Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in males and the second most commonly diagnosed
cancer in females, with over 1.3 million new cancer cases, and
693,900 mortalities, estimated to have occurred in 2012 (1). China, similarly to several other
developing countries in Asia, has been experiencing a significant
rise in the incidence of CRC over the recent decades (2–4).
It is widely accepted that the adenoma-carcinoma
sequence represents the process by which most cases of CRC arise
(5). Several studies have revealed
that age is one of the most important influential factors for
colorectal adenoma: An older age (≥65 years) is associated with a
higher prevalence of adenoma and advanced adenoma (6,7). In
addition, the prevalence of adenoma and advanced adenoma in persons
76–80 years of age is more than double that of persons aged 40–49
years (8). The comparatively high
rate of incidence of adenomatous and advanced adenomatous polyps in
the older population makes this group an important CRC screening
target.
Features of polyps in the colorectum may affect the
selection of screening and surveillance modalities for CRC
(9). However, few studies have been
published on the features of colorectal polyps in elderly.
Therefore, the aim of the present study was to investigate the
clinical, enteroscopic and pathological characteristics of
colorectal polyps in Chinese elderly patients in a single center
(The Central Hospital of Wuhan, Hubei, China).
Patients and methods
Study design and patients
The present retrospective study was based on the
colonoscopic database information from all colonoscopic
examinations performed at the Central Hospital of Wuhan, Hubei,
China between January 2013 and December 2014. The study protocol
was reviewed and approved by the Ethics and Research Committee of
the Central Hospital of Wuhan.
For the analysis of the features of colorectal
polyps in elderly patients, the following inclusion criteria were
used: (1) the patient was ≥65 years
of age; (2) the patient had received
an endoscopic resection of colorectal polyps; (3) complete medical records were available.
Exclusion criteria were as follows: i) the patient was <65
years; ii) there was an absence of polyps; iii) the polyps were
unresected (due to taking anticoagulant drugs, multiple comorbid
conditions, income inadequacy or low social support), or the polyps
were observed, but not retrieved (by reason of small, sessile, and
proximal colon polyps); iv) the patients were suffering from
melanosis coli, colorectal cancer, inflammatory bowel disease,
active gastrointestinal bleeding or familial adenomatous polyposis;
v) there was a history of colectomy or rectectomy; vi) the
colonoscopy did not reach the cecum; and vii) the bowel preparation
was poor (semi-solid stool that could not be suctioned or washed
away, and <90% of mucosal visualization) (10).
Procedures and definitions
Procedures were performed by 14 colonoscopists:
Seven experienced colonoscopists, each of whom had performed in
excess of 2,000 colonoscopies and had been in colonoscopy practice
for >10 years, and seven less experienced colonoscopists, who
had had 3–5 years of colonoscopy practice, during which each had
performed between 300 and 500 colonoscopies. Our center used
polyethylene glycol-electrolyte powder (PEG-ELP; WanHe
Pharmaceutical Co., Ltd., Shenzhen, China) as a purgative for all
patients who underwent a colonoscopy. Colonoscopies were performed
following bowel preparation with 3 l PEG-ELP.
The colonoscopes used in the present study [an
OLYMPUS GIF-XQ240 (Pro Scope Systems, Blue Ash, OH, USA) and a
PENTAX EC-3890Fi (PENTAX Medical Co., Montvale, NJ, USA)] are both
utilized in standard electronic colonoscopies. Additional
technologies, including narrow band imaging and i-Scan, were not
regularly used. Polypectomies were performed using standard biopsy
forceps (for polyps <5 mm) or polypectomy snares for larger
polyps (>5 mm). Polyp size was estimated by comparing a polyp to
the fully opened biopsy forceps (7 mm in length; JHY-FB-23-180-O-O;
Changzhou Jiuhong Medical Instrument Co., Ltd., Changzhou, China)
or polypectomy snares (15 mm in diameter; NOE342214-C; Endo-Flex
GmbH, Voerde, Germany).
Precise characteristics of the colorectal polyps
(i.e., number, size, form, and location) were documented in the
colonoscopy reports by endoscopists.
Following polypectomy, the samples were sent to the
pathology department of the Central Hospital of Wuhan, and
processed for routine histological examination. Two experienced
gastrointestinal pathologists evaluated the histopathology of the
samples, and entered details of the histological features of polyps
in the pathology reports.
All the demographic data, including the age and sex
of the patients and information regarding the colorectal polyps,
were collected from the endoscopy and pathology databases of our
center. The indications for colonoscopy were reviewed manually from
the medical files.
For the purpose of the present analysis, colorectal
polyps were divided into two groups: The right-sided and left-sided
lesions. Polyps located proximal to the splenic flexure were
considered right-sided (including the cecum, ascending colon and
transverse colon), whereas those that were distal to the splenic
flexure were considered left-sided (including the descending colon,
sigmoid colon and rectum).
In the present study, colorectal polyps were divided
into two types based on their histological findings:
Non-adenomatous polyps (NAPs; benign mucosa, inflammatory,
hyperplasic, lymphoid, lipomatous, and so forth) and adenomatous
polyps (APs; tubular, villous, tubulovillous, and serrated
adenoma). APs were further grouped as non-advanced adenomas (NAAs)
and advanced adenomas (AAs). AAs were those possessing the
following features: ≥10 mm in diameter, having villous or
tubulovillous histology, having high-grade dysplasia (HGD), or any
combination of these features (11).
The morphology of the colorectal polyps was
determined according to the Paris classification, being classified
into protruding lesions [elevated by >2.5 mm above the mucosal
layer: Pedunculated (0-Ip), sessile (0-Is) or semipedunculated
(0-Isp)], superficial lesions [slightly elevated by <2.5 mm
(0-IIa), flat (0-IIb) or slightly depressed (0-IIc)], and laterally
spreading tumors (LSTs) (12). The
colorectal polyps were classified according to their size as
follows: ≤5 mm, 6–9 mm, 10–20 mm and >20 mm.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Fisher's exact test was used for between-group comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
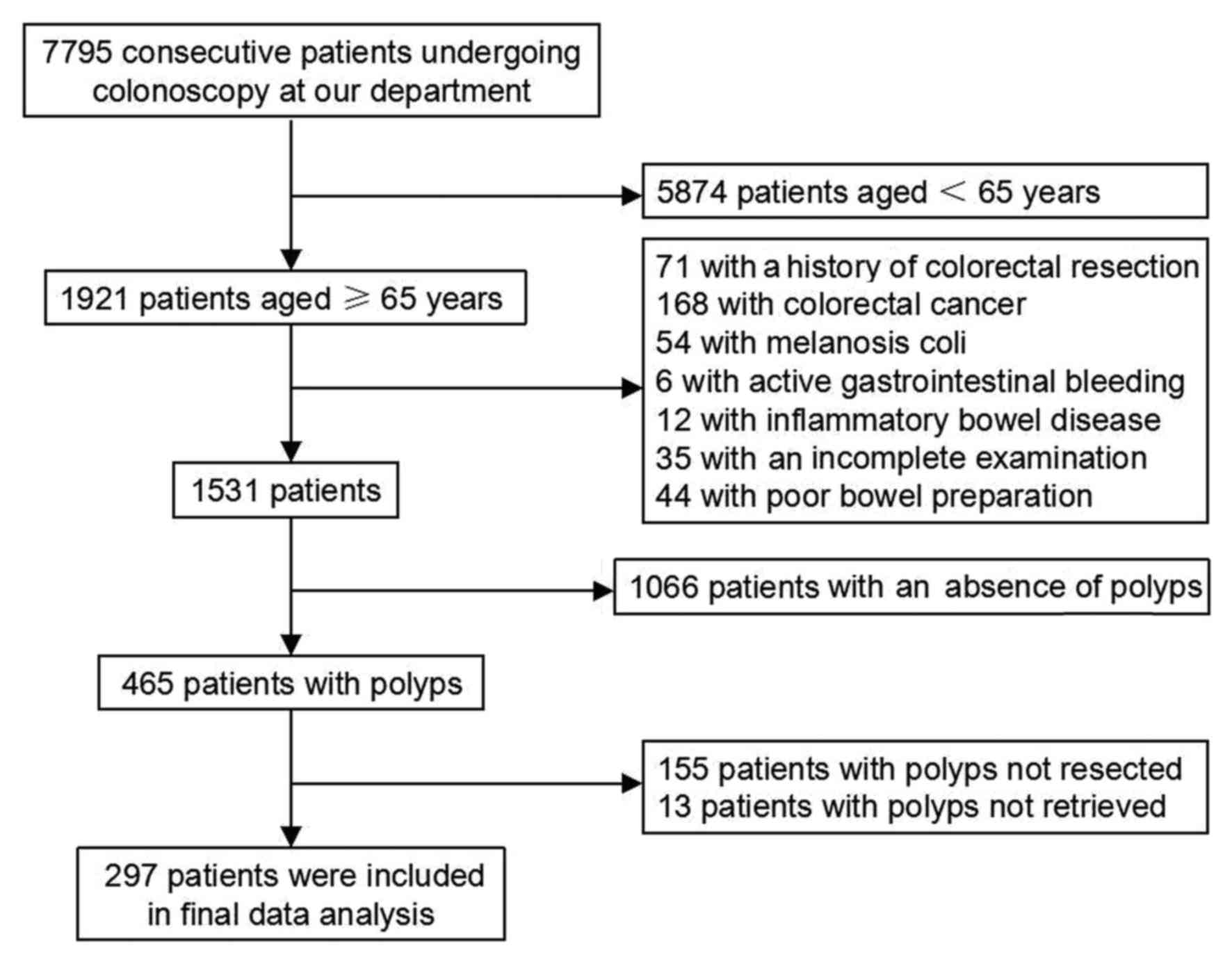
Between January 2013 and December 2014, a total of
7,795 consecutive patients undergoing colonoscopy at our center
(The Central Hospital of Wuhan, Hubei, China) were retrospectively
analyzed. A total of 7,498 patients were excluded who met the
exclusion criteria, and therefore 297 patients were included in the
present study (Fig. 1). The general
characteristics of these patients are summarized in Table I. The mean age was 71.4±5.2 years
(range, 65–87 years); 149 (50.2%) of the patients were male and 148
(49.8%) were female, and 161 patients (54.2%) were aged 70 years or
older.
 | Table I.General characteristics of the elderly
patients studied. |
Table I.
General characteristics of the elderly
patients studied.
| Characteristic | Results |
|---|
| Number of
patients | 297 |
| Sex |
|
| Male | 149 |
|
Female | 148 |
| Age (years) |
|
|
65–69 | 136 |
|
70–74 | 83 |
|
75–79 | 51 |
|
≥80 | 27 |
| Age range
(years) | 65–87 |
| Mean age
(years) |
71.4±5.2a |
The indications for colonoscopy examination included
a change in bowel habit (24.2%), abdominal pain (19.5%),
constipation (14.1%), rectal bleeding or hematochezia (10.1%),
positive fecal occult blood (7.4%), regular health examination
(7.1%), diarrhea (6.7%), abdominal distention (5.4%), and other
less common indications, including melena, anus bulge, weight loss,
anemia, an abdominal mass or anus fistula (Table II).
 | Table II.Indications for colonoscopy
examinations. |
Table II.
Indications for colonoscopy
examinations.
| Indication | Number of
patients |
|---|
| Rectal bleeding or
hematochezia | 30 |
| Positive fecal
occult blood | 22 |
| Constipation | 42 |
| Diarrhea | 20 |
| Abdominal pain | 58 |
| Abdominal
distention | 16 |
| Change in bowel
habit | 72 |
| Melena | 6 |
| Anus bulge | 3 |
| Weight loss | 3 |
| Anemia | 2 |
| Abdominal mass | 1 |
| Anus fistula | 1 |
| Regular health
examination | 21 |
| Total | 297 |
Altogether, a total of 509 colorectal polyps were
resected from 297 patients. The histological findings are shown in
Table III. Of all polyps, 263
(51.7%) were NAPs and 246 (48.3%) were APs. Of all the NAPs, 227
(44.6%) were inflammatory polyps, 24 (4.7%) were hyperplastic
polyps, 6 (1.2%) were normal mucosa and 6 (1.2%) were others. Of
all the APs, 104 (20.4%) were NAAs, and 142 (27.9%) were AAs. The
histological finding of NAAs was tubular adenoma. The 142 AAs
comprised 11 (2.2%) tubular adenomas, 14 (2.7%) villous adenomas,
115 (22.6%) tubulovillous adenomas and 2 (0.4%) serrated adenomas.
Among the AAs, 9 (1.8%) polyps were noted to have HGD.
 | Table III.Histopathological features of the 509
colorectal polyps. |
Table III.
Histopathological features of the 509
colorectal polyps.
| Histopathology of
resected polyps | Result (%) |
|---|
| Non-adenomatous
polyps | 263
(51.7) |
|
Inflammatory polyp | 227 (44.6) |
|
Hyperplastic polyp | 24 (4.7) |
| Normal
mucosa | 6 (1.2) |
|
Other | 6 (1.2) |
| Adenomatous
polyps | 246 (48.3) |
|
Non-advanced adenomas | 104 (20.4) |
|
Tubular
adenoma |
|
|
Size
<10 mm with LGD | 104 (20.4) |
|
Advanced adenomas | 142 (27.9) |
|
Tubular
adenoma | 11 (2.2) |
|
Size
<10 mm with HGD | 1 (0.2) |
|
Size
≥10 mm with LGD | 10 (2.0) |
| Villous
adenoma | 14 (2.7) |
|
Tubulovillous adenoma | 115 (22.6) |
|
With LGD | 107 (21.0) |
|
With HGD | 8 (1.6) |
|
Serrated adenoma |
|
|
Size ≥10 mm with
LGD | 2 (0.4) |
| Total | 509 (100) |
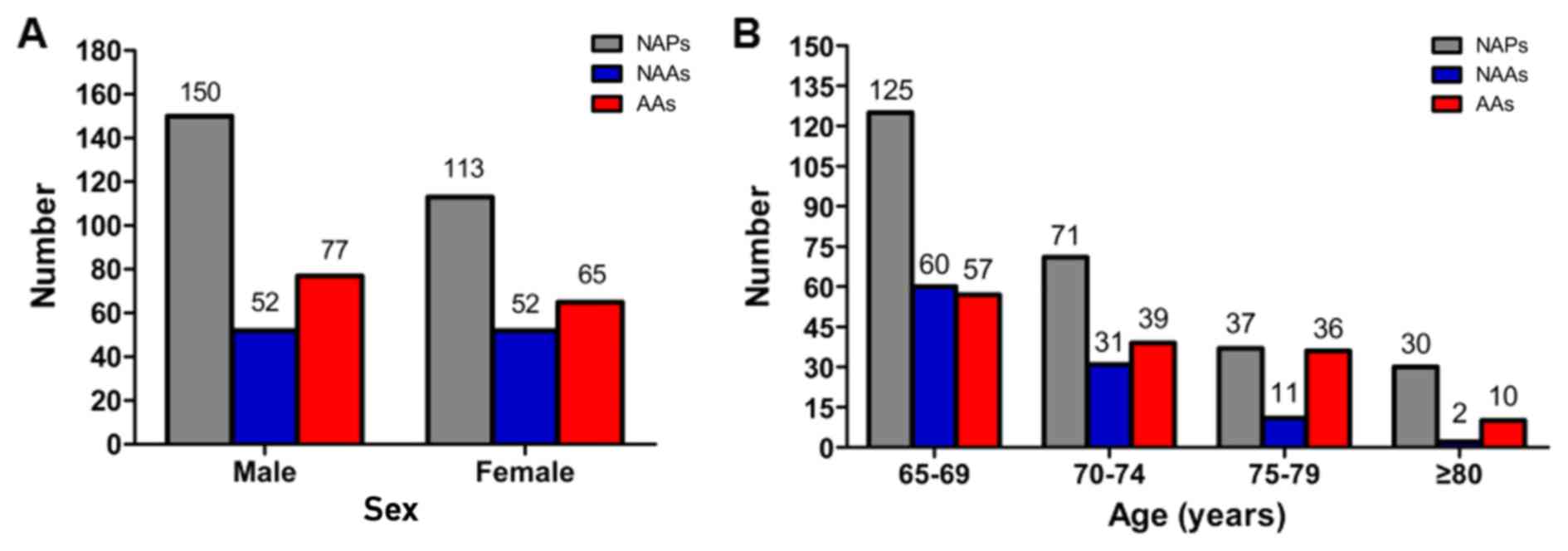
In men, 279 (54.8%) polyps were noted, of which 150
(29.5%) were NAPs, 52 (10.2%) were NAAs and 77 (15.1%) were AAs. In
women, 230 (45.2%) polyps were noted, of which 113 (22.2%) were
NAPs, 52 (10.2%) were NAAs and 65 (12.8%) were AAs. The number of
colorectal polyps according to sex is shown in Fig. 2A. No association was identified
between sex and the histological finding of colorectal polyps
(P>0.05; Fig. 2A).
Of all the polyps, 242 (47.5%), 141 (27.7%), 84
(16.5%) and 42 (8.3%) were identified in patients of 65–69, 70–74,
75–79, and ≥80 years of age, respectively. A comparison of the
polyps according to age, using 75 years as a cut-off, revealed that
36.5% (46/126) of the polyps in patients ≥75 years of age had an
advanced feature compared with 25.1% (96/383) of those in patients
aged 65–74 years (P<0.05; Fig. 2B
and Table IV), although there were
no between-group differences in the frequency of NAPs and APs
(P>0.05; Fig. 2B and Table IV).
 | Table IV.Histopathological features of 509
polyps according to the patients' age. |
Table IV.
Histopathological features of 509
polyps according to the patients' age.
|
| Patient age |
|
|---|
|
|
|
|
|---|
| Histopathology | 65–74 years N
(%) | ≥75 years N
(%) | P-value |
|---|
| NAPs | 196 (38.5) | 67 (13.2) | >0.05 |
| APs | 187 (36.7) | 59 (11.6) |
|
| NAAs | 91 (17.8) | 13 (2.6) | <0.05 |
| AAs | 96 (18.9) | 46 (9.0) |
|
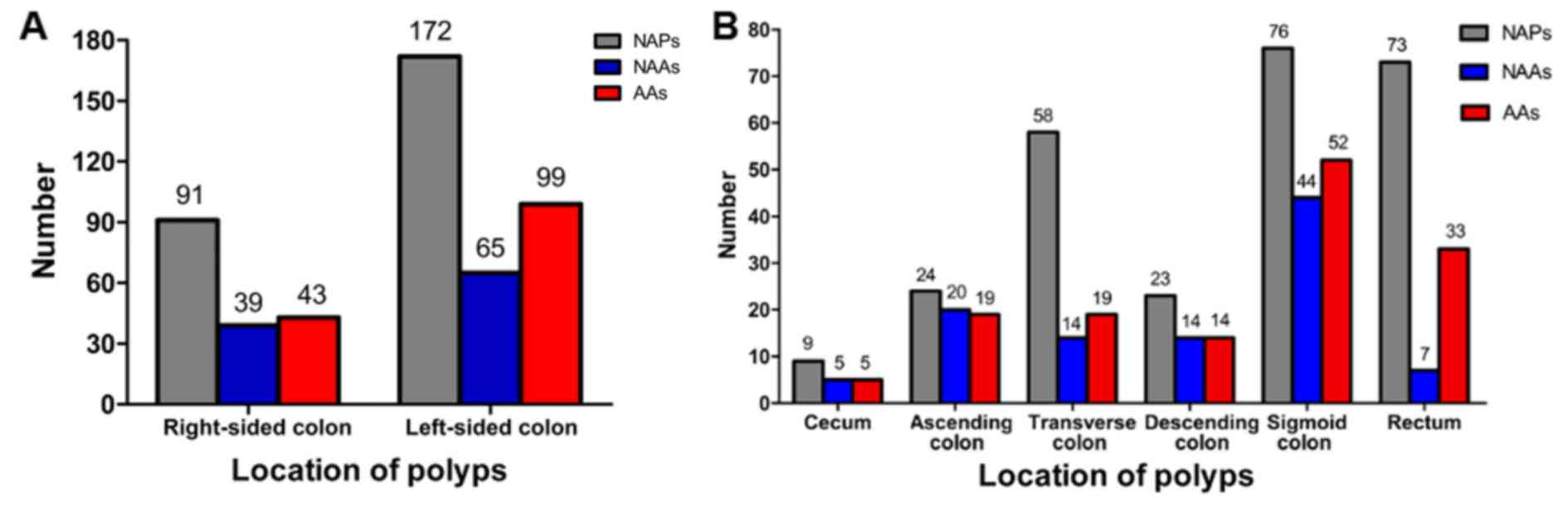
In the right-sided colon, 173 (34.0%) polyps were
identified, of which 91 (17.9%) were NAPs, 39 (7.7%) were NAAs and
43 (8.4%) were AAs. In the left-sided colon, 336 (66.0%) polyps
were identified, of which 172 (33.8%) were NAPs, 65 (12.8%) were
NAAs and 99 (19.4%) were AAs. The number of colorectal polyps
according to distribution is shown in Fig. 3. There was no association between
distribution and the histological findings of colorectal polyps
(P>0.05; Fig. 3A). It is worth
noting that the sigmoid colon was the most frequent site for the
three different types of polyps, and the rectum, ascending colon
and rectum were the second most frequent site for NAPs, NAAs, and
AAs, respectively (Fig. 3B).
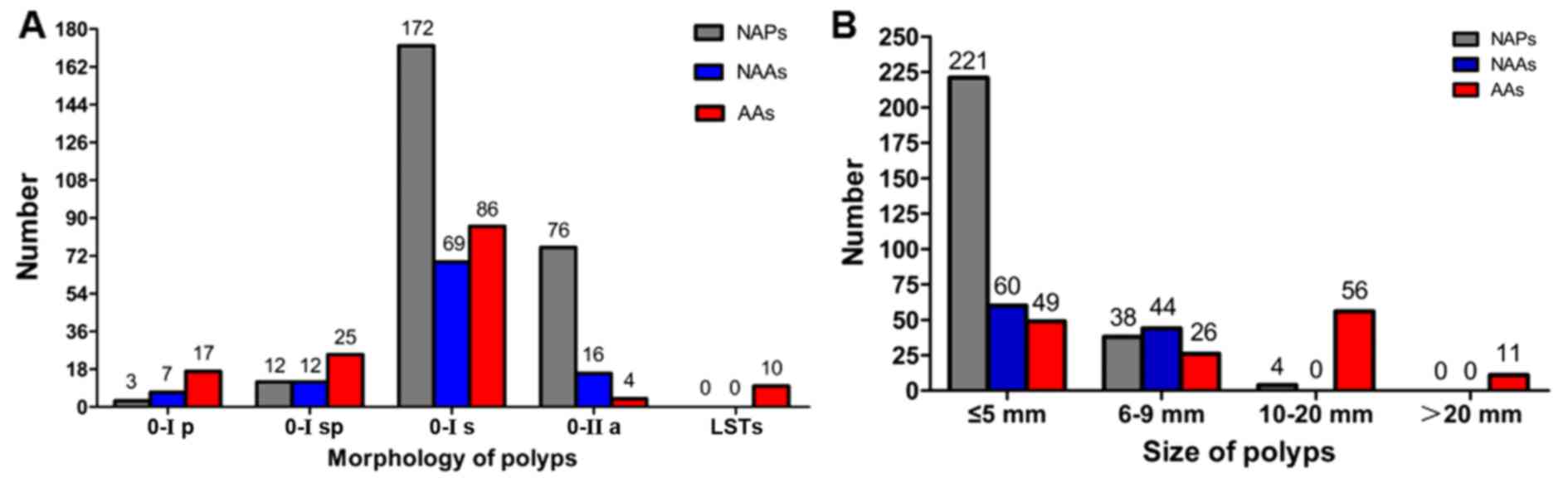
In terms of morphology, the colorectal polyps could
be classified as follows: 27 (5.3%) were type 0-Ip, 49 (9.6%) were
type 0-Isp, 327 (64.2%) were type 0-Is, 96 (18.9%) were type 0-IIa,
and 10 (2.0%) were LSTs. No completely flat or depressed lesions
(type 0-IIb or 0-IIc) were noted. The morphological appearance of
polyps according to the Paris classification is shown in Fig. 4A. It was identified that the sessile
type (0-Is) appeared the most frequently for the three different
histological features of polyps (Fig.
4A).
Regarding the polyps' size, 330 (64.8%) were ≤5 mm
in terms of their greatest dimension, 108 (21.2%) were 6–9 mm, 60
(11.8%) were 10–20 mm, and 11 (2.2%) were >20 mm. The size of
the polyps according to the histopathological findings is described
in Fig. 4B. A comparison of polyps
by size, using 10 mm as a cut-off, revealed that larger polyps were
more likely to exhibit an adenomatous component, and tended to be
advanced. Approximately 40.9% (179/438) of the polyps <10 mm in
size had an adenomatous component, compared with 94.4% (67/71) of
those ≥10 mm (P<0.0001; Fig. 4B
and Table V). In addition, only
17.1% (75/438) of the polyps that were <10 mm in size had an
advanced feature, compared with 94.4% (67/71) of those ≥10 mm
(P<0.0001; Fig. 4B and Table V). Similar findings were also
observed when the size cut-off was set at 5 mm. Only 33.0%
(109/330) of the polyps ≤5 mm in size had an adenomatous component,
compared with 76.5% (137/179) of those 6 mm or larger (P<0.0001;
Fig. 4B and Table V). Furthermore, only 14.8% (49/330)
of the polyps ≤5 mm in size had an advanced feature, compared with
52.0% (93/179) of those 6 mm or larger (P<0.0001; Fig. 4B and Table
V).
 | Table V.Histopathological features of the 509
polyps according to polyp size. |
Table V.
Histopathological features of the 509
polyps according to polyp size.
|
| Polyp size (N,
%)a |
| Polyp size (N,
%)b |
|
|---|
|
|
|
|
|
|
|---|
| Histopathology | 0–9 mm | ≥10 mm | P-value | 0–5 mm | ≥6 mm | P-value |
|---|
| NAPs | 259 (50.9) | 4 (0.8) | <0.0001 | 221 (43.4) | 42 (8.3) | <0.0001 |
|
APs | 179 (35.1) | 67 (13.2) |
| 109 (21.4) | 137 (26.9) |
|
|
NAAs | 104 (20.4) | 0 (0) | <0.0001 | 60 (11.8) | 44 (8.6) | <0.0001 |
|
AAs | 75 (14.7) | 67 (13.2) |
| 49 (9.6) | 93 (18.3) |
|
Discussion
The present study has retrospectively analyzed the
clinical, endoscopic and pathological characteristics of colorectal
polyps in Chinese elderly patients over a period of two years
through the analysis of endoscopic and pathology reports in a
single center (The Central Hospital of Wuhan, Hubei, China).
In the present study, it was observed that there
were no specific clinical symptoms in older patients with
colorectal polyps, and the majority of patients presented with
changes in bowel habit or other symptoms, including abdominal pain,
constipation, rectal bleeding or hematochezia, and positive fecal
occult blood, which was similar to findings reported in other
retrospective studies (13,14).
An advancing age is an independent risk factor for
developing colorectal adenomas, which may lead to higher rates of
colorectal cancer in the elderly (7). In one study, the prevalence of
colorectal adenomas increased markedly with age among participants
aged 20–79 years, although the increase was more marked for AAs
(15). In the present study, the AAs
were more common in patients ≥75 years of age, compared with
patients who were 65–74 years of age, but the incidence of APs was
not significantly associated with age, perhaps due to a more
selective and smaller sample size, or other biases.
Data published in recent studies have revealed that
males had a greater likelihood of developing a larger number of APs
and AAs compared with females (15,16),
whereas in the present study, no significant differences were
observed between the sexes. The findings of the present study are
similar to those reported in a previous study by Yamaji et
al (17), who reported that sex
was not to be considered as an independent risk factor for the
development of advanced colorectal adenomas (17). This may be due to the small sample
size in the present study, or an increasing risk in women as they
grow older.
The present study has shown that left-sided
colorectal polyps were more prevalent than right-sided ones, a
finding that is in agreement with previous studies (18,19). In
the present study, APs and AAs were detected predominantly in the
sigmoid colon, although there was also a significant number of
colon polyps and adenomas lying proximal to the splenic flexure.
Flexible sigmoidoscopy is recommended as a possible alternative to
colonoscopy (20), in which the
distal 40–60 cm of the colon (up to the splenic flexure) may be
inspected. It is anticipated that examination of the colon limited
to the splenic flexure would have ‘missed’ 34% of the proximal
polyps in our subjects. The incidence of adenomatous polyps in the
proximal colon, as well as AAs, has increased in the last few years
(16). In addition, Patel et
al (18) reported that there was
an increased right-sided prevalence of adenoma or carcinoma with
age. It is clear that evaluation of the whole bowel is particularly
important in older patients. However, during daily practice,
increasing adverse complications, poorer bowel preparation and more
incomplete examinations are observed in older patients undergoing
colonoscopy for diagnostic, screening and surveillance purposes
(21). In this case, the colonoscopy
test for the elderly should be addressed to the whole colon, in
preference to methods that evaluate only a part of the colon
according to specific factors, such as an elderly patient's
comorbid medical conditions, cognitive ability and mobility.
In the present study, sessile type (0-Is) appeared
the most frequently for the three different histological features
of polyps. No flat and depressed lesions (0-IIb and 0-IIc) were
identified in the present study. The possible reasons for this were
poorer bowel preparation, and a less frequent use of
dye-chromoendoscopy, such as indigo carmine, or electronic
chromoendoscopy, such as the i-Scan procedure (22–24).
Diminutive (1–5 mm in size) and small (6–9 mm in
size) colorectal polyps represent the majority of polyps that are
identifiable during colonoscopy (25,26). A
study from Taiwan revealed that 1.3% of the diminutive polyps had
an advanced histology (25). Chaput
et al (27) demonstrated an
advanced histology in 4.7% of the diminutive, and 35.2% of the
small polyps, mainly due to presence of a villous component
(27). Shapiro et al
(28) determined that 4.1% of the
diminutive polyps contained a villous component, and the rate of
advanced histology for small polyps was >15% (28). In a systematic review by Hassan et
al (29), AAs were identified in
4.6% of diminutive polyps, 7.9% of small polyps, and 12.5% of
sub-centimetre (<10 mm) polyps. The observations in the present
study revealed that 14.8% of the diminutive polyps, 24.1% of the
small polyps, and 17.1% (75/438) of sub-centimetre polyps had an
advanced histology, findings that were similar to those previously
reported by Tsai et al (30).
In that study, which included patients aged 40–89 years, the
prevalence of AAs was 10% in polyps ≤5 mm, and 27% in polyps 6–9 mm
in size (30). The prevalence of an
advanced histology in diminutive and small colorectal polyps may
vary widely in different studies, and it was suspected by the
present authors that the contributing factors would possibly
include sample size, the demographics of the screened population,
the geographic environment, and dietary habits. In the present
study, it was important to note that an increasing polyp size was
associated with an increased likelihood of adenoma and advanced
histology when the size cut-off for polyps was set at 5 mm or 10
mm. Therefore, one may conclude that diminutive and small
colorectal polyps should not be ignored in older people, and for
patients with multiple medical comorbidities, a failure to remove
those polyps may place the elderly at risk of progression to
advanced lesions and CRC.
A histopathological examination is considered as a
gold standard for polyp characterization, and it is essential to
recommend a surveillance interval following colonoscopy screening
and polypectomy (31). However, the
requirement for a post-polypectomy histological assessment leads to
a substantial exploitation of medical and economic resources
(32). In recent years, a ‘resect
and discard’ strategy based on the findings of image-enhanced
endoscopy (e.g., high-definition endoscopy, magnifying endoscopy
and chromoendoscopy) for diminutive colorectal polyps has been
proposed to save both the time and cost of histopathology (33–36).
However, there are several barriers to applying this strategy in
the clinical practice of the present authors. First, as the cost
for a pathology examination ($31 per specimen) is relatively
inexpensive in Wuhan, according to the regulation of Medicare
payment system, there would be no substantial economic benefit
compared with Europe and America. Secondly, current medical legal
regulation in China does not allow such management in clinical
practice. The standard of medical care remains to submit resected
polyps for pathological assessment according to the corresponding
expert consensus (37). Thirdly,
high-definition endoscopy, magnifying endoscopy,
dye-chromoendoscopy and electronic chromoendoscopy [e.g., narrow
band imaging (NBI) and i-Scan] have not been used routinely in the
clinical practice of the present authors. Considering the
development and widespread use of available modern image-enhanced
endoscopy, it is anticipated that the ‘resect and discard’ strategy
may be used only by our endoscopists trained with an appropriate
diagnostic method in the near future.
There are certain limitations associated with the
present study. First, since a single-center retrospective study was
performed, as the study subjects did not include adequate numbers
of patients from other regions, any generalization of the results
was limited by the small sample size and certain bias. The effect
of possible confounding factors, such as geographic distribution,
diet, physical activity, socioeconomic status and comorbid medical
conditions, should be considered. In addition, image-enhanced
endoscopy (e.g. high-definition colonoscopy, dye-chromoendoscopy
and electronic chromoendoscopy) might have markedly increased the
detection of small and flat polyps (22–24);
small or flat lesions may occasionally also have been missed due to
insufficient technical imaging methods in the present study.
Therefore, the study requires replication in other centers, and
with multiple technical imaging methods by experienced users.
In conclusion, the present study has revealed that a
significant number of colorectal polyps lie proximal to the splenic
flexure. Therefore, an evaluation of the whole bowel is
particularly important in colonoscopy for the elderly. In addition,
since polyp size was associated with the presence of adenoma and an
advanced component, the present authors consider that diminutive
and small colorectal polyps should not be ignored in elderly
patients, in order to decrease the prevalence of advanced lesions
and CRC.
Acknowledgments
The present study was supported by a grant from the
Research Funding for Health and Family Planning Commission of Wuhan
Municipality (grant no. WX16D38).
References
|
1
|
World Health Organization International
Agency for Research on Cancer, . GLOBOCAN 2012: Estimated Cancer
Incidence, Mortality and Prevalence Worldwide in 2012. http://www-dep.iarc.fr/August 10–2014
|
|
2
|
Yazdizadeh B, Jarrahi AM, Mortazavi H,
Mohagheghi MA, Tahmasebi S and Nahvijo A: Time trends in the
occurrence of major GI cancers in Iran. Asian Pac J Cancer Prev.
6:130–134. 2005.PubMed/NCBI
|
|
3
|
Khuhaprema T and Srivatanakul P: Colon and
rectum cancer in Thailand: An overview. Jpn J Clin Oncol.
38:237–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai Z, Zheng RS, Zou XN, Zhang SW, Zeng
HM, Li N and Chen WQ: Analysis and prediction of colorectal cancer
incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi.
46:598–603. 2012.(In Chinese). PubMed/NCBI
|
|
5
|
Leslie A, Carey FA, Pratt NR and Steele
RJ: The colorectal adenoma-carcinoma sequence. Br J Surg.
89:845–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neugut AI, Jacobson JS and De Vivo I:
Epidemiology of colorectal adenomatous polyps. Cancer Epidemiol
Biomarkers Prev. 2:159–176. 1993.PubMed/NCBI
|
|
7
|
Heitman SJ, Ronksley PE, Hilsden RJ, Manns
BJ, Rostom A and Hemmelgarn BR: Prevalence of adenomas and
colorectal cancer in average risk individuals: A systematic review
and meta-analysis. Clin Gastroenterol Hepatol. 7:1272–1278. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strul H, Kariv R, Leshno M, Halak A,
Jakubowicz M, Santo M, Umansky M, Shirin H, Degani Y, Revivo M, et
al: The prevalence rate and anatomic location of colorectal adenoma
and cancer detected by colonoscopy in average-risk individuals aged
40–80 years. Am J Gastroenterol. 101:255–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Short MW, Layton MC, Teer BN and
Domagalski JE: Colorectal cancer screening and surveillance. Am Fam
Physician. 91:93–100. 2015.PubMed/NCBI
|
|
10
|
Aronchick CA, Lipshutz WH, Wright SH,
Dufrayne F and Bergman G: A novel tableted purgative for
colonoscopic preparation: Efficacy and safety comparisons with
colyte and fleet phospho-soda. Gastrointest Endosc. 52:346–352.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong SN, Kim JH, Choe WH, Han HS, Sung IK,
Park HS and Shim CS: The prevalence and risk of colorectal neoplasm
in asymptomatic average-risk screenees aged 40 to 49 years of age.
Gastrointest Endosc. 72:480–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
The Paris endoscopic classification of
superficial neoplastic lesions: Esophagus, stomach, and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58 6
Suppl:S3–S43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fong TV, Chuah SK, Chiou SS, Chiu KW, Hsu
CC, Chiu YC, Wu KL, Chou YP, Ong GY and Changchien CS: Correlation
of the morphology and size of colonic polyps with their histology.
Chang Gung Med J. 26:339–343. 2003.PubMed/NCBI
|
|
14
|
Pullens HJ, Joosten M, Siersema PD and
Brink MA: Open-access flexible sigmoidoscopy frequently leads to
additional colonoscopy in symptomatic patients over 50 years. J
Gastrointestin Liver Dis. 23:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang MH, Rampal S, Sung J, Choi YH, Son
HJ, Lee JH, Kim YH, Chang DK, Rhee PL, Rhee JC, et al: The
prevalence of colorectal adenomas in asymptomatic Korean men and
women. Cancer Epidemiol Biomarkers Prev. 23:499–507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Oliveira AM, Anapaz V, Lourenço L,
Graça Rodrigues C, Alberto S Folgado, Martins A, de Deus J Ramos
and Reis J: Is there a proximal shift in the distribution of
colorectal adenomas? United European Gastroenterol J. 3:353–357.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaji Y, Mitsushima T, Ikuma H, Watabe H,
Okamoto M, Kawabe T, Wada R, Doi H and Omata M: Incidence and
recurrence rates of colorectal adenomas estimated by annually
repeated colonoscopies on asymptomatic Japanese. Gut. 53:568–572.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patel K and Hoffman NE: The anatomical
distribution of colorectal polyps at colonoscopy. J Clin
Gastroenterol. 33:222–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zare-Mirzaie A, Abolhasani M and
Aryamanesh A: Left sided colorectal adenomatous polyps have more
risk for high grade dysplasia. Acta Med Iran. 51:172–177.
2013.PubMed/NCBI
|
|
20
|
Levin B, Lieberman DA, McFarland B, Smith
RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR,
et al: Screening and surveillance for the early detection of
colorectal cancer and adenomatous polyps, 2008: A joint guideline
from the American cancer society, the US multi-society task force
on colorectal cancer, and the american college of radiology. CA
Cancer J Clin. 58:130–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Day LW and Velayos F: Colorectal cancer
screening and surveillance in the elderly: Updates and
controversies. Gut Liver. 9:143–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trecca A, Gaj F, Di Lorenzo GP, Ricciardi
MR, Silano M, Bella A and Sperone M: Improved detection of
colorectal neoplasms with selective use of chromoendoscopy in 2005
consecutive patients. Tech Coloproctol. 10:339–344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SY, Lee SK, Kim BC, Han J, Kim JH,
Cheon JH, Kim TI and Kim WH: Efficacy of chromoendoscopy with
indigocarmine for the detection of ascending colon and cecum
lesions. Scand J Gastroenterol. 43:878–885. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Testoni PA, Notaristefano C, Vailati C, Di
Leo M and Viale E: High-definition colonoscopy with i-Scan: Better
diagnosis for small polyps and flat adenomas. World J
Gastroenterol. 18:5231–5239. 2012.PubMed/NCBI
|
|
25
|
Chiu HM, Chang LC, Shun CT, Wu MS and Wang
HP: Current management of diminutive colorectal polyps in Taiwan.
Dig Endosc. 26 Suppl 2:S64–S67. 2014. View Article : Google Scholar
|
|
26
|
Matsuda T, Kawano H, Hisabe T, Ikematsu H,
Kobayashi N, Mizuno K, Oka S, Takeuchi Y, Tamai N, Uraoka T, et al:
Current status and future perspectives of endoscopic diagnosis and
treatment of diminutive colorectal polyps. Dig Endosc. 26 Suppl
2:S104–S108. 2014. View Article : Google Scholar
|
|
27
|
Chaput U, Alberto SF, Terris B, Beuvon F,
Audureau E, Coriat R, Roche H, Gaudric M, Prat F and Chaussade S:
Risk factors for advanced adenomas amongst small and diminutive
colorectal polyps: A prospective monocenter study. Dig Liver Dis.
43:609–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shapiro R, Ben-Horin S, Bar-Meir S and
Avidan B: The risk of advanced histology in small-sized colonic
polyps: Are non-invasive colonic imaging modalities good enough?
Int J Colorectal Dis. 27:1071–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hassan C, Pickhardt PJ, Kim DH, Di Giulio
E, Zullo A, Laghi A, Repici A, Iafrate F, Osborn J and Annibale B:
Systematic review: Distribution of advanced neoplasia according to
polyp size at screening colonoscopy. Aliment Pharmacol Ther.
31:210–217. 2010.PubMed/NCBI
|
|
30
|
Tsai FC and Strum WB: Prevalence of
advanced adenomas in small and diminutive colon polyps using direct
measurement of size. Dig Dis Sci. 56:2384–2388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lieberman DA, Rex DK, Winawer SJ,
Giardiello FM, Johnson DA and Levin TR; United States Multi-Society
Task Force on Colorectal Cancer, : Guidelines for colonoscopy
surveillance after screening and polypectomy: A consensus update by
the US multi-society task force on colorectal cancer.
Gastroenterology. 143:844–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hassan C, Repici A, Zullo A and Sharma P:
New paradigms for colonoscopic management of diminutive colorectal
polyps: Predict, resect, and discard or do not resect? Clin Endosc.
46:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ignjatovic A, East JE, Suzuki N, Vance M,
Guenther T and Saunders BP: Optical diagnosis of small colorectal
polyps at routine colonoscopy (Detect InSpect ChAracterise Resect
and Discard; DISCARD trial): A prospective cohort study. Lancet
Oncol. 10:1171–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hassan C, Pickhardt PJ and Rex DK: A
resect and discard strategy would improve cost-effectiveness of
colorectal cancer screening. Clin Gastroenterol Hepatol. 8:865–869.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McGill SK, Evangelou E, Ioannidis JP,
Soetikno RM and Kaltenbach T: Narrow band imaging to differentiate
neoplastic and non-neoplastic colorectal polyps in real time: A
meta analysis of diagnostic operating characteristics. Gut.
62:1704–1713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takeuchi Y, Hanafusa M, Kanzaki H, Ohta T
and Hanaoka N: Proposal of a new ‘resect and discard’ strategy
using magnifying narrow band imaging: Pilot study of diagnostic
accuracy. Dig Endosc. 26 Suppl 2:S90–S97. 2014. View Article : Google Scholar
|
|
37
|
Endoscopic Diagnosis and Treatment Group
of Early Digestive Cancer, Chinese Society of Digestive Endoscopy;
Gastrointestinal tumor group, Chinese Society of Gastroenterology
and Intestinal Study group, Chinese Society of Digestive Endoscopy,
. Chinese consensus: screening, diagnosis and treatment of early
colorectal cancer and precancerous lesions (2014, Chongqing). Chin
J Dig Endosc. 2:69–85. 2015.(In Chinese).
|