Introduction
Approximately 50% of breast cancer patients will
develop distant metastasis (1,2), which
is a major cause of cancer-related mortality among women (3). In most series, isolated liver
metastases are found in 5–25% of the cases (1,2,4–6). As the
majority of LMBC patients may have systemic disease, only a limited
number of patients are candidates for local treatment.
Although systemic chemotherapy regimens with new
molecular-targeted agents have been developed, LMBC is an
incurable, fatal disease, with a median survival of 3–15 months
(5,7,8) and the
management of liver metastases remains challenging. Thus, the
treatment of patients with LMBC is considered as palliative. To
improve the treatment outcome, various local treatments, such as
surgery and transcatheter arterial chemoembolization (TACE) have
been applied, in combination with chemotherapy or performed
alternatively. However, the clinical utility of local treatment has
not been established in LMBC; by contrast, surgery is widely
considered to be a useful treatment option in liver metastasis from
colorectal cancer (9).
Proton beam therapy (PBT) is characterized by
precisely delivering a high dose of radiation to the target, while
significantly limiting the exposure of regions beyond the target.
It is well known that PBT for primary liver cancer achieves
excellent local control rates with few adverse effects (10–14). The
preliminary outcome of 5 LMBC cases who received PBT during a
maximum follow-up period of 8 years in 2012 was previously reported
(15). The cases were further
followed up and new patients with LMBC who were treated using PBT
were included in order to investigate the safety and effectiveness
of PBT for LMBC patients.
Patients and methods
Patients
A total of 8 patients with LMBC who received PBT at
the University of Tsukuba (Tsukuba, Japan) between 2003 and 2013
were retrospectively investigated. All the patients were women and
had a median age of 47 years (range, 38–63 years). The tumors were
categorized as solitary or multiple tumors that could be included
within a few irradiation fields. Patients with extrahepatic tumors
were excluded.
The time interval from primary disease surgery to
PBT was 3–14 years (median, 5 years) in 7 patients with
metachronous metastasis and the surgery was performed after PBT in
the remaining patient who had synchronous metastasis. Of the 8
patients, 5 had solitary tumors and 3 had multiple tumors. The
tumor distribution was unilateral in all cases. The maximal
diameter of the tumors was 1.2–7 cm (median, 4 cm). All the
patients had received another form of treatment prior to PBT, such
as chemotherapy and/or hormone therapy. Two patients received
concurrent chemotherapy and hormone therapy. According to the
Eastern Cooperative Oncology Group Performance Status (PS) scale,
all the patients had a PS of 0–1 and a Child-Pugh score of 5 (class
A). The follow-up period after PBT was 1.1–12.5 years (median, 3.8
years). The treatment strategy was discussed with surgeons on an
individual basis, considering the patient's PS, tumor location and
tumor size, and was approved in an in-hospital conference. The
reason for selecting PBT was disease incurable by chemotherapy,
surgery or radiofrequency ablation (RFA) in 7 cases and on the
patient's request in 1 case. Written informed consent was obtained
from all patients prior to PBT. The characteristics of the patients
and tumors are summarized in Table
I.
 | Table I.Summary of patient
characteristics. |
Table I.
Summary of patient
characteristics.
| Case | Age,
years/gender | Number | Size, cm | Dose, Gy
(RBE)/fr | Previous therapy | Concurrent
therapy | Adjuvant therapy | Survival period,
years |
|---|
| 1 | 48/F | S | 2 | 66/10 | C, H | N | N | 12.5a |
| 2 | 39/F | S | 7 | 66/10 | C, H | N | N | 8.9a |
| 3 | 38/F | M | 4 | 66/10 | C | N | N | 7.8a |
| 4 | 63/F | S | 2.5 | 66/10 | C, H | N | N | 4.4a |
| 5 | 51/F | S | 4 | 72.6/22 | C | N | N | 3.2a |
| 6 | 46/F | M | 1.2 | 72.6/22 | C | N | N | 2.2 |
| 7 | 51/F | S | 4 | 72.6/22 | R, H | H | H | 1.8a |
| 8 | 32/F | M | 5.5 | 72.6/22 | C, H | C | C | 1.1 |
PBT
Computed tomography (CT) images were captured at
5-mm intervals during the expiratory phase under a respiratory
gating system (16). At the
treatment planning stage, an aperture margin of 5–10 mm, a depth
margin of 5–10 mm, and a 5-mm margin on the caudal axis were added
to cover the entire clinical target volume to compensate for
uncertainty resulting from respiration-induced hepatic movements.
These margins included the field margins. A bolus was fabricated
for the smearing process. Proton beams of 155–250 MeV, generated
through a linear accelerator and synchrotron, were spread out and
shaped with ridge filters, double-scattering sheets,
multicollimators and custom-made boluses to ensure that the beams
conformed to the treatment planning data. The patient's position
was registered using an implanted fiducial marker and orthogonal
fluoroscopy unit attached to the treatment unit. PBT was performed
using a respiratory gating system (16).
The proton beam schedule was selected according to
the tumor location and treatment strategy. Multiple tumors were
included in the same irradiation field. The total irradiation dose
was 66 Gray equivalent [Gy relative biological effectiveness (RBE)]
in 22 fractions in 5 patients, and 72.6 Gy (RBE) in 22 fractions in
4 patients. The maximum cumulative dose was set for the spinal
cord, stomach and duodenum to <50 Gy (RBE), and for the colon to
<60 Gy (RBE). The RBE of the PBT was considered to be 1.1
(17).
Treatment after PBT
A total of 4 patients received adjuvant chemotherapy
or hormone therapy following PBT. Moreover, a total of 5 patients
received additional treatment to the new or recurrent tumors (PBT,
X-ray radiotherapy, chemotherapy and/or hormone therapy).
Follow-up and evaluation criteria
During the treatment sessions, acute
treatment-related toxicities were assessed weekly in all patients.
After completion of PBT, the patients were evaluated by means of
physical examinations, blood tests, and CT or MRI scans. Assessment
of response was evaluated according to the Response Evaluation
Criteria in Solid Tumors, version 1.1 (18). Local failure was defined as an
increase in the maximal diameter of the treated target tumors of
>20% and 5 mm. Adverse events were assessed after every
procedure according to the Common Terminology Criteria for Adverse
Effects (CTCAE), version 4.0 (19).
Patients treated prior to 2010 were also retrospectively reviewed
using the CTCAE 4.0.
To determine safety, the treatment completion rate,
liver toxicity and late adverse effects were examined. To assess
the treatment effect, the OS, PFS and LC rates were calculated
using the Kaplan-Meier method.
Results
Treatment dose
All the patients completed treatment without
interruptions. The biologically effective dose for the liver
(α/β=3) was 11.3–31.1 Gy (RBE) [median, 18.5 Gy (RBE)], and the
volume that received >30 Gy (RBE) (V30) was 12–39%
(median, 23%) of the liver. A late grade 2 adverse effect was
observed in 1 patient (rib fracture 7 months after PBT).
Follow-up
A total of 5 patients remained alive at the final
follow-up between July 2015 and March 2016, whereas 3 patients had
succumbed to the disease. The follow-up period was 1.1–12.5 years
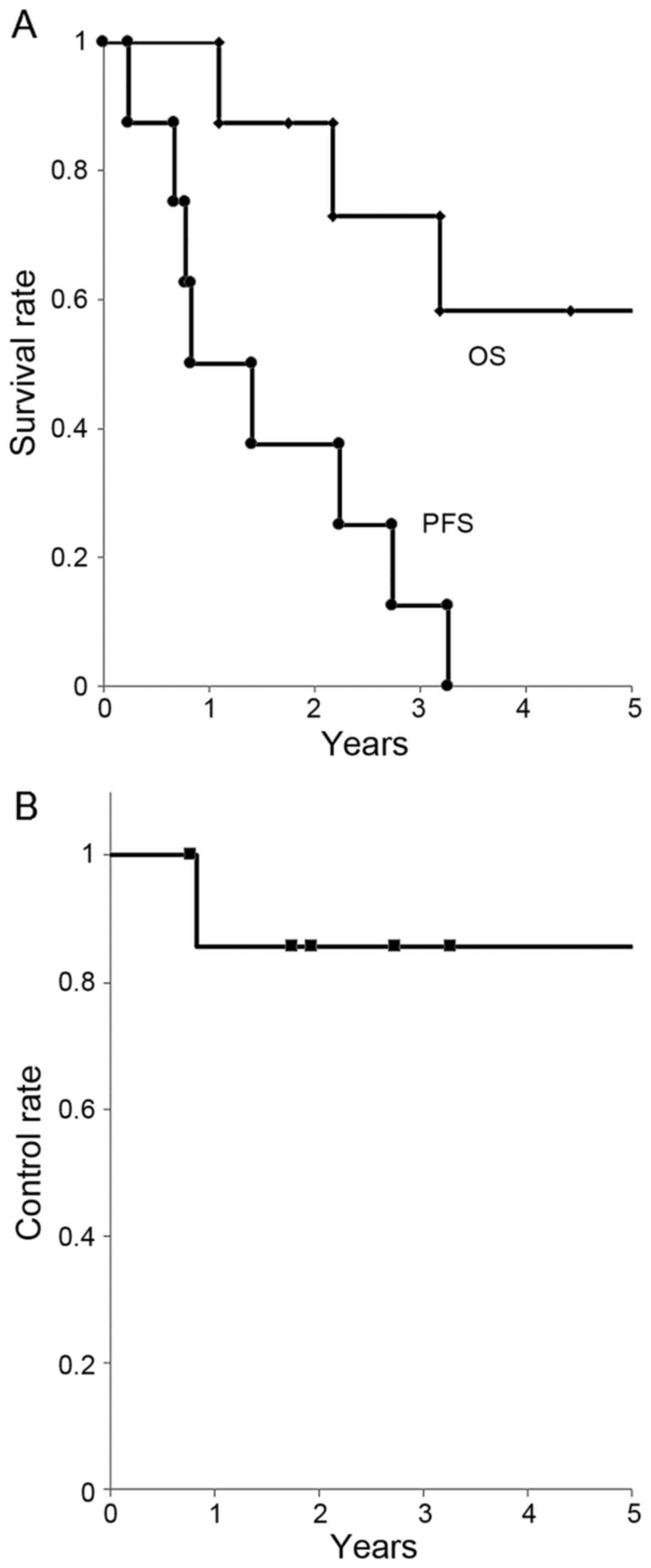
(median, 3.8 years). The OS rate was 88/73/58% at 1/3/5 years,
respectively. The PFS rate was 50/25/0% at 1/3/5 years,
respectively (median, 0.8 years). The LC rate was 86/86/86% at
1/3/5 years, respectively (Fig.
1).
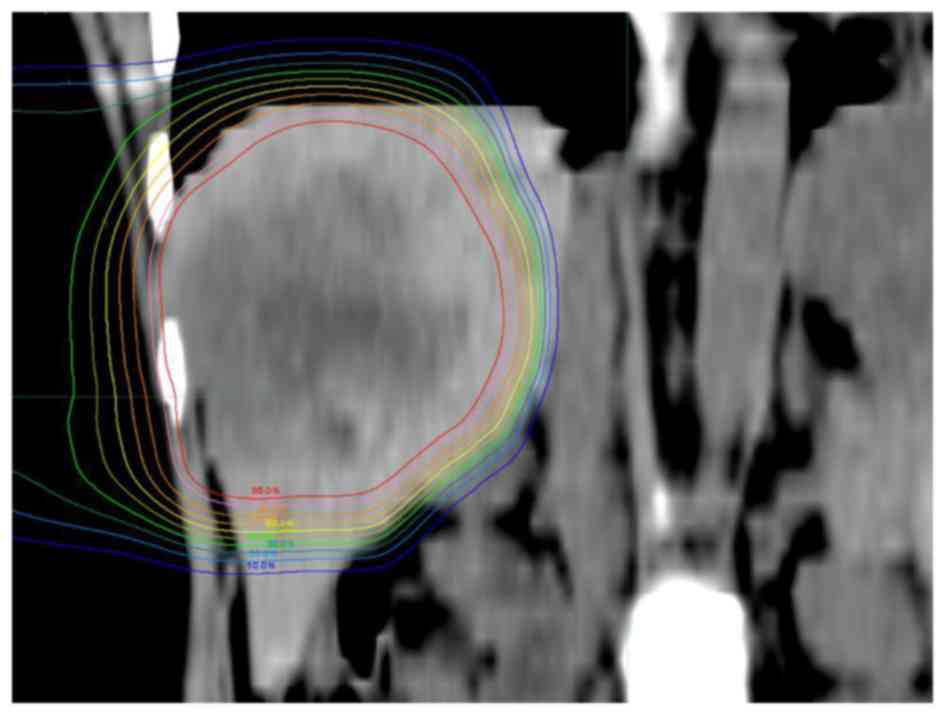
Fig. 2 shows a
39-years-old woman with LMBC. The patient had a 7-cm solitary tumor
in the right lobe of the liver that had not been controlled by
chemotherapy. PBT was administered at a dose of 66 Gy (RBE) in 10
fractions. Due to the superior physical characteristic of proton
beams, the tumor was conformally irradiated with high doses, while
the left lobe of the liver and digestive organs received extremely
low doses. No radiation-induced liver damage or gastrointestinal
disorders were observed. Additional chemotherapy and PBT were
performed to the new lesions after the initial PBT and the patient
remains alive 8.9 years after PBT (last follow-up, December,
2015).
Discussion
Reports on the local treatment of the patients with
LMBC are extremely rare. With surgery, the OS rate is 49–75/41–61%
at 3/5 years, respectively, with a median of 3.8–5.3 years
(20–24). With TACE, the OS rate was
63–76/13–48% at 1/3 years, respectively (25–27)
(Table II). Although severe adverse
effects are rare, Caralt et al reported that 2/12 patients
(17%) suffered from bile leakage postoperatively (28).
 | Table II.Local treatment outcome of liver
metastasis from breast cancer (review and present study). |
Table II.
Local treatment outcome of liver
metastasis from breast cancer (review and present study).
| First author | Patient population,
n | Number (S/M) | Distribution
(unilateral/bilateral) | Size, cm (cut-off
value, patient no.) | Treatment | 1/3/5-year OS rate,
% (median, years) | 1/3/5-year PFS
rate, % (median, years) | (Refs.) |
|---|
| Hoffmann | 29 |
|
|
| SU | −/75/59 |
| (21) |
| Adam | 85 | 32/53 | 52/33 | <5/≥5,
71/14 | SU | −/−/41 (3.8) | −/−/17 | (20) |
| Vlastos | 31 | 20/11 |
| <2/≥2,
11/20 | SU | −/−/61 (5.3) | −/−/31 | (22) |
| Abbott | 86 | 53/33 |
| <5/≥5,
73/13 | SU | (4.8) | (1.2) | (23) |
| Pocard | 52 | 36/16 | 28/24 | <3/≥3, 9/12 | SU | 86/49/− |
| (24) |
| Li | 29 |
|
|
| TACE | 63/13/− |
| (27) |
| Vogl | 159 |
|
|
| TACE | 64/36/− |
| (26) |
| Duan | 44 |
|
|
| TACE+C | 76/48/− (2.6) |
| (25) |
| Present study | 8 | 5/3 | 8/0 | <5/≥5, 5/2 | PBT | 88/73/58 | 50/25/0 (0.8) |
|
The OS rate in our study was 88/73/58% at 1/3/5
years, respectively, using PBT. Compared with the previous studies
on local treatment, the number and size of the tumors did not
differ significantly. However, our data on OS are consistent with
that of the surgery, which is associated with the highest OS rate
among optional treatments. As treatment of patients with LMBC is
not satisfactory and the survival period with chemotherapy is 3–15
months, PBT may be a useful treatment option if the metastasis is
confined to the liver. Moreover, although 1 patient suffered a late
grade 2 adverse effect (rib fracture), there were no reported
adverse event-related deaths, and no patients required
hospitalization. Thus, PBT appears to be a safe treatment for LMBC
patients.
The advantages of PBT for LMBC are considered to be
as follows: i) Few adverse effects, ii) high local control rate,
iii) treatment repeatability and iv) applicable to large tumors.
First, tolerable doses to the liver have been well-documented.
Austin-Seymour et al reported the tolerance dose as 30–35 Gy
to one-third of the liver volume (29), and Emami et al reported a 5%
risk of liver dysfunction 5 years after radiotherapy with 30 Gy
(30). The V30 was 15–39%
in our patients. It is well established that PBT for the treatment
of primary liver cancers has the distinct advantage of causing
relatively little damage to the healthy liver tissue (10,12,31,32). The
complications of hepatitis or liver cirrhosis are clearly less
frequent in breast cancer patients compared with primary liver
cancer patients. No patients exhibited a Child-Pugh score elevation
of >2 during the follow-up in the present study. Based on safety
data of previous studies on primary liver cancers, we consider the
extent of liver toxicity to be very limited during treatment of
LMBC (10,12,31,32).
Second, it was previously reported that the LC rate is 98/87/81% at
1/3/5 years, respectively, with PBT for primary liver cancers
(11). It appears that the LC rate
of LMBC is equal to that of primary liver cancers, possibly due to
the fact that breast cancer is as radiosensitive as primary liver
cancer. We consider that PBT may be applied as local treatment
based on its high local control rate, particularly when other
treatments have not proven to be useful. Third, breast cancer may
cause additional metastatic tumors, some of which may appear in the
liver. It is highly possible that additional local treatment is
required for the new metastatic tumors in breast cancer patients.
In the present study, 1 patient received additional PBT when new
liver metastases appeared. Repeated PBT is occasionally applied for
primary liver cancers and its safety has been proven (33). The most important factor for repeat
PBT is liver function. Surgery is one of the standard options in
local treatment. However, repeat surgery is not only difficult due
to adhesions or complications, but is also associated with
unacceptable risk in several patients. Considering the efficacy of
repeated treatment for primary liver cancers and the lower
frequency of complicated liver disease mentioned above, PBT is a
safe and effective option for repeated treatment. Fourth, RFA,
which is also one of the viable local treatment options in the view
of its safety and repeatability, is limited to tumors sized <5
cm. PBT may be applied to treat significantly larger tumors without
severe adverse effects. In the present study, 2 patients had tumors
sized >5 cm. The patient with the largest tumor (7 cm) has
received additional chemotherapy and radiotherapy and remains alive
8.8 years after treatment (last follow-up, December, 2015).
For patients with LMBC, chemotherapy is the first
choice of treatment; however, the treatment outcome is not
satisfactory. If the metastatic tumors are confined to the liver,
several optional treatments have been attempted, some of which may
achieve higher OS rates compared with conventional chemotherapy,
although there are currently no available evidence-rich data. Our
study was retrospective, and the number of patients was limited.
However, to the best of our knowledge, only one case report of LMBC
using PBT has been published to date (15), and ours is the first study to
investigate survival rate from several patients. The OS rate was
definitely not inferior to that of other local treatments,
(10–14,31,34). We
consider that PBT was sufficiently effective to be considered as a
viable local treatment option. Further investigation, with a larger
patient sample is expected to provide more detailed information on
the treatment of LMBC patients using PBT.
Acknowledgements
The present study was partially supported by
Grants-in-Aid for Scientific Research (17H04256) in Japan.
References
|
1
|
Hoe AL, Royle GT and Taylor I: Breast
liver metastases-incidence, diagnosis and outcome. J R Soc Med.
84:714–716. 1991.PubMed/NCBI
|
|
2
|
Zinser JW, Hortobagyi GN, Buzdar AU, Smith
TL and Fraschini G: Clinical course of breast cancer patients with
liver metastases. J Clin Oncol. 5:773–782. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue K, Ogawa M, Horikoshi N, Aiba K,
Mukaiyama T, Mizunuma N, Itami S, Hirano A, Matsuoka A and
Matsumura T: Evaluation of prognostic factors for 233 patients with
recurrent advanced breast cancer. Jpn J Clin Oncol. 21:334–339.
1991.PubMed/NCBI
|
|
5
|
O'Reilly SM, Richards MA and Rubens RD:
Liver metastases from breast cancer: The relationship between
clinical, biochemical and pathological features and survival. Eur J
Cancer. 26:574–577. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Insa A, Lluch A, Prosper F, Marugan I,
Martinez-Agullo A and Garcia-Conde J: Prognostic factors predicting
survival from first recurrence in patients with metastatic breast
cancer: Analysis of 439 patients. Breast Cancer Res Treat.
56:67–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldhirsch A, Gelber RD and Castiglione M:
Relapse of breast cancer after adjuvant treatment in premenopausal
and perimenopausal women: Patterns and prognoses. J Clin Oncol.
6:89–97. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wyld L, Gutteridge E, Pinder SE, James JJ,
Chan SY, Cheung KL, Robertson JF and Evans AJ: Prognostic factors
for patients with hepatic metastases from breast cancer. Br J
Cancer. 89:284–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folprecht G, Gruenberger T, Bechstein W,
Raab HR, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J,
Lang H, Trarbach T, et al: Survival of patients with initially
unresectable colorectal liver metastases treated with
FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary
concept (CELIM study). Ann Oncol. 25:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukumitsu N, Sugahara S, Nakayama H,
Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K and
Tokuuye K: A prospective study of hypofractionated proton beam
therapy for patients with hepatocellular carcinoma. Int J Radiat
Oncol Biol Phys. 74:831–836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizumoto M, Okumura T, Hashimoto T, Fukuda
K, Oshiro Y, Fukumitsu N, Abei M, Kawaguchi A, Hayashi Y, Ookawa A,
et al: Proton beam therapy for hepatocellular carcinoma: A
comparison of three treatment protocols. Int J Radiat Oncol Biol
Phys. 81:1039–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bush DA, Hillebrand DJ, Slater JM and
Slater JD: High-dose proton beam radiotherapy of hepatocellular
carcinoma: Preliminary results of a phase II trial.
Gastroenterology. 127 5 Suppl 1:S189–S193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawashima M, Furuse J, Nishio T, Konishi
M, Ishii H, Kinoshita T, Nagase M, Nihei K and Ogino T: Phase II
study of radiotherapy employing proton beam for hepatocellular
carcinoma. J Clin Oncol. 23:1839–1846. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi WX, Fu S, Zhang Q and Guo XM: Charged
particle therapy versus photon therapy for patients with
hepatocellular carcinoma: A systematic review and meta-analysis.
Radiother Oncol. 114:289–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanemoto A, Ishikawa H, Mizumoto M,
Okumura T, Hashimoto T, Oshiro Y, Fukumitsu N, Tsuboi K, Sakae T
and Sakurai H: Proton beam therapy for liver metastasis from breast
cancer: Five case reports and a review of the literature. Int Canc
Conf J. 1:pp. 210–214. 2012; View Article : Google Scholar
|
|
16
|
Fukumitsu N, Hashimoto T, Okumura T,
Mizumoto M, Tohno E, Fukuda K, Abei M, Sakae T and Sakurai H:
Investigation of the geometric accuracy of proton beam irradiation
in the liver. Int J Radiat Oncol Biol Phys. 82:826–833. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paganetti H, Niemierko A, Ancukiewicz M,
Gerweck LE, Goitein M, Loeffler JS and Suit HD: Relative biological
effectiveness (RBE) values for proton beam therapy. Int J Radiat
Oncol Biol Phys. 53:407–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Cancer Institute, . Common
terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htmMay
28–2009
|
|
20
|
Adam R, Aloia T, Krissat J, Bralet MP,
Paule B, Giacchetti S, Delvart V, Azoulay D, Bismuth H and Castaing
D: Is liver resection justified for patients with hepatic
metastases from breast cancer? Ann Surg. 244:897–908. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoffmann K, Franz C, Hinz U, Schirmacher
P, Herfarth C, Eichbaum M, Büchler MW and Schemmer P: Liver
resection for multimodal treatment of breast cancer metastases:
Identification of prognostic factors. Ann Surg Oncol. 17:1546–1554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlastos G, Smith DL, Singletary SE, Mirza
NQ, Tuttle TM, Popat RJ, Curley SA, Ellis LM, Roh MS and Vauthey
JN: Long-term survival after an aggressive surgical approach in
patients with breast cancer hepatic metastases. Ann Surg Oncol.
11:869–874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbott DE, Brouquet A, Mittendorf EA,
Andreou A, Meric-Bernstam F, Valero V, Green MC, Kuerer HM, Curley
SA, Abdalla EK, et al: Resection of liver metastases from breast
cancer: Estrogen receptor status and response to chemotherapy
before metastasectomy define outcome. Surgery. 151:710–716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pocard M, Pouillart P, Asselain B and
Salmon R: Hepatic resection in metastatic breast cancer: Results
and prognostic factors. Eur J Surg Oncol. 26:155–159. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan XF, Dong NN, Zhang T and Li Q:
Treatment outcome of patients with liver-only metastases from
breast cancer after mastectomy: A retrospective analysis. J Cancer
Res Clin Oncol. 137:1363–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vogl TJ, Naguib NN, Nour-Eldin NE, Eichler
K, Zangos S and Gruber-Rouh T: Transarterial chemoembolization
(TACE) with mitomycin C and gemcitabine for liver metastases in
breast cancer. Eur Radiol. 20:173–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li XP, Meng ZQ, Guo WJ and Li J: Treatment
for liver metastases from breast cancer: Results and prognostic
factors. World J Gastroenterol. 11:3782–3787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caralt M, Bilbao I, Cortés J, Escartín A,
Lázaro JL, Dopazo C, Olsina JJ, Balsells J and Charco R: Hepatic
resection for liver metastases as part of the ‘oncosurgical’
treatment of metastatic breast cancer. Ann Surg Oncol.
15:2804–2810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Austin-Seymour MM, Chen GT, Castro JR,
Saunders WM, Pitluck S, Woodruff KH and Kessler M: Dose volume
histogram analysis of liver radiation tolerance. Int J Radiat Oncol
Biol Phys. 12:31–35. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Emami B, Lyman J, Brown A, Coia L, Goitein
M, Munzenrider JE, Shank B, Solin LJ and Wesson M: Tolerance of
normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol
Phys. 21:109–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizumoto M, Tokuuye K, Sugahara S,
Nakayama H, Fukumitsu N, Ohara K, Abei M, Shoda J, Tohno E and
Minami M: Proton beam therapy for hepatocellular carcinoma adjacent
to the porta hepatis. Int J Radiat Oncol Biol Phys. 71:462–467.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizumoto M, Okumura T, Hashimoto T, Fukuda
K, Oshiro Y, Fukumitsu N, Abei M, Kawaguchi A, Hayashi Y, Ohkawa A,
et al: Evaluation of liver function after proton beam therapy for
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
82:e529–e535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto T, Tokuuye K, Fukumitsu N, Igaki
H, Hata M, Kagei K, Sugahara S, Ohara K, Matsuzaki Y and Akine Y:
Repeated proton beam therapy for hepatocellular carcinoma. Int J
Radiat Oncol Biol Phys. 65:196–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukumitsu N, Okumura T, Takizawa D,
Makishima H, Numajiri H, Murofushi K, Ohnishi K, Mizumoto M, Aihara
T, Ishikawa H, et al: Proton beam therapy for metastatic liver
tumors. Radiother Oncol. 117:322–327. 2015. View Article : Google Scholar : PubMed/NCBI
|