka H, Bojanowski K, Oka M and Saijo N: Synergism
between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38,
in human small cell lung cancer cells. Cancer Res. 56:789–793.
1996.PubMed/NCBI
|
|
18
|
Sugiyama T, Yakushiji M, Nishida T,
Ushijima K, Okura N, Kigawa J and Terakawa N: Irinotecan (CPT-11)
combined with cisplatin in patients with refractory or recurrent
ovarian cancer. Cancer Lett. 128:211–218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kigawa J, Takahashi M, Minagawa Y, Oishi
T, Sugiyama T, Yakushiji M and Terakawa N: Topoisomerase-I activity
and response to second-line chemotherapy consisting of
camptothecin-11 and cisplatin in patients with ovarian cancer. Int
J Cancer. 84:521–524. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Markman M and Bookman MA: Second-line
treatment of ovarian cancer. Oncologist. 5:26–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rustin GJ, Nelstrop AE, McClean P, Brady
MF, McGuire WP, Hoskins WJ, Mitchell H and Lambert HE: Defining
response of ovarian carcinoma to initial chemotherapy according to
serum CA 125. J Clin Oncol. 14:1545–1551. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE).
https://evs.
nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdfMarch
17–2017
|
|
25
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: the AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsubamoto H, Kawaguchi R, Ito K, Shiozaki
T, Takeuchi S, Itani Y, Arakawa A, Tabata T and Toyoda S: Phase II
study of carboplatin and weekly irinotecan combination chemotherapy
in recurrent ovarian cancer: a Kansai clinical oncology group study
(KCOG0330). Anticancer Res. 33:1073–1079. 2013.PubMed/NCBI
|
|
27
|
Misawa T, Kikkawa F, Maeda O, Obata NH,
Higashide K, Suganuma N and Tomoda Y: Establishment and
characterization of acquired resistance to platinum anticancer
drugs in human ovarian carcinoma cells. Jpn J Cancer Res. 86:88–94.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takano M, Kato M, Yoshikawa T, Sasaki N,
Hirata J, Furuya K, Takahashi M, Yokota H, Kino N, Horie K, et al:
Clinical significance of UDP-glucuronosyltransferase 1A1*6 for
toxicities of combination chemotherapy with irinotecan and
cisplatin in gynecologic cancers: a prospective multi-institutional
study. Oncology. 76:315–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ichikawa W, Uehara K, Minamimura K, Tanaka
C, Takii Y, Miyauchi H, Sadahiro S, Fujita K, Moriwaki T, Nakamura
M, et al: An internally and externally validated nomogram for
predicting the risk of irinotecan-induced severe neutropenia in
advanced colorectal cancer patients. Br J Cancer. 112:1709–1716.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cannistra SA, Matulonis UA, Penson RT,
Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D,
Wenham R and McGuire W: Phase II study of bevacizumab in patients
with platinum-resistant ovarian cancer or peritoneal serous cancer.
J Clin Oncol. 25:5180–5186. 2007. View Article : Google Scholar : PubMed/NCBI
|