Introduction
Head and neck cancer is mostly of squamous cell
origin (squamous cell carcinoma of the head and neck; SCCHN) and is
the 10th most common type of cancer, with >630,000 cases
diagnosed annually (1). Over 350,000
deaths from head and neck cancer were estimated to have occurred in
2008 worldwide (2). The frequency,
incidence rates and locations of SCCHN vary widely among countries
and continents (2–4).
Following the introduction of the TAX 323 and 324
studies, induction chemotherapy drew significant scientific and
clinical interest, leading to further investigations (3,5,6). The use of docetaxel in the treatment
regimen was evaluated in those studies. Docetaxel promotes tubulin
polymerization and affects the formation of stable microtubules,
which leads to cell death (7).
Recent results from multicenter studies and a current meta-analysis
demonstrated that the combination of docetaxel with 5-fluorouracil
(5-FU) and cisplatin (TPF regimen) is more efficacious compared
with the classic regimen of cisplatin plus 5-FU (PF regimen) as
induction chemotherapy for advanced head and neck cancer (8). The TPF regimen achieved longer
progression-free survival, better locoregional control and higher
response rates (9,10). An explanation for this advantage may
be a better control of local and metastatic disease. Another aspect
is the possibility of functional organ preservation when primary
radiochemotherapy is used. Such approaches appear to be promising
and are likely to improve the quality of life (QoL) of the
patients, provided that survival is comparable to that with classic
treatments and the side effects are manageable. Thus far, only a
limited number of studies have investigated the effect of the
organ-preserving TPF regimen on the QoL of SCCHN patients.
Radiochemotherapy is currently the cornerstone of
treatment for SCCHN (11,12), either alone or combined with surgery.
However, a major disadvantage of induction chemotherapy is severe
treatment-induced toxicity. TPF treatment was associated with a
high percentage of patients experiencing myelosuppression, with
grade 3 or 4 neutropenia (5,6,13,14), as
recently reported by a meta-analysis (8). The high incidence of toxicity may be
the cause for the limited use of this combination treatment in the
palliative setting. Moreover, the PARADIGM study that compared the
addition of induction chemotherapy to radiochemotherapy vs.
radiochemotherapy alone did not report an improvement in survival
with induction chemotherapy prior to radiochemotherapy (15).
There are several different therapy schedules for
the treatment of SCCHN, but a definitive therapeutic strategy has
not yet been established. Promising results were obtained from only
a few studies in which docetaxel was used as part of induction
chemotherapy. Therefore, the clinical effectiveness and toxicity
profile of docetaxel in combination with 5-FU and cisplatin was
evaluated in a defined setting in patients with curable and
metastatic/recurrent SCCHN in our hospital.
Patients and methods
Patient population
The medical records of patients with pathologically
confirmed SCCHN, who received treatment with docetaxel at the
hospitals of Charité-Universitätsmedizin Berlin, Germany, between
April 2007 and May 2012, were retrospectively reviewed. Data were
retrieved from the archives of Charité or from the attending
physicians.
Treatment plan
The established treatment schedule consisted of an
intravenous infusion of docetaxel at a dose of 75 mg/m2
followed by an intravenous infusion of cisplatin at 75
mg/m2 and a continuous 5-day infusion of 5-FU at a dose
of 750 mg/m2. The cycles were repeated after 21 days.
The treatment regimen was adapted when severe side effects
occurred. In case of reduced kidney function, cisplatin was
substituted by carboplatin.
Premedication with dexamethasone was administered in
the evening of the day prior to the first TPF application. One hour
prior to the initiation of therapy, antiemetic medication and
mannitol with 0.9% saline solution were administered to prevent
acute renal failure. As an additional preventive measure for
agranulocytosis, granulocyte colony-stimulating factor (GCSF) was
administered on day 6 to accelerate the recovery of therapy-induced
neutropenia.
TPF induction chemotherapy was most frequently
followed by targeted therapy with an anti-epidermal growth factor
receptor antibody (cetuximab; loading dose of 400 mg/m2
with 250 mg/m2 over the subsequent weeks) with
simultaneous intensity-modulated fractionated radiotherapy with a
total dose of 54–79.2 Gy over 7 weeks, 5 days per week.
Clinical examination
To assess the disease status prior to and following
therapy, the clinical evaluation routinely included panendoscopy
and radiological evaluation consisting of computed tomography (CT)
scans, abdominal sonography and additional magnetic resonance
imaging and positron emission tomography/CT, or bone scintigraphy,
as necessary. With this information, the decision on treatment
strategy was made by an interdisciplinary tumor board consisting of
oncologists, otolaryngologists and head and neck surgeons,
maxillofacial surgeons, radiotherapists and pathologists.
The Karnofsky scale was used in this study in
addition to chronic ailments, such as pain, ototoxicity,
neurotoxicity, permanent tracheostoma or percutaneous endoscopic
gastrostomy, to assess patient status.
Toxicity
The toxicity profile was divided into hematological
and non-hematological events and was scored according to version 4
of the National Cancer Institute Common Terminology Criteria for
Adverse Events (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
Treatment outcome
The response rate (RR), overall survival (OS),
progression-free survival (PFS), cumulative incidence of distant
disease and locoregional control, toxicity, side effects and QoL
were analyzed. The response to therapy was assessed according to
the definitions of the Response Evaluation Criteria In Solid
Tumors, version 1.1, published by the European Organization for
Research and Treatment of Cancer (EORTC) (12,16),
National Cancer Institute of the United States, and the National
Cancer Institute of Canada Clinical Trials Group: Complete response
(CR) was defined as the disappearance of all signs of the current
disease recorded from the start of the treatment. Partial response
(PR) was defined as a decrease in the size of the tumor of ~30%.
Stable disease (SD) was defined as no disease progression or
regression. Progressive disease (PD) was defined as an increase in
the tumor size of >20% during or after treatment. Finally, a
mixed response was defined as decrease at one site and progression
at a different site. Patients with a mixed response were not
included in the calculation of overall response rate (ORR)
(16).
ORR was calculated based on CR, PR and SD, until the
first sign of recurrence or PD was objectively recorded. The time
of response evaluation in this study was calculated from the first
day of treatment with TPF until the first follow-up examination
after treatment. The OS was defined from the beginning of the
treatment until the last date of contact or death.
Statistical analysis
All survival parameters were calculated using SPSS
software, version 23 (IBM Corp., Armonk, NY, USA).
Results
Patient population
According to the abovementioned criteria, 45
patients with a median age of 57 years were included in the present
study. Of those patients, 35 received curative therapy (CT) and 10
received palliative therapy (PT). A total of 6 patients received a
modified therapy schedule with curative intent with reduced/adapted
dosage due to a worsening general condition or pre-existing
comorbidities. Data from all patients were available for evaluation
of toxicity and survival time. The majority of the patients (87%)
were male. The review from the patient' charts revealed that nearly
>50% had a history of chronic alcohol abuse. Nicotine use was
reported in >60% in all groups and a simultaneous use of both
substances in 90% in the curative and 100% in the palliative group.
Approximately >50% of the SCCHNs were diagnosed at an advanced
stage (T4) with metastases in the regional lymph nodes (N2). The
patient characteristics are summarized in Table I.
 | Table I.Patient characteristics according to
the different therapy approaches. |
Table I.
Patient characteristics according to
the different therapy approaches.
| Characteristics | CT (n=30) | PT (n=9) | CTrD (n=6) | Total (n=45) |
|---|
| Gender, no. (%) |
|
|
|
|
| Male | 26 (87) | 7 (78) | 6 (100) | 39 (87) |
|
Female | 4 (13) | 2 (22) | 0 (0) | 6 (13) |
| Alcohol consumption,
no. (%) |
|
|
|
|
| Yes | 14 (47) | 9 (100) | 3 (50) | 26 (58) |
| No | 16 (53) | 0 (0) | 3 (50) | 19 (42) |
| Recovering
alcoholic | 2 (7) | 0 (0) | 0 (0) | 2 (4) |
| Nicotine consumption,
no. (%) |
|
|
|
|
| Yes | 27 (90) | 9 (100) | 4 (67) | 40 (89) |
| Never
smoker | 3 (10) | 0 (0) | 2 (33) | 5 (11) |
| Former
smoker | 7 (23) | 0 (0) | 0 (0) | 7 (16) |
| Alcohol
and nicotine consumption, no. (%) | 14 (47) | 9 (100) | 2 (33) | 25 (56) |
| Comorbidities, no.
(%) |
|
|
|
|
|
Hypertension | 2 (7) | 4 (44) | 1 (17) | 7 (16) |
| Diabetes
mellitus | 3 (10) | 1 (11) | 1 (17) | 5 (11) |
|
Trauma | 5 (17) | 2 (22) | 0 (17) | 7 (16) |
| Heart
disease | 2 (7) | 2 (22) | 2 (33) | 6 (13) |
| Previous
malignancy | 5 (17) | 0 (0) | 1 (17) | 6 (13) |
| Localization, no.
(%) |
|
|
|
|
|
Epipharynx | 5 (17) | 0 (0) | 0 (0) | 5 (11) |
|
Oropharynx | 6 (20) | 5 (56) | 3 (50) | 14 (31) |
|
Hypopharynx | 4 (13) | 1 (11) | 1 (17) | 6 (13) |
|
Larynx | 9 (30) | 0 (0) | 0 (0) | 9 (20) |
| Floor of
mouth | 4 (13) | 0 (0) | 1 (17) | 5 (11) |
|
Tongue | 2 (7) | 2 (22) | 1 (17) | 5 (11) |
|
Other | 0 (0) | 1 (11) | 0 (0) | 1 (2) |
| T classification,
no. (%) |
|
|
|
|
| T1 | 0 (0) | 0 (0) | 1 (17) | 1 (2) |
| T2 | 4 (13) | 2 (22) | 1 (17) | 7 (16) |
| T3 | 5 (17) | 3 (33) | 2 (33) | 10 (22) |
| T4 | 21 (70) | 3 (33) | 2 (33) | 26 (58) |
| Tx | 0 (0) | 1 (11) | 0 (0) | 1 (2) |
| N classification,
no. (%) |
|
|
|
|
| N0 | 6 (20) | 1 (11) | 0 (0) | 7 (16) |
| N1 | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| N2 | 16 (53) | 5 (56) | 2 (33) | 23 (51) |
| N3 | 6 (20) | 2 (22) | 4 (67) | 12 (27) |
| Nx | 1 (3) | 1 (11) | 0 (0) | 2 (4) |
| Recurrence prior to
TPF therapy, no. (%) |
|
|
|
|
|
Yes | 0 (0) | 5 (56) | 0 (0) | 5 (11) |
| No | 30 (100) | 4 (44) | 6 (100) | 40 (89) |
Patient status
The Karnofsky score was ranked as 60–100% before and
0–100% after therapy. No information on general conditions was
available in 10 patients. In 8 patients, the score decreased to 31%
after treatment. Patients with an initial score of <70% were at
risk for further decrease, which was already visible during
treatment. Patients treated with a palliative intent had lower
scores during TPF treatment. The Karnofsky scores and chronic
ailments are listed in Table
II.
 | Table II.Karnofsky score measured among
patients in different groups before, during and after TPF
therapy. |
Table II.
Karnofsky score measured among
patients in different groups before, during and after TPF
therapy.
|
|
Karnofsky
score (%) |
|---|
|
|
|
|---|
|
| Before
treatment | During
treatment | After
treatment |
|
|---|
|
|
|
|
|
|
|---|
| Type of
treatment | ≥90 | <90 | <50 | ≥90 | <90 | <50 | ≥90 | <90 | <50 | Missing |
|---|
| CT | 23 | 3 | 0 | 17 | 9 | 0 | 22 | 4 | 0 | 4 |
| PT | 2 | 4 | 0 | 2 | 2 | 2 | 2 | 1 | 3 | 3 |
| CTrD | 2 | 1 | 0 | 2 | 0 | 1 | 2 | 0 | 1 | 3 |
| Total | 27 | 8 | 0 | 21 | 11 | 3 | 26 | 5 | 4 | 10 |
Treatment characteristics
A total of 87% of the patients received chemotherapy
based on the treatment schedule described in the methods section,
and 80% of all patients completed the therapy. A total of 4
patients required a dose reduction after the first and second
cycles due to severe mucositis, nephrotoxicity and ototoxicity. The
most common reason for discontinuing treatment was a poor treatment
response. To reduce the side effects, the treatment regimen was
individualized in 6 patients, who received platinum agents other
than cisplatin, such as carboplatin, oxaliplatin or lipoplatin, or
by reducing the dosage of docetaxel to 50 mg/m2. By
contrast, the dosage of 5-FU was increased to 1,000
mg/m2 from 750 mg/m2 (Table III).
 | Table III.Treatment completion and subsequent
therapy strategies. |
Table III.
Treatment completion and subsequent
therapy strategies.
| Treatment | CT (n=30) | PT (n=9) | CTrD (n=6) | Total (n=45) |
|---|
| Completed
treatment, no. (%) | 26 (87) | 4 (44) | 5 (83) | 35 (77) |
| Subsequent
treatment, no. (%) |
|
|
|
|
| RT with
cetuximab | 13 (43) | 2 (22) | 4 (67) | 19 (42) |
|
CRT | 13 (43) | 2 (22) | 2 (33) | 17 (38) |
| RT | 2 (7) | 2 (22) | 0 (0) | 7 (16) |
|
Resection | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| None
possible | 1 (3) | 3 (33) | 0 (0) | 1 (2) |
| Reduced dosage, no.
(%) | 2 (7) | 3 (33) | 2 (33) | 7 (16) |
Efficacy
An ORR of 86% was registered (93% in the curative
group, 75% in the palliative group and 67% in the modified regimen
group). Over 50% of the patients treated with a curative intent
reached a CR after completing chemotherapy and further treatment.
The data on response to treatment are summarized in Table IV. One patient treated with a
palliative intent succumbed to the disease after the second cycle
of TPF, due to the advanced stage and poor general condition.
 | Table IV.Response to treatment. |
Table IV.
Response to treatment.
| Variables | CT (n=30) | PT (n=9) | CTrD (n=6) | Total (n=45) |
|---|
| Response to TPF,
% |
|
|
|
|
| Overall
response | 93 | 75 | 67 | 86 |
|
Complete response | 10 | 0 | 17 | 9 |
| Partial
response | 66 | 25 | 33 | 53 |
| Stable
disease | 17 | 50 | 17 | 23 |
| Response to TPF and
subsequent therapy, % |
|
|
|
|
| Overall
response | 93 | 75 | 67 | 86 |
|
Complete response | 51 | 25 | 33 | 44 |
| Partial
response | 7 | 0 | 0 | 5 |
| Stable
disease | 0 | 0 | 0 | 0 |
Patients were followed up for a median of 44.9
months. The median OS was not reached, except for the palliative
group, with a median OS of 9 months.
The estimated 1-year survival rate was 78.6%, the
2-year survival rate was 75% and the 3-year survival rate was 65.1%
for the entire patient population. In total, 12 patients (27%)
succumbed to the disease during follow-up. The median PFS was 32
months. Estimations of the PFS at 1 year were 63%, at 2 years 57%
and at 3 years 36%. The cumulative incidence rate of locoregional
failure at 1 year was 8%. In total, distant metastases occurred
retrospectively in 3 patients. The cumulative incidence rate of
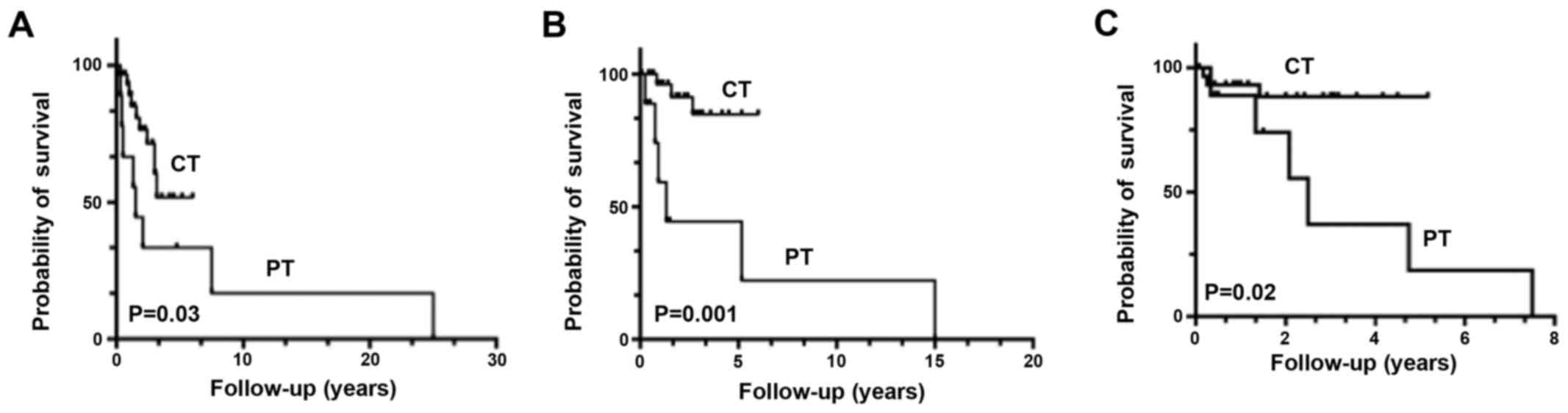
distant metastases at 1 year was 4%. Kaplan-Meier curves showed
better OS (P=0.03), Recurrence-free survival (P=0.001) and PFS
(P=0.02) in the CT group compared with those in the PT group
(Fig. 1).
Toxicity and side effects
Hematological toxicity was documented in all
patients. The grade 3 and 4 adverse events were leukopenia and
lymphopenia. The duration of the hematological toxic effects was
relatively brief and they were alleviated after a median of 1 week.
The low leukocyte count may be explained by the documented
bacterial infections, which were treated with antibiotics.
Additionally, an oropharyngeal infection with Candida albicans was
diagnosed and treated with topical antimycotics.
Severe neutropenia was not very common (13%) and was
managed by human GCSF, which was part of the treatment schedule.
Non-hematological toxicities occurred in >50% of the study
population. A total of 51% patients exhibited a small increase in
liver enzyme levels, which was the most common toxicity in this
category.
The most frequent side effects were gastrointestinal
reactions, including nausea (27%), emesis (13%) and diarrhea (11%).
All adverse events were more severe in the patient cohort treated
with a palliative intent and they are summarized in Tables V and VI.
 | Table V.Hematological and non-hematological
adverse events during TPF therapy. |
Table V.
Hematological and non-hematological
adverse events during TPF therapy.
| Adverse events | Grade I–II | Grade III–IV |
|---|
| Hematological,
% |
|
|
|
Anaemia | 49 | 2 |
|
Leukopenia | 25 | 33 |
|
Lymphopenia | 11 | 22 |
|
Neutropenia | 0 | 13 |
|
Thrombopenia | 16 | 2 |
| Non-hematological,
% |
|
|
|
Hypertransaminasemia | 51 | 2 |
|
Hypercreatininaemia | 24 | 2 |
|
Hypokaliaemia | 11 | 2 |
|
Hyponatriaemia | 36 | 7 |
|
Hypomagnesaemia | 2 | 4 |
|
Hyperglycaemia | 13 | 0 |
|
Hypocalcaemia | 2 | 2 |
 | Table VI.Side effects during TPF therapy. |
Table VI.
Side effects during TPF therapy.
| Side effects | CT, n (%)
(n=30) | PT, n (%)
(n=9) | CTrD, n (%)
(n=6) | Total, n (%)
(n=45) |
|---|
| Nausea | 10 (33) | 1 (11) | 1 (17) | 12 (27) |
| Emesis | 4 (13) | 1 (11) | 1 (17) | 6 (13) |
| Diarrhea | 2 (7) | 2 (22) | 1 (17) | 5 (11) |
| Mucositis | 2 (7) | 3 (33) | 3 (50) | 8 (18) |
| Algesia | 3 (10) | 3 (33) | 2 (33) | 8 (18) |
| Fever | 6 (20) | 1 (11) | 1 (17) | 8 (18) |
| Infection | 3 (10) | 2 (22) | 0 (0) | 5 (11) |
| Ototoxicity | 2 (7) | 0 (0) | 0 (0) | 2 (4) |
| Neurotoxicity | 1 (3) | 0 (0) | 1 (17) | 2 (4) |
| Acute renal
failure | 1 (3) | 1 (11) | 1 (17) | 3 (7) |
| Atrial
fibrillation | 1 (3) | 0 (0) | 0 (0) | 1 (2) |
| Thrombosis | 2 (7) | 1 (11) | 0 (0) | 3 (7) |
Discussion
Based on the observations and evaluations in this
study cohort, it may be concluded that docetaxel applied in
combination with cisplatin and 5-FU is an effective therapy for
locally advanced, recurrent or metastatic SCCHN, in accordance with
previous clinical trials.
SCCHNs are locally aggressive and may develop
regional metastases. Induction chemotherapy with platinum-based
agents and 5-FU has achieved improved results compared with the
current classic therapies in terms of survival, locoregional
disease control and organ function and, most importantly, in terms
of swallowing function maintenance and voice preservation (17). In the TAX 323 trial, 358 patients
with inoperable SCCHN were enrolled. The group treated with TPF
(n=177) experienced a significantly longer PFS of 11.0 months,
compared with 8.2 months for patients treated with PF alone
(n=181), with an ORR of 68%. At a median follow-up period of 51.1
months, patients receiving the docetaxel-based regimen achieved a
median OS improvement of 4.3 months compared with those receiving
standard chemotherapy with PF. Treatment with TPF resulted in a 27%
reduction in the mortality risk. The incidence of grade 3/4
toxicity was higher if patients were treated with PF alone. During
treatment with TPF, severe neutropenia (76.9 vs. 52.5%) and
alopecia (11.6 vs. 0%) were observed more often, whereas the rate
of vomiting and stomatitis was lower compared with the PF regimen.
Other modified designs reached comparable results, with ORRs of up
to 100% (6). In a recent
meta-analysis, seven clinical trials that investigated 3- and
5-year efficacy were reviewed, and ORR was found to be better in
the TPF induction chemotherapy group compared with that in the
PF-based therapy group (8). In the
present retrospective study, an ORR of 86% was achieved; therefore,
the data are comparable with the results of already published
reports.
Different to other studies, the present study also
included patients treated with a palliative intent, while the
majority of other studies only analyzed patients treated with a
curative intent (5,6,8,18). The ORR in the palliative treatment
group was 75%, while the group treated with a curative intent
reached an ORR of 93%. In contrast to investigations with
potentially curable SCCHN, studies on TPF and the outcome of
patients with diagnosed metastatic and recurrent SCCHN are scarce.
In a previous study that included 55 patients treated in the
palliative setting with 60 mg/m2 docetaxel, 50
mg/m2 cisplatin and 500 mg/m2 5-FU, an ORR of
56% was observed (19), which is
significantly lower compared with the palliative group in the
present study, which achieved a ORR of 75%.
Considering the survival rates, Paccagnella et
al reported a 2-year survival of 61% and a median PFR of 30.4
months in the TPF group of patients treated with a curative intent
(20). Compared with the published
data, our data show a higher 2-year survival rate of 75% and a
longer median PFR of 32 months in the entire cohort. In the TAX 324
study, better locoregional control was achieved in the TPF group
(30% failure) compared with that in the PF group (38% failure)
(5). However, the occurrence of
distant metastases did not differ significantly between the two
groups (5% in the TPF and 9% in the PF group). In the present
study, an incidence rate of 8% per year for locoregional failure
and 4% per year for distant metastatic disease was observed.
The Karnofsky score was decreased in 8 patients
(31%) following treatment. The decrease in the Karnofsky score was
particularly observed among patients with a Karnofsky score of
<70% at the beginning of treatment with docetaxel. Van Herpen
et al measured the QoL using the EORTC Quality of Life
Questionnaire C30 (QLQ-C30) and the EORTC QLQ Head and Neck
Cancer-Specific Module (EORTC QLQ-H&N35) (20). The standardized questionnaires and
documentation of symptoms, such as prolonged cough and dysphagia,
were recorded at 2, 4, 6 and 9 months following completion of
therapy. In contrast to our observations made with the Karnofsky
score, which is hypothesized to be correlated with the perceived
QoL, QoL appeared to improve during therapy. After 6 months of
therapy, the QoL in patients receiving TPF was improved compared
with those receiving PF (35.1 vs. 27.2%, respectively).
Furthermore, a lower incidence of dysphagia and chronic cough and
an improvement in speech were observed in the TPF cohort. A
compromise in QoL was observed in 23.6%, which was similar to our
results of the curative patient cohort.
Consistent with other clinical trials, hematological
and non-hematological treatment-related side effects (particularly
grade 3 and 4 leukopenia and gastrointestinal reactions) were
observed in nearly all investigated patients (5,6,8,18,19).
Another concern is the high rate of neutropenia during chemotherapy
with TPF. The TAX 323 and 324 studies reported increasing rates of
neutropenia (77–83%). Due to prophylactic treatment with GCSF in
our study, the rates of neutropenia were reduced. However, these
side effects were diminished after a few days of the chemotherapy
and are therefore considered acceptable weighed against the
survival benefit. In addition, the number and severity of
non-hematological side effects such as vomiting, ototoxicity or
neurotoxicity were lower than expected, whereas a higher-grade
mucositis was documented.
These encouraging data must be interpreted with
caution due to the relatively limited number of patients included
in this study; in addition, comparing phase II and III with
retrospective data may be a source of misinterpretation.
In conclusion, based on our experience with treating
patients with advanced SCCHN, a combination chemotherapy with
docetaxel for patients with locally advanced and recurrent or/and
metastatic SCCHN appears to be advantageous when the side effects
are tolerable. As regards the high toxicity rates, a careful
risk-benefit-analysis for each individual patient is recommended,
whereas patient compliance is crucial. A deterioration of the
general condition may be expected in 31% of the patient population,
particularly for patients with a Karnofsky score of <70% at
treatment initiation.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coordes A, Lenz K, Qian X, Lenarz M,
Kaufmann AM and Albers AE: Meta-analysis of survival in patients
with HNSCC discriminates risk depending on combined HPV and p16
status. Eur Arch Otorhinolaryngol. 273:2157–2169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tinhofer I, Jöhrens K, Keilholz U,
Kaufmann A, Lehmann A, Weichert W, Stenzinger A, Stromberger C,
Klinghammer K, Becker ET, et al: Contribution of human papilloma
virus to the incidence of squamous cell carcinoma of the head and
neck in a European population with high smoking prevalence. Eur J
Cancer. 51:514–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartsch V: Assault on the mitotic spindle.
The action mechanism of taxane. Pharm Unserer Zeit. 34:104–108.
2005.(In German). View Article : Google Scholar
|
|
8
|
Qian X, Ma C, Hoffmann TK, Kaufmann AM and
Albers AE: Taxane-cisplatin-fluorouracil as induction chemotherapy
for advanced head and neck cancer: A meta-analysis of the 5-year
efficacy and safety. Springerplus. 4:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blanchard P, Baujat B, Holostenco V,
Bourredjem A, Baey C, Bourhis J and Pignon JP; MACH-CH
Collaborative group, : Meta-analysis of chemotherapy in head and
neck cancer (MACH-NC): A comprehensive analysis by tumour site.
Radiother Oncol. 100:33–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J, Liu Y, Yang X, Zhang CP, Zhang ZY
and Zhong LP: Induction chemotherapy in patients with resectable
head and neck squamous cell carcinoma: A meta-analysis. World J
Surg Oncol. 11:672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Mamgani A, van Rooij P, Verduijn GM,
Mehilal R, Kerrebijn JD and Levendag PC: The impact of treatment
modality and radiation technique on outcomes and toxicity of
patients with locally advanced oropharyngeal cancer. Laryngoscope.
123:386–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper JS, Zhang Q, Pajak TF, Forastiere
AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Long-term follow-up of the RTOG 9501/intergroup phase III
trial: Postoperative concurrent radiation therapy and chemotherapy
in high-risk squamous cell carcinoma of the head and neck. Int J
Radiat Oncol Biol Phys. 84:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Billan S, Kaidar-Person O, Atrash F,
Doweck I, Haim N, Kuten A and Ronen O: Toxicity of induction
chemotherapy with docetaxel, cisplatin and 5-fluorouracil for
advanced head and neck cancer. Isr Med Assoc J. 15:231–235.
2013.PubMed/NCBI
|
|
14
|
Lorch JH, Goloubeva O, Haddad RI, Cullen
K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE and Posner
MR; TAX 324 Study Group, : Induction chemotherapy with cisplatin
and fluorouracil alone or in combination with docetaxel in locally
advanced squamous-cell cancer of the head and neck: Long-term
results of the TAX 324 randomised phase 3 trial. Lancet Oncol.
12:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong LP, Zhang CP, Ren GX, Guo W, William
WN Jr, Sun J, Zhu HG, Tu WY, Li J, Cai YL, et al: Randomized phase
III trial of induction chemotherapy with docetaxel, cisplatin, and
fluorouracil followed by surgery versus up-front surgery in locally
advanced resectable oral squamous cell carcinoma. J Clin Oncol.
31:744–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paccagnella A, Ghi MG, Loreggian L,
Buffoli A, Koussis H, Mione CA, Bonetti A, Campostrini F, Gardani
G, Ardizzoia A, et al: Concomitant chemoradiotherapy versus
induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by
concomitant chemoradiotherapy in locally advanced head and neck
cancer: A phase II randomized study. Ann Oncol. 21:1515–1522. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin JT, Lai GM, Chang TH, Liu MT, Bi CP,
Wang JW and Chen MK: Chemotherapy with modified docetaxel,
cisplatin, and 5-fluorouracil in patients with metastatic head and
neck cancer. Adv Ther. 29:71–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Herpen CM, Mauer ME, Mesia R, Degardin
M, Jelic S, Coens C, Betka J, Bernier J, Remenar E, Stewart JS, et
al: Short-term health-related quality of life and symptom control
with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF),
5-fluorouracil (PF) for induction in unresectable locoregionally
advanced head and neck cancer patients (EORTC 24971/TAX 323). Br J
Cancer. 103:1173–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|