Introduction
Paclitaxel is a member of the taxane agents that has
demonstrated efficacy in ovarian cancer, both in first- and in
second-line therapy (1).
Neurological disorders are counted among the side-effects of this
drug (2). In the present study, a
rare case of a non-neuropathic ocular disorder known as cystoid
macular edema (CME), due to the use of paclitaxel in patients
treated for ovarian cancer, is described.
Case report
In September 2007, a 49-year-old woman underwent
optimal debulking surgery for FIGO stage IIIC ovarian serious
cancer, with residual disease following surgery (a tumor measuring
<1 cm). The patient received adjuvant chemotherapy based on
carboplatin and paclitaxel for six cycles every three weeks,
achieving a complete response. The cumulative dose of paclitaxel
was 1,680 mg. After 13 months, the patient presented with hepatic
metastasis and peritoneal nodules. In view of the recurrence of the
disease, the patient received chemotherapy based on carboplatin and
pegylated liposomal doxorubicin. After 8 cycles of the
chemotherapy, based on the disease progression the patient
subsequently received Topotecan for 13 cycles. However, the patient
then presented with pulmonary progression disease, and consequently
she was treated with paclitaxel (60 mg/m2) administered
intravenously once a week. At the 45th cycle of chemotherapy, the
patient was referred to the ophthalmologist for progressively
decreasing bilateral visual acuity. The visual acuity was 20/200 in
the right eye (RE) and 20/100 in the left eye (LE), and it did not
improve through the use of lenses. The slit lamp biomicroscopy of
the anterior segment and the intraocular pressure of both eyes were
normal, but fundus ophthalmoscopy revealed a bilateral CME.
Spectral domain optical coherence tomography (OCT) (Cirrus OCT;
Zeiss GmbH, Jena, Germany) scans of the two eyes revealed an
increased macular thickness due to intraretinal fluid accumulation;
fluorescein angiography exhibited normal filling of the choroidal
and retinal vessel, with an intact parafoveal capillary net. These
findings were consistent with the diagnosis of CME without leaking
vessels. The patient's optical history was negative for previous
eye diseases. After collegial counseling based on paclitaxel
side-effects, the chemotherapy treatment was discontinued. Thirty
days later, the visual acuity increased (20/50 in the RE and 20/40
in the LE). Three months after the discontinuation of paclitaxel,
visual acuity increased to 20/20 in both eyes, and a further OCT
examination revealed the restoration of macular shape, with
regression of macular edema. The patient remains alive with stable
disease, and without treatment (Fig.
1).
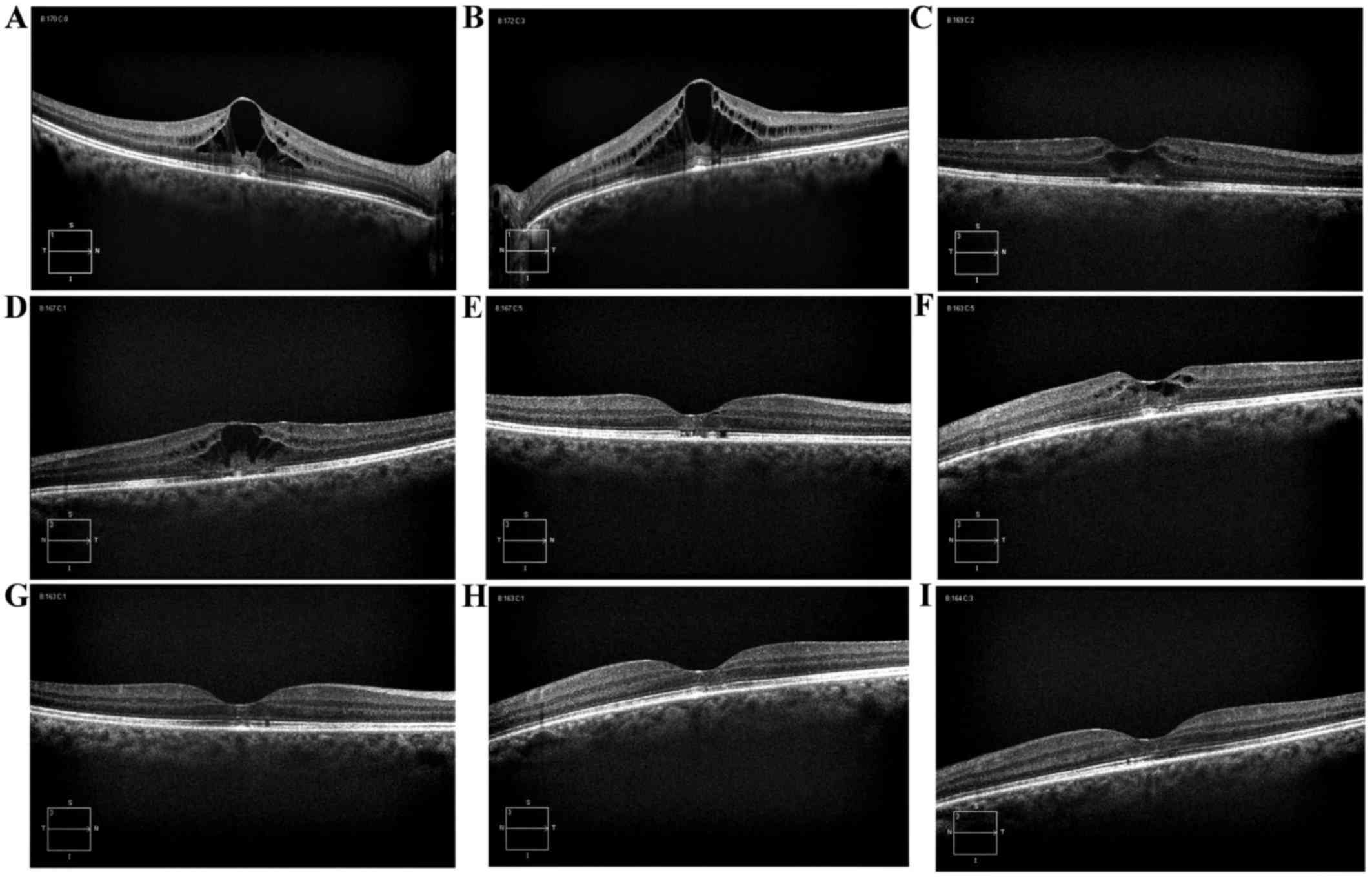 | Figure 1.Spectral domain optical coherence
tomography images derived from the present case study. (A)
Baseline: Macular edema with a CRT of 515 µm in the RE. (B)
Baseline: Macular edema with a CRT of 584 µm in the LE. (C) At 40
days after the interruption of plaxitel systemic therapy, CRT
decreased to 320 µm in the RE. (D) At 40 days after the
interruption of plaxitel systemic therapy, CRT decreased to 391 µm
in the LE. (E) Three months later, the CRT was 232 µm in the RE,
with the restoration of macular shape. (F) Three months later, the
CRT was 316 µm in the LE, with the restoration of macular shape.
(G) Four months later, the CRT was 230 µm in the RE. (H) Four
months later, the CRT was 227 µm in the LE. (I) Eight months later,
the CRT was 235 µm in the RE, with a normal macula shape. (J) Eight
months later, the CRT was 234 microns in the LE, with a normal
macula shape. CRT, central retinal thickness; LE, left eye; RE,
right eye. |
Discussion
Paclitaxel is an anticancer drug produced by the
plants of the genus Taxus, which acts by restricting microtubule
mobility and inhibiting mitosis. The largest and heaviest adverse
effect of this reagent is toxic effects to the bone marrow and
neurological disorders. The frequency of peripheral neuropathy has
been reported to increase with a cumulative dose. Reports
concerning ophthalmic adverse effects, including impaired visual
acuity, dry eyes, scintillating scotomas, keratitis, photopsia,
development of open-angle glaucoma and optic neuropathy, are scarce
(3,4). Furthermore, bilateral CME has been
described as a highly unusual adverse event after the
administration of taxanes. Macular edema, or CME, is a common cause
of visual impairment, and has been classically demonstrated using
fluorescein angiograms, showing capillary leakage. CME without
fluorescein leakage occurs only rarely, and its most common causes
are juvenile X-linked retinoschisis, Goldmann-Favre syndrome, and
niacin toxicity (3,5,6). At the
present time, the mechanism of CME associated with no fluorescein
leakage has not been elucidated, since the necessary
histopathological studies have not been performed. Several
mechanisms have been proposed, although it is considered to result
from disruption of the normal blood-retinal barrier by molecules
having a molecular weight lower than that of fluorescein. This
leads to fluid accumulation in the intracellular space. It has been
well established that taxane agents cause fluid retention,
represented by edema, weight gain, and third-space fluid collection
(pericardial, pleural, ascites), and this appears to be associated
with their cumulative dose (7,8).
Considering the cases where there was no indication of systemic
fluid retention, Koo and Kim (9)
have suggested that CME was presumed to occur as a result of
cellular toxicity derived from the suppression of intracellular
microtubule reorganization (9).
It has yet to be determined why, even assuming a
specific pathogenetic mechanism, this side-effect occurs so
infrequently. In the literature, there are 13 cases of CME
associated with the use of taxanes, including 6 caused by Taxol, 5
due to nanoparticle albumin-bound paclitaxel, and only 2 linked
with Taxotere (docetaxel) (3,10–17).
It remains an open question as to why paclitaxel is more toxic than
docetaxel. Possibly, a certain amount of cumulative dose is
necessary to induce CME, and this may be achieved less easily
through the use of docetaxel. To the best of our knowledge, the
present case study is the first that has been reported in the field
of ovarian cancer, since those previously described in literature
occurred during the course of treatment of lung or breast cancer.
The second peculiarity of the present case report lies in the fact
that the toxicity was presented only during the use of Taxol in the
second line of chemotherapy, and only when it had reached a very
high cumulative dose, such as 4,380 mg.
Considering the very late manifestation reported in
the present case study, it is possible to envisage a sensitizing
effect, although further studies are required in order to confirm
this. Based on the literature, it is evident that the treatment of
CME has not uniquely been based upon paclitaxel therapy: Cases have
also been described of the use of acetazolamide, prednisolone,
triamcinolone and dorzolamide. However, to the best of our
knowledge, the present case study has confirmed that macular edema
associated with paclitaxel use exhibited spontaneous resolution
following discontinuation of the causative agent. Taxane-associated
maculopathy has been scarcely reported in the literature, but the
gynecological oncologist should be alert to its possible
development, and an ophthalmologic evaluation should be offered to
all patients using paclitaxel.
References
|
1
|
Boere IA and van der Burg ME: Review of
dose-intense platinum and/or paclitaxel containing chemotherapy in
advanced and recurrent epithelial ovarian cancer. Curr Pharm Des.
18:3741–3753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oishi R and Egashira N: Fukuoka.
Peripheral neuropathy induced by anticancer drugs. IgakuZasshi.
104:71–80. 2013.
|
|
3
|
Hofstra LS, de Vries EG and Willemse PH:
Ophthalmic toxicity following paclitaxel infusion. Ann Oncol.
8:10531997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Tripathi RC and Tripathi BJ:
Drug-induced ocular disorders. Drug Saf. 31:127–141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semb KA, Aamdal S and Oian P: Capillary
protein leak syndrome appears to explain fluid retention in cancer
patients who receive docetaxel treatment. J Clin Oncol.
16:3426–3432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spirn MJ, Warren FA, Guyer DR, Klancnik JM
Jr and Spaide RF: Optical coherence tomography findings in
nicotinic acid maculopathy. Am J Ophthalmol. 135:913–914. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Telander DG and Sarraf D: Cystoid macular
edema with docetaxel chemotherapy and the fluid retention syndrome.
Semin Ophthalmol. 22:151–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaud LB, Valero V and Hortobagyi G:
Risks and benefits of taxanes in breast and ovarian cancer. Drug
Saf. 23:401–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Béhar A, Pujade-Lauraine E, Maurel A, Brun
MD, Chauvin FF, de Chauvin F Feuilhade, Oulid-Aissa D and Hille D:
The pathophysiological mechanism of fluid retention in advanced
cancer patients treated with docetaxel, but not receiving
corticosteroid comedication. Br J Clin Pharmacol. 43:653–658. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koo NK and Kim YC: A case of
paclitaxel-induced maculopathy treated with methazolamide. Korean J
Ophthalmol. 26:394–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teitelbaum BA and Tresley DJ: Cystic
maculopathy with normal capillary permeability secondary to
docetaxel. Optom Vis Sci. 80:277–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy CG, Walsh JB, Hudis CA, Lake D and
Theodoulou M: Cystoid macular edema secondary to nab-paclitaxel
therapy. J Clin Oncol. 28:e684–e687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith SV, Benz MS and Brown DM: Cystoid
macular edema secondary to albumin-bound paclitaxel therapy. Arch
Ophthalmol. 126:1605–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joshi MM and Garretson BR: Paclitaxel
maculopathy. Arch Ophthalmol. 125:709–710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Risard SM, Pieramici DJ and Rabena MD:
Cystoid macular edema secondary to Paclitaxel (abraxane). Retin
Cases Brief Rep. 3:383–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Georgakopoulos CD, Makri OE, Vasilakis P
and Exarchou A: Angiographically silent cystoid macular oedema
secondary to paclitaxel therapy. Clin Exp Optom. 95:233–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baskin DE and Garg SJ: Abraxane-induced
cystoid macular edema refractory to concomitant intravenous
bevacizumab. Can J Ophthalmol. 46:200–201. 2011. View Article : Google Scholar : PubMed/NCBI
|















