Introduction
Differentiated thyroid carcinoma (DTC) typically has
a good prognosis; however, effective systemic chemotherapy for
advanced DTC had not existed until recently, when lenvatinib, a
multi-tyrosine kinase inhibitor was proven an effective treatment
option (1). Subsequently, lenvatinib
has been introduced into clinical practice for advanced DTC.
Unfortunately, there are little safety data for lenvatinib,
particularly regarding elderly patients.
Numerous adverse effects of lenvatinib have been
reported, including hypertension, hand-foot syndrome (HFS) diarrhea
and thrombocytopenia. In the SELECT trial, ~70% of patients
receiving lenvatinib presented with hypertension (1); similar results have also been
demonstrated in other clinical trials (2–4). Zhu
et al (5) analyzed the safety
and efficacy profiles of lenvatinib in patients with cancer in a
systematic review and meta-analysis; in an analysis of 978 patients
treated with lenvatinib, the most frequently observed adverse
events of grade 3 or higher were thrombocytopenia (25.4%),
hypertension (17.7%) and peripheral edema (15.5%). Additionally,
incidences of all-grade and high-grade hypertension were
significantly increased (5).
Numerous problems have been reported regarding the
use of chemotherapy in elderly patients (6–8). Elderly
patients more frequently suffer from the adverse side effects of
anticancer chemotherapy than younger patients. There are many
clinical reports on the safety and efficacy of lenvatinib (1,5), but
none specifically study its safety profile in elderly patients. In
clinical practice, lenvatinib is commonly used to treat elderly
patients, and there is significant experience in administrating
lenvatinib to elderly patients in Kumamoto University Hospital. The
present study examined the side effects of lenvatinib in 18
patients grouped by age (younger, <75 years and elderly, ≥75
years) to analyze differences in the adverse events associated with
lenvatinib treatment in elderly patients.
Patients and methods
Patients
This retrospective, observational, cross-sectional
study was designed to evaluate the safety of lenvatinib for elderly
patients. A total of 18 consecutive patients with
histopathologically-proven DTC treated with lenvatinib at Kumamoto
University Hospital between July 2015 and July 2016 were enrolled
in the study. Written informed consent was obtained from all
patients prior to enrollment. Information regarding adverse events
was obtained from medical charts and compared between younger
(<75 years) and elderly (≥75 years) patients. When collecting
blood pressure (BP) data, the hospital BP was used rather than the
home BP. Adverse events were evaluated using the Common Terminology
Criteria for Adverse Events version 4.0 (9). The study protocol has been approved by
the institutional review board of Kumamoto University.
Statistical analysis
The Mann-Whitney U test was used to compare the
degree of each adverse event (such as BP), and χ2 tests
were used to compare the proportions of variables between groups.
All statistical analyses were conducted using SAS JMP Pro v12.1.0
(SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of the 18 enrolled patients
are presented in Table I. The
proportion of younger and elderly patients and of males and females
were the same in the overall cohort and groups. All patients had
histologically-confirmed papillary thyroid carcinoma as their
primary diagnosis and were administered 24 mg per day lenvatinib,
with the exception of 5 patients whose tumors showed vessel
invasion (Table II).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | No. of patients
(%) |
|---|
| Total | 18 (100) |
| Age, median (range),
years | 74 (59–87) |
| ≥75 | 9 (50) |
| Sex |
|
|
Female | 9 (50) |
| Male | 9 (50) |
| ECOG performance
status |
|
| 0 | 12 (67) |
| 1 | 5 (28) |
| 2 | 1 (5) |
| Histology
subtype |
|
|
Papillary | 18 (100) |
| Prior treatment |
|
| None | 13 (72) |
|
Sorafenib | 5 (28) |
| History of
hypertension |
|
| Yes | 11 (61) |
| No | 7 (39) |
| Using
antihypertensive drugs |
| Yes | 10 (56) |
| No | 8 (44) |
 | Table II.Starting dose of lenvatinib. |
Table II.
Starting dose of lenvatinib.
| Starting dose,
mg | No. of patients
(%) | Reason |
|---|
| 24 | 13 (72) | Standard dose |
| 20 | 1 (5) | Vessel invasion |
| 14 | 3
(18) | Vessel invasion |
| 10 | 1 (5) | Vessel invasion |
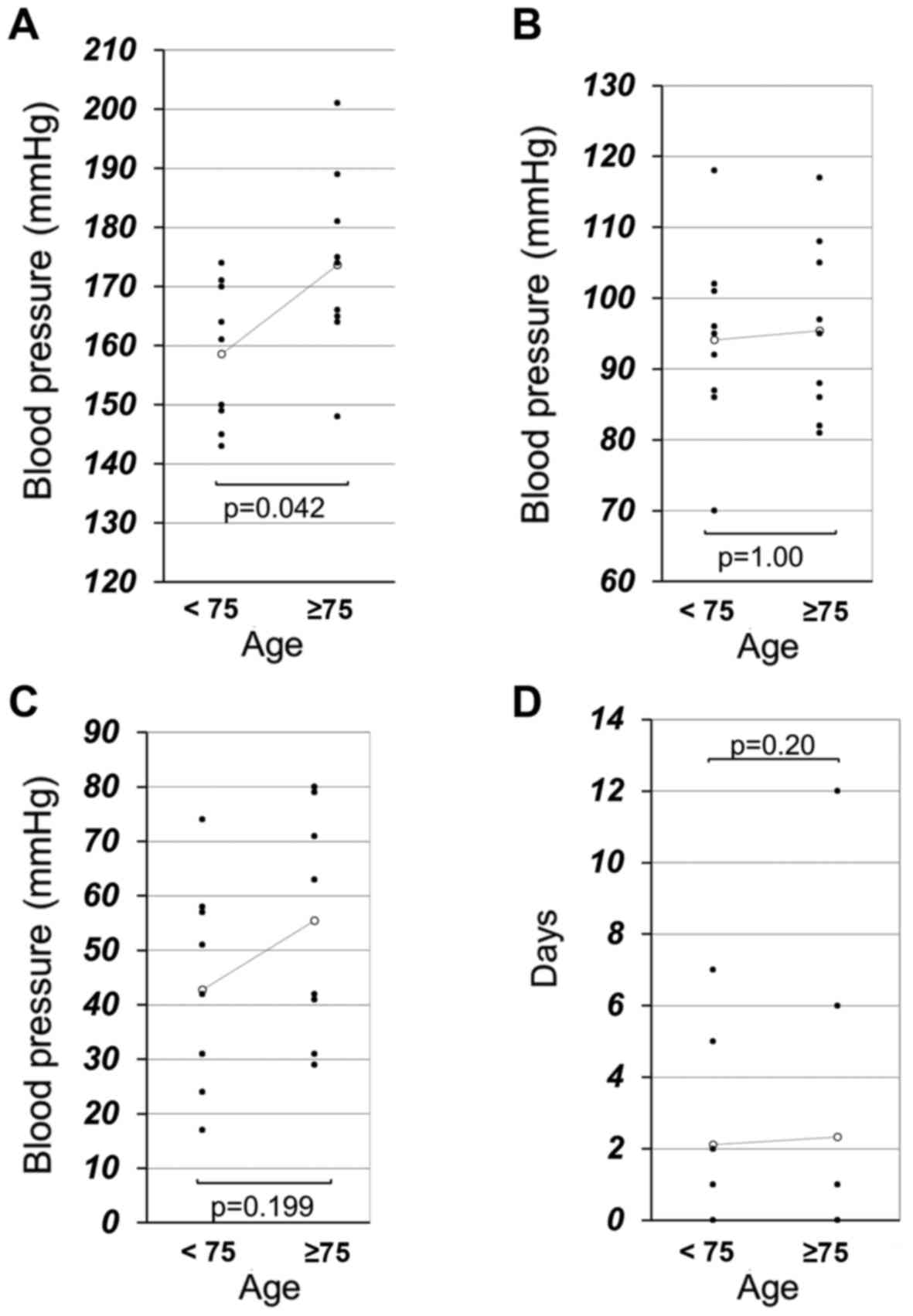
The median maximum systolic BP (sBP) was
significantly different between the younger patient and elderly
patient groups (158 vs. 173 mmHg; P=0.042; Fig. 1A). There were no significant
differences between the younger and elderly patients in median
maximum diastolic BP (dBP; 94 vs. 95 mmHg; P=1.00; Fig. 1B), median degree of sBP elevation (43
vs. 55 mmHg; P=0.199; Fig. 1C) or
median days until a clinical hypertension diagnosis (2.11 vs. 2.33
days; P=0.436; Fig. 1D). There was
also no significant difference in the baseline sBP between elderly
and young patients. Furthermore, the frequency of other adverse
events (grade ≥1 HFS, proteinuria and diarrhea) exhibited no
significant differences between groups (Table III).
 | Table III.Effects of lenvatinib on HFS,
proteinuria and diarrhea. |
Table III.
Effects of lenvatinib on HFS,
proteinuria and diarrhea.
| A, HFS |
|---|
|
|---|
| Age | HFS (yes/no) | P-value |
|---|
| <75 | 3/6 | 0.08 |
| ≥75 | 5/4 |
|
|
| B, Proteinuria |
|
| Age | Proteinuria
(yes/no) | P-value |
|
| <75 | 6/3 | 0.08 |
| ≥75 | 2/7 |
|
|
| C, Diarrhea |
|
| Age | Diarrhea
(yes/no) | P-value |
|
| <75 | 0/9 | 1.0 |
| ≥75 | 1/8 |
|
Between the two groups, there were no significant
differences in the proportion of patients with hypertension history
and using antihypertensive drugs.
Discussion
The present study performed a retrospective analysis
of 18 consecutive lenvatinib-treated advanced DTC patients from
Kumamoto University Hospital. The analysis was focused on comparing
the degree of adverse events associated with lenvatinib treatment
between elderly and younger patient groups. Among several adverse
events, hypertension was focused upon, as it has previously been
demonstrated to be a major clinical concern for elderly patients
receiving chemotherapy (6–8). The results revealed that sBP was
significantly elevated in elderly patients compared with younger
patients, but the change from baseline to maximum sBP was not
significant. As presented in Fig.
1C, the elderly patients had at least the trend to have more
elevated sBP than young patients following lenvatinib therapy. This
trend may be one of the reasons for elevated sBP in elderly
patients. Conversely, there were no indications of increases in
other lenvatinib-specific toxicities such as HFS, proteinuria,
diarrhea, fatigue and thrombocytopenia among elderly patients.
Elderly patients suffer more chemotherapy-induced
toxicities than younger patients with cancer (6–8). In
clinical trials the majority of patients are relatively young and
in good condition; thus, there is often a lack of information from
these studies regarding efficacy and safety for elderly patients.
Among studies investigating the efficacy of lenvatinib for thyroid
carcinoma, to the best of our knowledge there have been no studies
analyzing the degree of lenvatinib-specific adverse effects in
elderly patients. The present results revealed a trend of increased
maximum sBP in elderly patients treated with lenvatinib compared
with younger patients; however, the frequency of other toxicities
did not increase, indicating that lenvatinib is relatively safe for
elderly patients.
In general, elderly patients tend to have increased
sBP and decreased dBP, caused by decreased elasticity and
extensibility of large arteries occurring due to the extension of
arteriosclerosis associated with increasing age. Although the
precise mechanisms of BP elevation following lenvatinib treatment
are unclear, one possible explanation may be lenvatinib-induced
vascular endothelial cell injury (10). The underlying mechanism of the high
sBP in elderly patients receiving lenvatinib may involve effects on
the vascular endothelial cells that surround arteriosclerotic
vessels. There is no evidence that demonstrates that elderly
patients tend to have HFS, diarrhea and other toxicities, which
might explain the lack of increased frequencies of these adverse
events in patients over 75 in the present study.
There were several limitations to the current study.
First, this was a retrospective observational study, not a
case-controlled study; therefore, the study design cannot avoid
confounding and selection biases. Second, the number of patients
was small, the results are not able to be extrapolated without
subsequent studies with larger patient cohorts. Thus, we cannot
regard this study as high quality.
In conclusion, a trend of hypertension in elderly
patients receiving lenvatinib but not in younger patients was
observed, suggesting that lenvatinib should be introduced carefully
to elderly patients. However, lenvatinib-induced hypertension may
easily be controlled using anti-hypertensive drugs or adjusting the
dose of lenvatinib. Overall, lenvatinib was tolerable, even in
elderly patients >75.
References
|
1
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boss DS, Glen H, Beijnen JH, Keesen M,
Morrison R, Tait B, Copalu W, Mazur A, Wanders J, OBrien JP, et al:
A phase I study of E7080, a multitargeted tyrosine kinase
inhibitor, in patients with advanced solid tumours. Br J Cancer.
106:1598–1604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamichi S, Nokihara H, Yamamoto N,
Yamada Y, Honda K, Tamura Y, Wakui H, Sasaki T, Yusa W, Fujino K
and Tamura T: A phase 1 study of lenvatinib, multiple receptor
tyrosine kinase inhibitor, in Japanese patients with advanced solid
tumors. Cancer Chemother Pharmacol. 76:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabanillas ME, Schlumberger M, Jarzab B,
Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner
DL, Topliss D, et al: A phase 2 trial of lenvatinib (E7080) in
advanced, progressive, radioiodine-refractory, differentiated
thyroid cancer: a clinical outcomes and biomarker assessment.
Cancer. 121:2749–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K
and Xiao Y: Safety and efficacy profile of lenvatinib in cancer
therapy: a systematic review and meta-analysis. Oncotarget.
7:44545–44557. 2016.PubMed/NCBI
|
|
6
|
Chrischilles EA, Pendergast JF, Kahn KL,
Wallace RB, Moga DC, Harrington DP, Kiefe CI, Weeks JC, West DW,
Zafar SY and Fletcher RH: Adverse events among the elderly
receiving chemotherapy for advanced non-small-cell lung cancer. J
Clin Oncol. 28:620–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Townsley CA, Selby R and Siu LL:
Systematic review of barriers to the recruitment of older patients
with cancer onto clinical trials. J Clin Oncol. 23:3112–3124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clough-Gorr KM, Stuck AE, Thwin SS and
Silliman RA: Older breast cancer survivors: geriatric assessment
domains are associated with poor tolerance of treatment adverse
effects and predict mortality over 7 years of follow-up. J Clin
Oncol. 28:380–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
US Department of Health and Human
Services, . National Cancer Institute Common Terminology Criteria
for Adverse Events v4.0. https://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02
_2009-09-15_QuickRef erence_5x7_Locked.pdfSeptember 1–2016
|
|
10
|
Keizer RJ, Gupta A, Mac Gillavry MR,
Jansen M, Wanders J, Beijnen JH, Schellens JH, Karlsson MO and
Huitema AD: A model of hypertension and proteinuria in cancer
patients treated with the anti-angiogenic drug E7080. J
Pharmacokinet Pharmacodyn. 37:347–363. 2010. View Article : Google Scholar : PubMed/NCBI
|