Introduction
The use of endoscopic surgery for the treatment of
breast cancer has been widely investigated by multiple medical
centers worldwide. These minimally invasive techniques may be
associated with various benefits, such as better cosmetic outcome
and improved upper limb function (1–3).
However, despite the advantages of endoscopic breast
cancer surgery, it has raised certain concerns regarding additional
cost in terms of operative time, equipment and training and, most
importantly, the risk of port-site metastases (4–6).
Previous studies have confirmed that port-site metastases had
developed following endoscopic breast cancer surgery (7,8).
Some of these problems may arise from the associated
liposuction, which is commonly used in mastoscopic axillary lymph
node dissection (MALND) in order to create working space.
Liposuction partly limits the development of video-assisted breast
surgery (VABS) and it has been reported that metastatic nodes were
found in the filtered liposuction fluid (7). Moreover, liposuction increases the
operative time and the working space created is limited.
We hereby describe a novel method for creating
endoscopic working space without prior liposuction, in order to
overcome the abovementioned limitations and to improve the
oncological safety of endoscopic techniques.
Patients and methods
Patients
Between June, 2014 and October, 2015, a total of 106
female breast cancer patients were treated by endoscopic breast
cancer surgery at the Department of General Surgery of Guiping
People's Hospital (Guangxi, China). The patients' age ranged from
31 to 78 years, with a median age of 48 years. A total of 15, 68
and 23 patients had stage I, II and III disease, respectively; 6
patients with stage III disease were administered neoadjuvant
therapy for 2–3 courses. The location of the primary tumor was as
follows: Lower inner quadrant, n=21; upper inner quadrant, n=16;
lower outer quadrant, n=39; upper outer quadrant, n=27; and central
area, n=3. Written informed consent was obtained from all the
patients and the study protocol was approved by the Institutional
Ethics Committee of Guiping People's Hospital.
Surgical procedures
VABS was performed as described below.
Patient's position
Under general anesthesia, the patient was placed in
the supine position with the ipsilateral arm in a 90° abduction.
Securing the patient's forearm to the frame of the bed was not
considered necessary.
Trocar placement and establishment of
gas-space
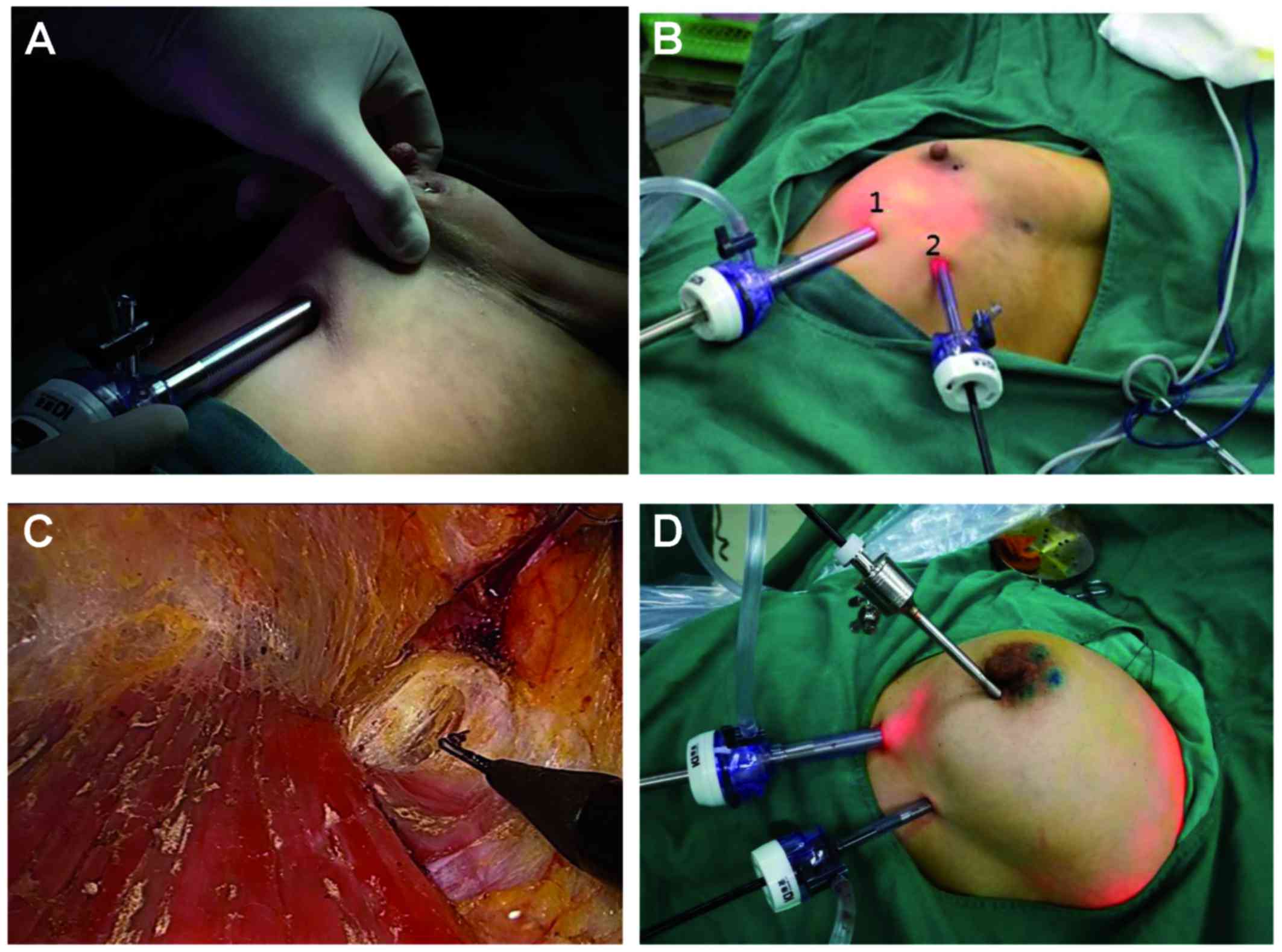
Using left VABS as an example, two incisions were
performed: The first incision was located at the intersection of
the vertical nipple line and the horizontal line at the level of 2
cm below the inframammary fold (Fig.
1A). This step is important for the removal of the lower part
of the mammary gland and axillary lymphatic tissue. The second
incision was located in the anterior line of the axilla and
parallel to the level of 2 cm below the inframammary fold (Fig. 1B). A 10-mm trocar was inserted via
the first incision. From this trocar, CO2 was infused
into the internal space between the posterior breast and pectoralis
fascia. The pressure was ~8 mmHg. A 30° 10-mm endoscope was
inserted via this first trocar. An additional 5-mm trocar was
placed in the second incision, through which a 5mm long
electrocautery probe was inserted (Fig.
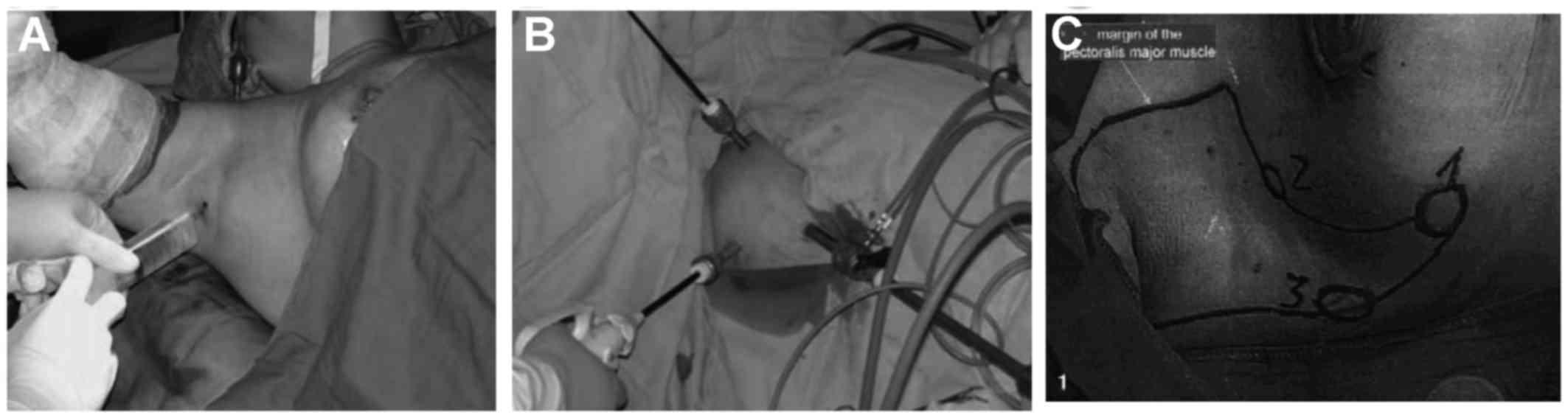
1B). This type of developmental operative approach was
different from other operations relying on liposuction to create
working space (Fig. 2A and B)
(9–12). Additionally, it is also different
from a previous study investigating MALND without liposuction
(Fig. 2C) (13).
Our endoscopic surgery started with dissection
between the posterior breast and pectoralis fascia. Tissue
dissection and vessel coagulation were achieved using an
electrocautery probe via the second trocar. Due to the expansion of
the CO2 gas, dissection was easily performed in the
space between the posterior breast and pectoralis fascia (Fig. 1C). This is critical for the
generation of working space to enable ALND.
Endoscopic ALND
Following establishment of gas-space, additional
skin incisions were placed in the lateral periareolar area. A third
5-mm trocar was inserted through the lateral region of the breast
to the space between the posterior breast and pectoralis fascia
(Fig. 1D). Endoscopic ALND was then
performed via this space.
A 30° 10-mm endoscope was introduced through the
first 10-mm trocar to allow viewing the axilla from the floor to
the apex, bordered by the latissimus dorsi muscle laterally and the
pectoralis major muscle superiorly.
The second and the third 5-mm trocars at the level
of 2 cm below the inframammary fold were the main and supplementary
operating positions, respectively. The 5-mm long-arm ultrasonic
knife and forceps were placed in these positions to facilitate the
performance of complex handling in the axilla under endoscopic
view. The adipose tissue and lymph nodes attached to the blood
vessels and nerves were dissected with an ultrasonic scalpel.
During endoscopic surgery with the ultrasonic scalpel, the blood
loss volume and operative time were significantly reduced compared
with those with the dipolar dissecting forceps and scissors.
This step described above differs from the most
commonly performed MALND, in which lipolysis, balloon expansion and
other lifting devices were omitted in endoscopic surgery to help
establish a gas space in the axilla (9,14). We
were able to establish sufficient working space, including the
internal space between the posterior breast and pectoralis fascia
and inflation region created by sustained air pressure of ~8 mmHg.
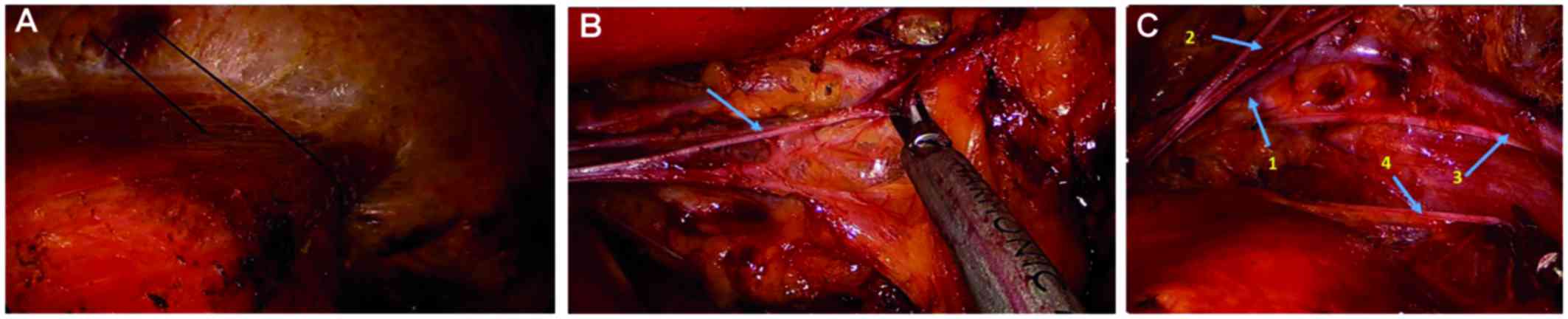
In cases where dissection of the axillary level II and/or level III
lymph nodes was required, we were able to create a suitable
endoscopic working space by elevating the lateral part of the
pectoralis major muscle with anchored sutures to penetrate the skin
and fixed the anchored suture outside the skin with a fixing device
(Fig. 3A).
Identification and preservation of
important anatomical structures
In principle, the axilla was dissected from the
lateral pectoralis major muscle and axillary floor to the apex,
until the axillary vein was identified. From there onwards, the
operation proceeded laterally and downwards. Strip-like and thick
structures, likely to be nerves and blood vessels, were preserved.
The dissection around some important anatomical structures is
further described below:
Intercostobrachial nerve. The intercostobrachial
nerve was the first main structure seen under the endoscope and it
was dissected from the lateral pectoralis major muscle. This nerve
runs from the serratus anterior and intercostal muscle at the
junction of the anterior and lateral side of the chest wall and it
looks like a ‘crossbeam’ in the axillary cavity. Adipose tissue and
the lymphatics around the nerve were peeled out using an ultrasonic
scalpel (Fig. 3B).
Axillary vein
We were able to identify the middle part of the
axillary vein when the endoscope was passed over the
intercostobrachial nerve. This vein is located in the
anterior-lower side of the intercostobrachial nerve. Once the
axillary vein was unmasked, we were able to complete the operation
safely.
Space between the major and minor pectoralis
muscles. The surgical procedure was directed inwards and upwards to
enter the space between the major and minor pectoralis muscles
(where the Rotter's lymph node is located). The medial thoracic
nerve was seen extending from the middle-upper part of the minor
pectoralis muscle to the major pectoralis muscle.
Dissection of axillary level II. The lateral
thoracic artery and part of the axillary vein were identified when
the procedure was advanced medially to dissect the adipose tissue
and the lymphatics behind the minor pectoralis muscle. Anchoring
sutures were always used to elevate the lateral part of the
pectoralis major muscle in order to establish a larger working
space when the dissection of these regions and/or axillary level
III was performed (Fig. 3A). This
procedure must be performed with caution, as the smaller and
thinner blood vessel branches are easily cut by the ultrasonic
scalpel with ensuing bleeding, while the larger structures are
preserved.
Long thoracic nerve
When the procedure was directed medially and
downwards, the long thoracic nerve (2–3 cm below the
intercostobrachial nerve) could only be found at the floor of the
axilla, in the deepest layer of the boundaries of the
lateral-posterior side of the chest wall. The adipose tissue and
lymphatics surrounding the nerve were dissected by the ultrasonic
scalpel (Fig. 3C).
Thoracodorsal nerve and vessels and subscapular
vessels. The thoracodorsal nerve and vessels and the subscapular
vessels were found under the middle part of the axillary vein when
the procedure was advanced laterally. Underneath those structures,
the subscapular, teres major and latissimus dorsi muscles were
identified (Fig. 3C).
Thoracoepigastric vein. It was occasionally seen
from the endoscopic view that the thoracoepigastric vein extended
from the inferior part of the axillary vein in the lateral part of
the minor pectoralis muscle to the anterior chest wall.
Finally, the dissected tissues were temporarily
collected at the floor or the apex of the axilla to be removed
later. Blood and lymphatic fluid was aspirated using a 5-mm suction
device.
The axilla was washed with warm distilled water,
which was continuously drained by a suction tube placed in the
inferior trocar hole.
Mastectomy
At the extended periareolar skin incision, the
dissection between the breast and subcutaneous tissue and along the
lateral and medial resection margins was performed using scissors
and a scalpel. The blood supply was significantly decreased due to
the isolation between the posterior breast and pectoralis fascia,
which was performed during the former step. Therefore, excessive
bleeding was not expected and haemostasis was achievable through
electrical coagulation.
Retrieved specimen
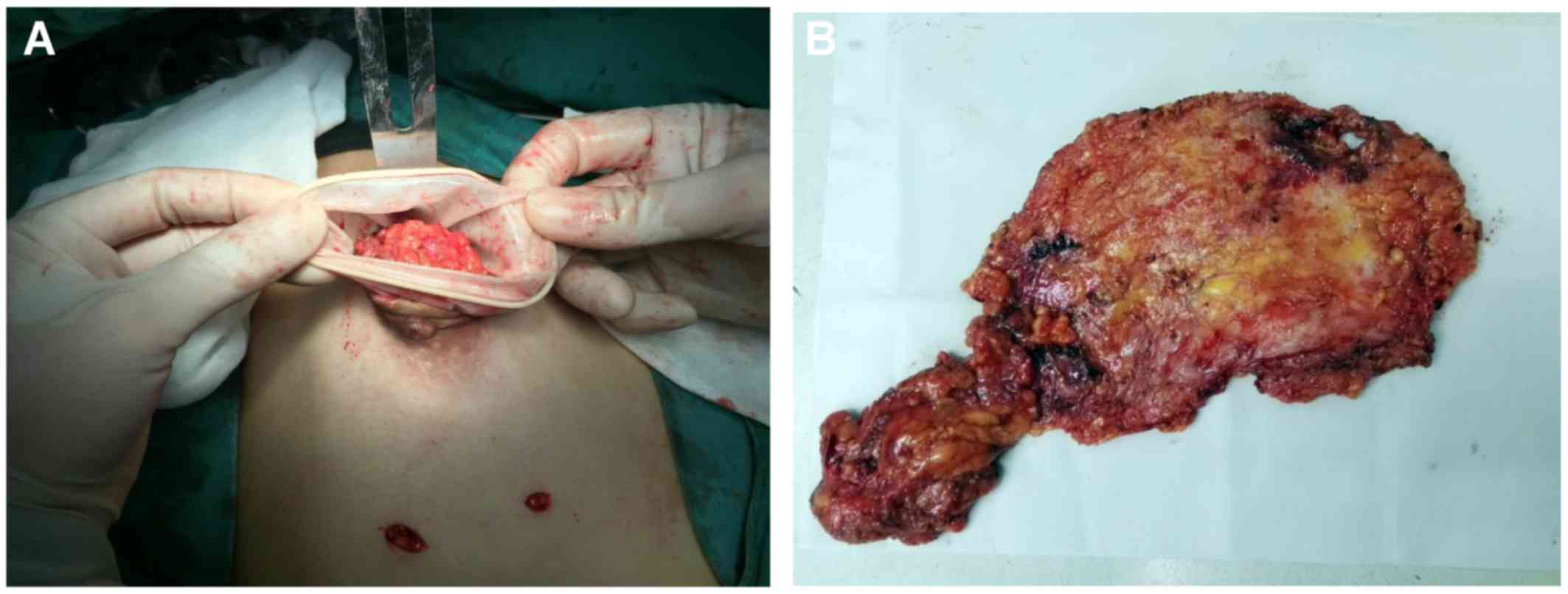
The periareolar skin incision through which the
trocar was formally placed was extended, and the entire breast
specimen was removed, along with extirpated adipose and lymphatic
tissues dissected in the axilla, through the extended skin incision
and sent for histological assessment (Fig. 4A).
If there was no need to preserve periareolar skin,
including the nipple-areola complex, an elliptical incision was
performed, incorporating the nipple-areola complex and extending
towards the axilla. After that, the entire specimen was retrieved
directly through this site.
In order to prevent tumor relapse, the axilla and
the space under the skin flap were washed with warm distilled water
which was then continuously drained by a suction tube. Finally, the
chest and axilla were bandaged.
Results
Intra- and postoperative
parameters
During the entire endoscopic procedure, the blood
loss volume was in the range of 20–100 ml, with a mean of 51 ml.
The mean number of harvested lymph nodes was 12.5 (range, 6–31). A
total of 85.8% (91/106) cases exhibited lymphatic invasion.
The operative time for the entire VABS lasted 55–180
min, with a mean of 85.5 min. The operative time was longer in the
earlier procedures, but was shortened once we had become
familiarized with the optimal operative pathway and the detailed
anatomy of the axilla under the endoscope.
The total volume of drainage fluid in each case
ranged from 15 to 250 ml. The time to removal of the drainage tubes
ranged from 3 to 5 days postoperatively.
There were only 2 trocar holes in the inframammary
fold and one incision in the periareolar skin, which were small and
easily concealed. Therefore, the majority of the patients were
satisfied with the cosmetic results.
No cases were intraoperatively converted to open
surgery due to uncontrolled bleeding.
Complications and outcome
There were no serious intraoperative complications,
such as injury to the axillary vein, subcutaneous accumulation of
fluid, or hematoma. No postoperative complications were reported
and postoperative infections were not observed. The shoulder range
of motion was not affected in any of the patients. There has been
no axillary tumor relapse, trocar site tumor implantation or upper
limb edema reported to date.
Discussion
The rates of complications, including injury to the
blood vessels, pain, edema and paraesthesia of the upper limb,
continuous lymphedema and limitation of shoulder movement, are
comparatively high in conventional ALND (13,15).
Furthermore, the incision scar from the anterior chest wall to the
axilla may be unsightly and they may also significantly restrict
the range of motion of the shoulder joint (16–19).
Endoscopic techniques have become a promising trend
in breast surgery. This technique makes operative anatomy easier
with the help of the magnifying function of endoscopic systems,
allowing for accurate identification and preservation of the
important blood vessels and nerves. In addition, endoscopic
techniques are associated with advantages such as satisfactory
cosmetic outcome, minimal invasiveness and improved shoulder
function. However, in addition to the disadvantages of higher cost
due to longer operative time, specialized equipment and training,
there are increasing concerns regarding the risk of port-site
metastases, which limit the development of endoscopic breast cancer
surgery. Some of these issues may be associated with liposuction,
which is commonly adopted in MALND to create working space.
In order to create working space, liposuction is
commonly used in VABS (2). As a
result of this technique, there is no need to extend the breast
incision intentionally or unintentionally towards the axilla for
performing ALND. Isolation and dissection may then be easily
performed with endoscopic instruments (9,12).
Liposuction is associated with problems that may limit the use of
VABS. There are several disadvantages: First, the amount of fluid
injected should be adjusted according to the proportion of fat
tissue of the patient. If the liposuction around the axillary vein
is not sufficient, the vein cannot be clearly seen and isolation
and dissection are difficult to perform with the endoscopic
instruments.
Second, the quality of liposuction directly affects
the efficiency and efficacy of the endoscopic surgical procedure.
When injecting lipolysis liquid and performing lipoaspiration, the
surgeon must thoroughly examine all parts of the axilla,
particularly the axillary apex and the space between the major and
minor pectoralis muscles. These regions are difficult to visualize,
which may affect the endoscopic procedure in the axilla and prolong
the operative time.
Third, MALND with liposuction is not suitable for
obese patients (9). The reason is
that, even if the axillary fat is well-aspirated, the created gas
space is quite limited, and important structures, such as the
axillary vein, lateral thoracic vessels, thoracic epigastric vein
and intercostobrachial nerve, are not easily identifiable in the
axilla of obese patients.
Fourth, liposuction is a time-consuming procedure,
and a sufficient lipolytic effect may require a long time to
achieve when MALND is applied (13).
Fifth, if the working trocars and camera are placed
too high, some lymphatic tissue may remain in the lower part of the
axilla near the insertion site of the trocar.
Finally, the most important limitation may be the
risk of port-site tumor implantation. It remains controversial
whether endoscopic ALND with liposuction overlooks the general
surgical oncological principle of en bloc resection of potentially
metastatic lymph nodes. Previous studies confirmed that metastatic
nodes were found in the filtered liposuction fluid (7) and histologically confirmed port-site
metastases were detected following endoscopic ALND with liposuction
(7,8).
To overcome these limitations, a new method for
performing video-assisted breast cancer surgery was developed,
which may be able to create working space without prior
liposuction. In our surgery, the blood loss volume, endoscopic
surgery time and total volume of drainage fluid are all obviously
more favorable compared with other endoscopic breast surgery
studies, as previously reported in a review (20).
In summary, the present study establishes an
improved method associated with certain benefits: As regards the
choice of endoscope, the 30° long (±33 cm) endoscope was selected,
as it meets the operative needs, particularly in axillary level II
and/or III. Regarding the selection of the surgical instruments,
our choices were different from those of other studies regarding
the use of short-arm dissecting forceps and scissors (9,14,21). As
the distance between the incision port and the dissection working
space is somewhat long, long-arm instruments were selected for the
endoscopic surgery. The 5-mm long-arm (±33 cm) dissecting forceps
and ultrasonic scalpel are considered suitable for this complex
work and they may be more comfortable for the surgeon performing
the surgery.
As regards the change of the placement of trocar and
creation of working space, in a large number of studies, axillary
incisions were the most common point of access to facilitate
endoscopic subcutaneous mastectomy, as well as axillary node biopsy
(20). In those studies, a total of
3 holes in the axilla were required for MALND (Fig. 2). This placement of the trocars would
result in a narrow working space in the axilla. Large or mixed
lymph nodes may create more intraoperative difficulties and, due to
the narrow space of the axilla, these lymph nodes cannot be easily
removed. Therefore, in the majority of MALNDs, enlarged and mixed
lymph nodes (diameter, >1 cm) should be excluded. Furthermore,
if the working trocars and camera are placed too high, some
lymphatic tissue may remain in the lower part of the axilla near
the insertion of the trocar.
From our perspective, the choice of trocar placement
and the created working space are crucial for creating a successful
operative environment. We recommend three incisions as follows: The
first incision is located at the intersection of the vertical line
of the nipple and the level of 2 cm below the inframammary fold;
the second incision is located at the anterior line of the axilla
and parallel to the level of 2 cm below the inframammary fold; and
additional skin incisions are placed in a periareolar lateral
location (Fig. 1D). The reason for
placing trocars at these sites is that the lower part of the
mammary gland and axillary lymphatic tissue cannot be missed during
the following procedure, and these positions may help create
adequate operative space in the next steps.
As regards working space, our technique did not
require other special equipment to create an operative space, such
as balloon expansion or a lifting device. The endoscopic procedure
started with dissection between the posterior breast and pectoralis
fascia. Due to the expansion with CO2 gas, the
dissection was simply performed between the posterior breast and
pectoralis fascia and sufficient working space was easily created
(Fig. 1C). This working space with 8
mmHg CO2 pressure made dissection of the axilla easier.
This type of developmental operative approach was different from
other operations, which used liposuction to create the initial
working space.
As regards the change in the order of endoscopic
ALND, creating an internal space between the posterior breast and
pectoralis fascia was considered the critical step of endoscopic
ALND. Unlike the most common MALND (9,14),
lipolysis and balloon expansion or other lifting devices were not
required in our endoscopic surgery to help establish a gas space in
axilla. In addition, the risk of trocar site tumor implantation
during liposuction was eliminated.
With sufficient working space, which included the
internal space between the posterior breast and pectoralis fascia,
and the inflation region created by a sustained air pressure of ~8
mmHg, this method is considered more suitable compared with other
MALNDs. If the dissection of the axillary level II and/or III is
difficult to perform, the lateral part of pectoralis major muscle
may be elevated with an anchoring suture that penetrates through
the skin and is fixed outwards (Fig.
3A).
With our technique, the axilla was first dissected
from the lateral pectoralis major muscle and axillary floor to the
apex, until the axillary vein was identified. This surgical order
was suitable for identifying the intercostobrachial nerve followed
by the axillary vein. Thereafter, the operation proceeded
laterally, medially and downwards. This procedure prevents injury
to the important structures in the axilla. In addition, some
lymphatic tissue remaining in the lower part of the axilla may be
completely dissected, unlike with other MALNDs with 3 trocars
restricted to the axilla (13).
It is noteworthy that, in several MALND studies, the
ultrasonic knife was not recommended, as the short-arm ultrasonic
knife is not sufficiently thin or sharp and, therefore, is not
considered suitable for severing small and thin strips, or for
peeling off the fat and lymphatic tissues around the important
nerves and vessels. We consider the 5-mm long-arm ultrasonic knife
to be better suited for the complex work in the axilla under
endoscopic view. The limited application of ultrasonic scalpel is
considered to be largely due to the narrow surgical working space
and the type of ultrasonic knife. Although the knife head of the
long-arm ultrasonic scalpel is thinner compared with that of the
short-arm scalpel, the endoscopic working space created was
sufficiently larger to allow use of the long-arm ultrasonic
scalpel. During endoscopic surgery with the ultrasonic scalpel, the
blood loss volume and the operative time were significantly reduced
compared with the bipolar dissecting forceps and scissors.
Regarding the change in the method of the
mastectomy, for the majority of VABS procedures, the most commonly
employed technique is referred to as the ‘subcutaneous tunneling
method’ (20), which involved
creating a number of subcutaneous tunnels using the endoscopic or
bladeless trocar. The working planes were created by subcutaneous
and sub-mammary elevation, balloon dissection and/or retraction
device. Our mastectomy procedure differed significantly from this
‘subcutaneous tunnelling method’. Mastectomy is usually performed
at the final step. Via the extended periareolar skin incision,
scissors and a scalpel were used to perform dissection between the
breast and subcutaneous tissue and along the lateral and medial
resection margins. After that, the entire breast specimen and
extirpated adipose/lymphatic tissues were completely removed
through the extended skin incision (Fig.
4B). This procedure is consistent with the general surgical
oncological principle and reduces the risk of port-site
metastases.
This surgical procedure was familiar to the majority
of the surgeons; thus, it was simpler and faster compared with the
‘subcutaneous tunneling method’. In addition, the risk of excessive
bleeding was reduced due to the blood supply isolation, which was
performed during the first step of creating a working pace between
the posterior breast and the pectoralis fascia. Furthermore, the
cosmetic outcome was as satisfactory as that with the ‘subcutaneous
tunneling method’.
This new endoscopic technique was associated with
several advantages: The total operative time, including mastectomy
and ALND (mean, 85.5 min), was shorter compared with that of other
VABS (mean ± standard deviation, 192.6±38.5 min) (20). The intraoperative blood loss volume
(mean, 48 ml) was also lower compared with other types of VABS
(mean, 189 ml) (20). The total
volume of drainage fluid (range, 10–250 ml) and the time to removal
of the drainage tubes (range, 3–5 days) were similar to those
recorded for other procedures (9,21). The
incidence of intraoperative and postoperative complications was
similar to that of other VABS. These advantages were achieved
without compromising tumor dissection and operative safety. Most
importantly, port-site tumor implantation was not reported, as the
method followed the oncological principle of en bloc resection of
potentially metastatic lymph nodes.
In the earlier stages, this type of surgery required
more time. However, the modified endoscopic technique was optimized
after performing ~15–30 procedures, which was similar with other
endoscopic methods (9,12). Provided the surgeon and the camera
operator were experienced, the time was even shorter compared with
other types of endoscopic breast surgery.
The limitations of the present study include lack of
level I evidence to support this type of VABS, as well as lack of
highquality clinical studies, which are required to confirm the
oncological success of this type of VABS.
In conclusion, our version of VABS differs from
other types of endoscopic surgery regarding the selection of the
working instruments, trocar placement, creation of working space,
order of ALND and mastectomy method. As it is applied without prior
liposuction, this new VABS technique reduces the risk of port-site
metastases and it has great clinical potential and good prospects
for future development of endoscopic breast surgery.
Acknowledgements
The authors wish to thank Dr Chiliang Chen and Mrs.
Kuili Liang for the helpful discussions. This study was supported
by Guangxi Colleges and Universities Science and Technology
Research Projects (project number, KY2015LX054) and the Youth
Science Foundation of Guangxi Medical University (project number,
GXMUYSF201643).
Glossary
Abbreviations
Abbreviations:
|
VABS
|
video-assisted breast surgery
|
|
MALND
|
mastoscopic axillary lymph node
dissection
|
|
ALND
|
axillary lymph node dissection
|
References
|
1
|
Yang W, Sejdinaj F, Shen L, Zhu W, Wang H
and Zhang H: Abstract P2-12-05: Trans-peri-areolar breast
conservative surgery followed by endoscopic axillary lymph node
dissection: A novel surgical option. Cancer Research.
76:P2-12-05–P12-12-05. 2016. View Article : Google Scholar
|
|
2
|
Wang ZH, Qu X, Teng CS, Ge ZC, Zhang HM,
Yuan Z, Gao YG, Lu C, Yu JA and Zhang ZT: Preliminary results for
treatment of early stage breast cancer with endoscopic subcutaneous
mastectomy combined with endoscopic sentinel lymph node biopsy in
China. J Surg Oncol. 113:616–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai H-W, Chen S-T, Chen D-R, Chen SL,
Chang TW, Kuo SJ, Kuo YL and Hung CS: Current Trends in and
Indications for Endoscopy-Assisted Breast Surgery for Breast
Cancer: Results from a Six-Year Study Conducted by the Taiwan
Endoscopic Breast Surgery Cooperative Group. PLoS One.
11:e01503102016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamaki Y, Tsukamoto F, Miyoshi Y, Tanji Y,
Taguchi T and Noguchi S: Overview: Video-assisted breast surgery.
Biomed Pharmacother. 56 Suppl 1:187s–191s. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keshtgar MR and Fukuma E: Endoscopic
mastectomy: What does the future hold? Wom Health Lond. 5:107–109.
2009. View Article : Google Scholar
|
|
6
|
Ingram D: FRCS DIMBMF: Is it time for
breast cancer surgeons to embrace endoscopic-assisted mastectomy?
ANZ J Surg. 78:837–838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langer I, Kocher T, Guller U, Torhorst J,
Oertli D, Harder F and Zuber M: Long-term outcomes of breast cancer
patients after endoscopic axillary lymph node dissection: A
prospective analysis of 52 patients. Breast Cancer Res Treat.
90:85–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salvat J, Knopf J-F, Ayoubi J-M, Slamani
L, Vincent-Genod A, Guilbert M and Walker D: Endoscopic exploration
and lymph node sampling of the axilla. Preliminary findings of a
randomized pilot study comparing clinical and anatomo-pathologic
results of endoscopic axillary lymph node sampling with traditional
surgical treatment. Eur J Obstet Gynecol Reprod Biol. 70:165–173.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chengyu L, Jian Z, Xiaoxin J, Hua L, Qi Y
and Chen G: Experience of a large series of mastoscopic axillary
lymph node dissection. J Surg Oncol. 98:89–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Momeni A, Bannasch H, Torio-Padron N,
Borges J and Stark GB: [The application of endoscopy in aesthetic
breast surgery]. Handchirurgie, Mikrochirurgie, plastische
Chirurgie. Organ Deutschsprachigen Arbeitsgemeinschaft
Handchirurgie Organ Deutschsprachigen Arbeitsgemeinschaft Mikrochir
Peripheren Nerven Gefasse. 38:144–148. 2006.
|
|
11
|
Ozaki S, Ohara M, Shigematsu H, Sasada T,
Emi A, Masumoto N, Kadoya T, Murakami S, Kataoka T, Fujii M, et al:
Technical feasibility and cosmetic advantage of hybrid
endoscopy-assisted breast-conserving surgery for breast cancer
patients. J Laparoendosc Adv Surg Tech A. 23:91–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chengyu L, Yongqiao Z, Hua L, Xiaoxin J,
Chen G, Jing L and Jian Z: A standardized surgical technique for
mastoscopic axillary lymph node dissection. Surg Laparosc Endosc
Percutan Tech. 15:153–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamprath S, Bechler J, Kühne-Heid R,
Krause N and Schneider A: Endoscopic axillary lymphadenectomy
without prior liposuction. Development of a technique and initial
experience. Surg Endosc. 13:1226–1229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai HW, Wu HS, Chuang KL, Chen DR, Chang
TW, Kuo SJ, Chen ST and Kuo YL: Endoscopy-Assisted Total Mastectomy
Followed by Immediate Pedicled Transverse Rectus Abdominis
Musculocutaneous (TRAM) Flap Reconstruction: Preliminary Results of
48 Patients. Surg Innov. 22:382–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cangiotti L, Poiatti R, Taglietti L, Re P
and Carrara B: A mini-invasive technique for axillary
lymphadenectomy in early breast cancer: a study of 15 patients.
Journal of experimental & clinical cancer research: CR.
18:295–298. 1999.
|
|
16
|
Ho WS, Ying SY and Chan AC:
Endoscopic-assisted subcutaneous mastectomy and axillary dissection
with immediate mammary prosthesis reconstruction for early breast
cancer. Surg Endosc. 16:302–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brun JL, Rousseau E, Belleannée G, de
Mascarel A and Brun G: Axillary lymphadenectomy prepared by fat and
lymph node suction in breast cancer. Eur J Surg Oncol. 24:17–20.
1998.(EJSO). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kühn T, Santjohanser C, Koretz K, Böhm W
and Kreienberg R: Axilloscopy and endoscopic sentinel node
detection in breast cancer patients. Surg Endosc. 14:573–577. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malur S, Bechler J and Schneider A:
Endoscopic axillary lymphadenectomy without prior liposuction in
100 patients with invasive breast cancer. Surg Laparosc Endosc
Percutan Tech. 11:38–41; discussion 42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leff DR, Vashisht R, Yongue G, Keshtgar M,
Yang GZ and Darzi A: Endoscopic breast surgery: Where are we now
and what might the future hold for video-assisted breast surgery?
Breast Cancer Res Treat. 125:607–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozaki S and Ohara M: Endoscopy-assisted
breast-conserving surgery for breast cancer patients. Gland Surg.
3:94–108. 2014.PubMed/NCBI
|