Introduction
Bevacizumab is a humanized monoclonal antibody that
inhibits vascular endothelial growth factor (VEGF) activity
(1). When it is used in combination
with cytotoxic drugs, high clinical utility is predicted for many
cancer types including non-small cell lung cancer (NSCLC) (2,3).
Evidence for its efficacy when used as an additional drug in
first-line chemotherapy for NSCLC has been produced by numerous
randomized phase III clinical trials (2,3).
Ramucirumab, a new anti-VEGF antibody, combined with docetaxel, has
recently been introduced as a second-line chemotherapy for
recurrent NSCLC (4,5). Therefore, it is of importance to
determine which is a more effective treatment, bevacizumab or
ramucirumab, in combination with docetaxel, as a second- or
later-line chemotherapy for patients with NSCLC. To the best of our
knowledge, there have been no clinical trials that compared
first-line bevacizumab plus docetaxel and ramucirumab plus
docetaxel, In addition, the results of a comparison between
second-line bevacizumab plus docetaxel and docetaxel alone for
patients with NSCLC have never been reported. In order to evaluate
the combined effect of bevacizumab and docetaxel as a second- or
later-line chemotherapy for NSCLC, a retrospective study was
performed.
Patients and methods
Patients and treatments
Patients with non-small cell lung cancer were
admitted to three tertiary hospitals (Tsukuba University Hospital,
Tsukuba Medical Center Hospital, and Mito Medical Center,
University of Tsukuba, Tsukuba, Japan) between November 2009 and
April 2016. The medical records of all the patients <75 years
old, who were treated with docetaxel (60 mg/m2, day 1,
q3 or 4 weeks) plus bevacizumab (15 mg/kg, day 1, q3 or 4 weeks) as
a second- or later-line chemotherapy were retrospectively reviewed.
The clinical data in these patients were compared with those in
patients <75 years old who were treated with docetaxel (60
mg/m2, day 1, q3 or 4 weeks) alone as a second- or
later-line chemotherapy during the same study period. All patients
were required to have had a pathological or cytological diagnosis
of NSCLC. Pathological diagnosis of lung cancer was defined by the
World Health Organization classification (6). A tumor-node-metastasis staging
(7) procedure using head computed
tomography (CT) or magnetic resonance imaging, bone scans and
ultrasonography and/or CT of the abdomen was performed for all
patients prior to starting bevacizumab treatment. Patient
demographic data at the time of bevacizumab therapy (age, sex,
smoking history, histology and stage) and objective tumor response
were obtained. The tumor response was evaluated as complete
response (CR), partial response (PR), stable disease (SD),
progressive disease (PD) or not evaluable (NE), according to the
Response Evaluation Criteria in Solid Tumors (8). Toxicity was graded according to the
National Cancer Institute Common Toxicity Criteria, version 3.0
(9). This observational study
conformed to the Ethical Guidelines for Clinical Studies issued by
the Ministry of Health, Labor and Welfare of Japan. Informed
consent was obtained from patients for their inclusion in this
study. Analysis of the medical records of lung cancer patients was
approved by the Ethics Committee of Mito Medical Center, University
of Tsukuba Hospital (NO 16–19).
Statistical analysis
Differences in proportions between 2 independent
groups were compared by the χ2 test. Survival
probability was estimated with the Kaplan-Meier method and compared
using the log-rank test. All statistical analysis was conducted
using SPSS 10.1 for Windows (SPSS Corp., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Complete data sets were obtained from 15 patients
treated with docetaxel plus bevacizumab, and 55 patients treated
with docetaxel alone. The patient characteristics are listed in
Table I. With regards to age, sex,
performance status, and pathology, there were no statistically
significant differences between the groups. The median treatment
line of docetaxel plus bevacizumab, docetaxel alone was third-line
in the two treatment groups. The mean number of courses of
docetaxel plus bevacizumab was 3.2 (range, 1–15), and that of
docetaxel alone was 3.1 (range, 1–13). Dose reduction was performed
in 9 (33.3%) of the patients treated with docetaxel plus
bevacizumab, and in 25 (45.5%) of those treated with docetaxel
alone.
 | Table I.Comparison of clinicopathological
features between patients treated with docetaxel plus bevacizumab
and with docetaxel alone. |
Table I.
Comparison of clinicopathological
features between patients treated with docetaxel plus bevacizumab
and with docetaxel alone.
| Variables | Patients treated with
docetaxel and bevacizumab | Patients treated with
docetaxel alone |
|---|
| Number of
patients | 55 | 15 |
| Age, years (median,
range) | 62 (35–74) | 63 (40–75) |
| Male: female | 39:16 | 8:7 |
| Performance
status |
|
|
| 0-1:2-4,
N (%) | 50 (90.9):5
(9.1) | 13 (86.7):2
(13.3) |
| Pathology |
|
|
| Ad: LA, N
(%) | 52 (94.5):3
(5.5) | 15 (100):0 (0) |
| Treatment line of
DOC-containing therapy (median, range) | 3 (2–6) | 3 (2–8) |
| Number of treatment
courses, mean (range) | 3.2 (1–13) | 5.3 (1–15) |
| Dose reduction
present/absent, N (%) | 25 (45.5)/30
(54.5) | 5 (33.3)/10
(66.7) |
Response to treatment
The overall response rate to docetaxel plus
bevacizumab therapy was 26.7% [0 CR; 4 PR; 95% confidence interval
(CI), 4.3–49.0%), and 53.3% of these patients had SD, amounting to
a disease control rate of 80.0%. On the other hand, the overall
response rate to docetaxel alone therapy was 9.1% (0 CR; 5 PR; 95%
CI, 1.5–16.7%), and 38.2% of these patients had SD, amounting to a
disease control rate of 47.3%.
Toxicity
Table II presents
the adverse events associated with docetaxel plus bevacizumab
therapy. All the patients treated with docetaxel plus bevacizumab
therapy had grade 3 or 4 ‘neutropenia’ or ‘febrile neutropenia’,
which developed in 100% and 26.7% of patients, respectively. The
rates of these adverse events in patients treated with docetaxel
alone were 63.6%, and 10.9%, respectively.
 | Table II.Comparison of clinicopathological
features between patients treated with docetaxel plus bevacizumab
and with docetaxel alone. |
Table II.
Comparison of clinicopathological
features between patients treated with docetaxel plus bevacizumab
and with docetaxel alone.
| Variables | Patients treated with
docetaxel and bevacizumab, N (%) | Patients treated with
docetaxel alone, N (%) |
|---|
| Number of
patients | 15 | 55 |
| Response |
|
|
| Complete
response | 0 (0) | 0 (0) |
| Partial
response | 4 (26.7) | 5 (9.1) |
| Stable
disease | 8 (53.3) | 21 (38.2) |
|
Progressive disease | 3 (20) | 29 (52.7) |
| Complete response +
partial response | 4 (26.7) | 5 (9.1) |
| Complete response +
partial response + stable disease | 12 (80) | 26 (47.3) |
Survival analysis
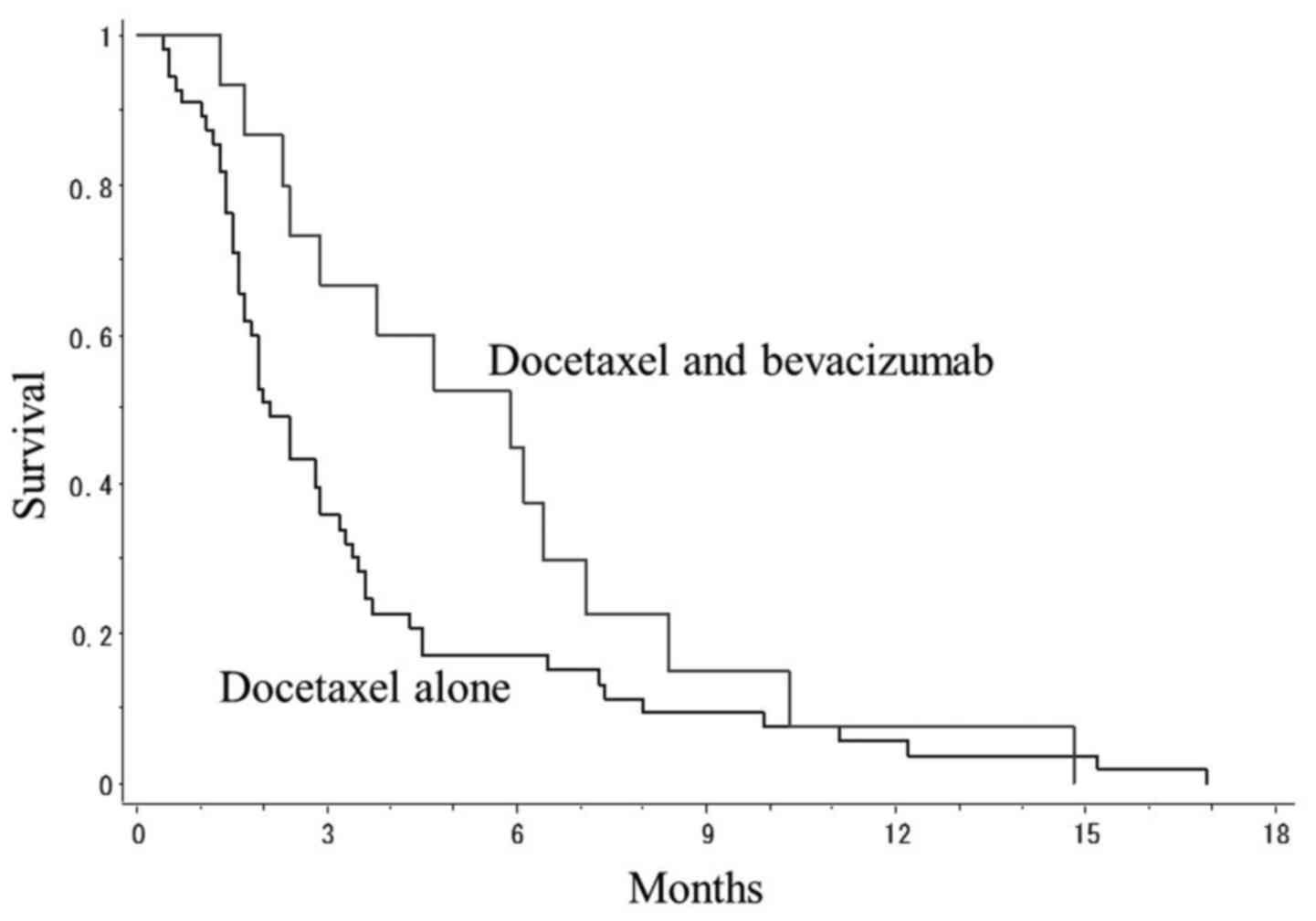
Fig. 1 presents PFS
curves of patients treated with docetaxel plus bevacizumab and
patients treated with docetaxel alone. The mean PFS in patients
treated with docetaxel plus bevacizumab and that of patients with
docetaxel alone was 5.9 and 2.1 months, respectively. There was a
non-significant tendency towards a difference in survival between
the two treatment groups (P=0.081, log-rank test).
Discussion
Docetaxel, pemetrexed and erlotinib have been
recommended as second-line chemotherapy for NSCLC in clinical
practice (3). However, there have
been insufficient results to comprehensively evaluate their
efficacy. Bevacizumab is a monoclonal antibody against VEGF; in
combination with cytotoxic agents, its efficacy in improvement of
response and prolongation of PFS has been demonstrated (2,3). In
addition, the usefulness of bevacizumab in patients with colon
cancer beyond PD has been reported (10). The results of clinical trials of
second-line docetaxel and ramucirumab for patients with NSCLC have
been demonstrated (4,5). Therefore, it is of importance to
determine which is the most effective drug, bevacizumab or
ramucirumab, in combination with docetaxel, as a second- or
later-line chemotherapy for patients with NSCLC. To the best of our
knowledge, there have been no clinical trials that compared
first-line bevacizumab plus docetaxel and ramucirumab plus
docetaxel, In addition, comparative results of second-line
bevacizumab plus docetaxel vs. docetaxel alone for patients with
NSCLC have not been reported. However, there have been several
studies of the efficacy of chemotherapy for patients with recurrent
NSCLC. In the JMEI trial, which compared the efficacy of docetaxel
and pemetrexed, the response rate (RR) and PFS were 9.0% and 3
months, and 11.5% and 3.1 months, respectively (11). The TAILOR trial with erlotinib
therapy revealed that the RR and PFS were 3.0% and 2.4 months
(12). The Lux-Lung-4 trial with
afatinib, the RR and PFS were 8.2% and 4.4 months (13). The NCCTG-SWOG N0426 trial with
pemetrexed and bevacizumab revealed that the RR and PFS were 10.4%
and 4.0 months (14). Herbst et
al (15) reported a difference
between patients treated with chemotherapy alone with pemetrexed or
docetaxel and those treated with bevacizumab plus chemotherapy with
pemetrexed or docetaxel; the median PFS and 6-month survival rate
in the former group of patients was 3 months and 21.5%,
respectively. On the on the other hand, in the latter group of
patients these values were 4.8 months and 30.5%, respectively
(15). In the present study, in 15
patients treated with docetaxel and bevacizumab, the RR and PFS
were 26.7% and 5.9 months, which appears comparable to the
aforementioned previous reports.
The current study has certain limitations. The
retrospective design without a large number of patients limited the
generalizability of the results. This was not a randomized
controlled trial and the indications of chemotherapy, whether
docetaxel and bevacizumab or docetaxel only, were clearly
determined. As a result, there was no statistical significant
difference in clinical characteristics between the two treatment
groups of patients. In the two treatment groups, the median line of
docetaxel-containing chemotherapy was third-line. However, the
results obtained in this study were those in unselected consecutive
patients in daily clinical practice.
The present retrospective study was performed to
evaluate the clinical efficacy of docetaxel and bevacizumab as a
second- or later-line chemotherapy for NSCLC. To exclude the effect
of uncertain factors on the results, the efficacy and adverse
events of docetaxel and bevacizumab therapy were evaluated in
patients <75 years old.
In conclusion, the possibility of an improvement of
response and prolongation of PFS in patients receiving second- or
later-line docetaxel and bevacizumab chemotherapy may have been
suggested by this study. However, the higher risk of febrile
neutropenia must be noted for this combination of drugs.
References
|
1
|
Khosravi Shahi P and Fernández Pineda I:
Tumoral angiogenesis: Review of the literature. Cancer Invest.
26:104–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Behera M, Pillai RN, Owonikoko TK, Kim S,
Steuer C, Chen Z, Saba NF, Belani CP, Khuri FR and Ramalingam SS:
Bevacizumab in combination with taxane versus non-taxane containing
regimens for advanced/metastatic nonsquamous non-small-cell lung
cancer: A systematic review. J Thorac Oncol. 10:1142–1147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carnio S, Novello S, Mele T, Levra MG and
Scagliotti GV: Extending survival of stage IV non-small cell lung
cancer. Semin Oncol. 41:69–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du L and Morgensztern D: Chemotherapy for
Advanced-Stage Non-Small Cell Lung Cancer. Cancer J. 21:366–370.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper MR, Binkowski C, Hartung J and
Towle J: Profile of ramucirumab in the treatment of metastatic
non-small-cell lung cancer. Onco Targets Ther. 9:1953–1960. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Crowley J, van Houtte P,
Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw P;
International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions, :
The International Association for the Study of Lung Cancer lung
cancer staging project: Proposals regarding the clinical staging of
small cell lung cancer in the forthcoming (seventh) edition of the
tumor, node, metastasis classification for lung cancer. J Thorac
Oncol. 2:1067–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pohl M and Schmiegel W: Therapeutic
Strategies in Diseases of the Digestive Tract-2015 and Beyond
Targeted Therapies in Colon Cancer Today and Tomorrow. Dig Dis.
34:574–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garassino MC, Martelli O, Broggini M,
Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F,
Moscetti L, et al: Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): A randomised controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katakami N, Atagi S, Goto K, Hida T, Horai
T, Inoue A, Ichinose Y, Koboyashi K, Takeda K, Kiura K, et al:
LUX-Lung 4: A phase II trial of afatinib in patients with advanced
non-small-cell lung cancer who progressed during prior treatment
with erlotinib, gefitinib, or both. J Clin Oncol. 31:3335–3341.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adjei AA, Mandrekar SJ, Dy GK, Molina JR,
Adjei AA, Gandara DR, Ziegler KL, Stella PJ, Rowland KM Jr, Schild
SE and Zinner RG: Phase II trial of pemetrexed plus bevacizumab for
second-line therapy of patients with advanced non-small-cell lung
cancer: NCCTG and SWOG study N0426. J Clin Oncol. 28:614–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbst RS, O'Neill VJ, Fehrenbacher L,
Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M and Sandler
A: Phase II study of efficacy and safety of bevacizumab in
combination with chemotherapy or erlotinib compared with
chemotherapy alone for treatment of recurrent or refractory
non-small-cell lung cancer. J Clin Oncol. 25:4743–4750. 2007.
View Article : Google Scholar : PubMed/NCBI
|