Introduction
The incidence of bladder cancer is increasing and it
is the most common urinary malignant tumor worldwide (1). It is the second most common urological
malignancy in the Mongolian population (2). A histological study examining 108
Mongolian patients with urinary bladder cancer revealed that 2, 12
and 86% of the patients were diagnosed with papilloma, papillary
urothelial neoplasm of low malignant potential and transitional
cell carcinoma, respectively (3).
Various environmental and genetic risk factors such as smoking,
occupational exposure to aromatic amines, male sex, older age and
bladder infections are associated with bladder cancer development
(4,5). Furthermore, certain genetic alterations
in bladder cancer including p53 tumor suppressor gene and its
regulating gene, human homolog of murine double minute (MDM2) are
considered to be associated with cancer susceptibility (6). Whether genetic polymorphisms in these
genes also has a role in the risk of bladder cancer remains to be
addressed.
P53 genomic sequence mutation is typically
concentrated in the region of exons 4–9 when it is detected in
human cancer (7,8). P53 codon 72 G to C polymorphism
(p53R72P) is found in exon 4 and is a well-known single nucleotide
polymorphism (SNP) that alters arginine to proline (9). The two forms are morphologically
wild-type, but such variants of p53 may target varying
transcriptional elements (10).
Previous meta-analyses have reported that p53R72P is associated
with the risk of bladder cancer in Asian but not Caucasian patients
(11,12). However, these studies did not include
Mongolian populations, and the risk association in this population
remains to be investigated.
A functional SNP was also identified at position 309
of the first intron at the P2 promoter region of human MDM2,
termed MDM2-SNP309 (rs2279744) (13). The conversion from the T to G allele
in this SNP increases the binding of the general transcription
activator Sp1 to the promoter and enhances the expression of the
MDM2 gene, which in turn promotes the degradation of p53 and leads
to a higher risk of carcinogenesis (14,15). For
bladder cancer, the homozygous variant of MDM2-SNP309 is not
associated with overall risk of bladder cancer but may influence
the invasive growth of bladder cancer in German populations
(16). A study examining Turkish
populations reported that the MDM2 T309G polymorphism is a
potential genetic susceptibility factor for bladder cancer
(17). Another previous study,
conducted in the USA, revealed that SNP309 is associated with
earlier onset of superficial tumors, poorer clinical outcomes and
TP53 mutation status in invasive bladder cancer (18). A study conducted in Japan revealed no
significant associations between each genotype of the MDM2 SNP309
and p53 Arg72Pro polymorphisms and bladder cancer risk, but the
polymorphisms may influence the clinical outcome of bladder cancer
(19). However, investigations have
been inconsistent, and the hypothesized association remains
controversial. These inconclusive and conflicting results may be
associated with the different patient subgroups and ethnicities
studied.
In the present study, the risk association between
p53R72P and MDM2-SNP309 and bladder cancer in Mongolian patients
was analyzed using the logistic regression method. The age onset of
patients with bladder cancer was also compared among the different
genotypes of these two genetic polymorphisms.
Patients and methods
Patients
A total of 79 cancer-free healthy controls and 63
patients with histologically confirmed bladder cancer who were
diagnosed in the First Central Hospital of Mongolia (Ulaanbaatar,
Mongolia) were enrolled in the study. Patients with bladder cancer
were not selected according to their age, sex or tumor stage. Age-
and gender-matched controls were enrolled in this study. All
patients provided informed consent and completed structured
questionnaires. The questionnaire included information about risk
factors for bladder cancer such as age, sex, body mass index,
socioeconomic status, occupational exposure, dietary factors,
smoking, alcohol drinking, drug usage, history of chronic urinary
tract diseases and exercising. This study was approved by the
Ethics Committee of the Ministry of Health of Mongolia (No. 4), and
the institutional review board of National Yang-Ming University,
Taiwan (YM102005). The approval documents will be provided upon
request. All participants were native Mongolians and aged >18
years.
Genotyping
In total, 3 ml whole blood was collected from
cancer-free healthy controls and patients with bladder cancer for
genotyping of p53R72P and MDM2-SNP309 after written informed
consent was obtained. The procedures of MDM2-SNP309 and p53R72P
genotyping was performed as described previously (18). In brief, DNA was extracted from 200
µl of blood using the Qiagen mini blood DNA extraction kit (Qiagen,
Inc., Valencia, CA, USA) and MDM2 SNP309 was amplified by
polymerase chain reaction (PCR) using the following primers:
Forward, 5′-CGGGAGTTCAGGGTAAAGGT-3′; and reverse,
5′-AGCAAGTCGGTGCTTACCTG-3′. The PCR reactions consisted of 100 ng
of genomic DNA, 0.2 µM primer, 200 µM dNTP, 1.5 mM
MgCl2, 20 mM Tris-HCl (pH 8.4), 50 mM KCl and 1 U of
Platinum Taq DNA polymerase (Invitrogen; Thermo Fisher Scientific,
Waltham, MA, USA). The thermal cycling conditions were 1 min at
94°C; 40 cycles of denaturing at 94°C, annealing at 58°C, and
elongation at 72°C for 30 sec each, followed by one cycle at 72°C
for 10 min. For restriction fragment length polymorphism analysis,
10–20 µl of the amplified 352-bp fragment was digested with 1 U of
MspA1I restriction enzyme (New England Biolabs, Inc., Ipswich, MA,
USA) at 37°C in a water bath for 30 min to 1 h. The T/T, T/G and
G/G genotypes were distinguished by bands with lengths of 233 and
88; 233, 187 and 88 bp; and 187 and 88 bp, respectively, following
electrophoresis. The same procedure was used for genotyping p53
codon 72 polymorphism (20). The
primers were as follows: Forward, 5′-TTTCACCCATCTACAGTCCC-3′;
reverse, 5′-CGGTGTAGGAGCTGCTG-3′. The length of the PCR product was
166 bp, then it was digested with BstU1 at 60°C for 1 h. The
digestion of the P/P variant yielded a 166-bp band, the R/R variant
yielded 135- and 31-bp bands and the P/R heterozygous variant
yielded 166, 135 and 31 bp bands.
Statistical analysis
The genotype and allele frequency were tested for
Hardy-Weinberg equilibrium. Discrepancies between controls and
cases were determined using 2×2 χ2 tables or two-sample
t-tests. Multivariate logistical regression analysis with
adjustment or stratification was used to determine odds ratios (OR)
and 95% confidence intervals (CI) for evaluating the risk
association of MDM2-SNP309 and p53R72P with the incidence of
bladder cancer. The Kaplan-Meier method with the log-rank test was
used to analyze the age at diagnosis of these genotypes.
Statistical evaluation was performed using the demo version of
Medcalc 9.5.1.0 software (MedCalc Software, Mariakerke, Belgium).
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of bladder cancer
cases and controls
One hundred and twenty-nine cancer-free controls and
141 patients with bladder cancer were initially enrolled in the
present study. However, characteristics of patients and controls
were only recorded in 79 controls and 63 patients with bladder
cancer, as they had provided the full information requested in the
questionnaires. The demography of 142 Mongolian volunteers awas
summarized in Table I. There were
3.5 times more male than female patients. Smoking, alcohol drinking
and exercise, as well as the history of chronic urinary tract
disease were significantly different between cases and controls
(P<0.05). Thus, these characteristics were considered
confounders that were adjusted for in the odds ratio in the
multivariate logistic regression analysis.
 | Table I.The demography of patients with
bladder cancer and cancer-free controls. |
Table I.
The demography of patients with
bladder cancer and cancer-free controls.
| Characteristics | Controls (%)
(n=79) | Cases (%) (n=63) | P-valuea |
|---|
| Age, years |
|
| 0.2263 |
| Mean | 58.3 | 55.6 |
|
| SD | 13.5 | 12. 7 |
|
| Sex |
|
| 0.9551 |
| Male | 60 (76) | 49 (78) |
|
|
Female | 19 (24) | 14 (22) |
|
| Smoking |
|
| <0.0001 |
| Yes | 19 (24) | 45 (73) |
|
| No | 60 (76) | 18 (27) |
|
| Alcohol drinking |
|
| 0.045 |
| Yes | 19 (24) | 26 (41) |
|
| No | 60 (76) | 37 (59) |
|
| HCI |
|
| 0.1697 |
| Yes | 79 (100) | 60 (95) |
|
| No | 0 (0) | 3 (5) |
|
| Coffee |
|
| 0.2292 |
| Yes | 25 (32) | 27 (43) |
|
| No | 54 (68) | 36 (57) |
|
| CVD |
|
| 0.8225 |
| Yes | 14 (18) | 13 (21) |
|
| No | 65 (82) | 50 (79) |
|
| History of CUTD |
|
| <0.0001 |
| Yes | 10 (13) | 34 (54) |
|
| No | 69 (87) | 29 (46) |
|
| Exercise |
|
| 0.0175 |
| Yes | 9 (11) | 18 (29) |
|
| No | 70 (89) | 45 (71) |
|
Allelic frequency analysis of
MDM2-SNP309 and p53R72P
The allelic frequencies of MDM2-SNP309 and p53R72P
were next analyzed in these 142 Mongolians. For MDM2-SNP309, the
number and percentage of wild-type (T/T), heterozygote (T/G) and
homozygote (G/G) alleles was 18 (22.7%), 44 (55.7%) and 17 (21.6%)
in the controls, respectively, whereas for bladder cancer cases,
these values were 13 (20.6%), 30 (47.6%) and 20 (31.7%),
respectively. The genotype frequency of MDM2-SNP309 in both
controls and cases obeyed the Hardy-Weinberg equilibrium. The
number and percentage of p53R72P genotypes were wild-type (R/R) 37
(46.8%), heterozygote (R/P) 23 (29.1%) and homozygote (P/P) 19
(24.1%) alleles in controls, whereas in bladder cancer cases the
R/R, R/P and P/P alleles were 35 (55.6%), 20 (31.7%) and 8 (12.7%),
respectively. Unlike MDM2-SNP309, the allelic frequency of p53R72P
in controls (R, 61%; P, 39%) was different from that of bladder
cancer cases (R, 71%; P, 29%). The genotype frequency of p53R72P in
bladder cancer cases obeyed the Hardy-Weinberg equilibrium, but not
in controls. The analysis of allelic frequencies of MDM2-SNP309 and
p53R72P is summarized in Table
II.
 | Table II.Numbers of donors categorized as
having different MDM2-SNP309 and p53R72P genotypes. |
Table II.
Numbers of donors categorized as
having different MDM2-SNP309 and p53R72P genotypes.
| A, MDM2-SNP309
(T→G) |
|---|
|
|---|
| Controls | Cases |
|---|
|
|
|---|
|
|
| Number | Allelic
frequency |
|
| Number | Allelic
frequency |
|---|
| Homozygote
reference | (T/T) | 18 | G% | T% | Homozygote
reference | (T/T) | 13 | G, % | T, % |
| Heterozygote | (T/G) | 44 | 49 | 51 | Heterozygote | (T/G) | 30 | 49 | 51 |
| Homozygote
variant | (G/G) | 17 |
|
| Homozygote
variant | (G/G) | 20 |
|
|
| H-Wa
consistence |
| χ2= | 1.0285 |
|
|
| χ2= | 0.080357 |
|
|
|
| χ2 test
P-value= | 0.3105 |
|
|
| χ2 test
P-value= | 0.7768 |
|
|
|
|
|
Obeyb |
|
|
|
| Obey |
|
|
| B, p53R72P (G→C for
R→P) |
|
| Controls | Cases |
|
|
|
|
| Number | Allelic
frequency |
|
| Number | Allelic
frequency |
|
| Homozygote
reference | (G/G) | 37 | G% | C% | Homozygote
reference | (G/G) | 35 | G, % | C, % |
| Heterozygote | (G/C) | 23 | 61 | 39 | Heterozygote | (G/C) | 20 | 71 | 29 |
| Homozygote
variant | (C/C) | 19 |
|
| Homozygote
variant | (C/C) | 8 |
| H-W
consistence |
| χ2= | 11.761 |
|
|
| χ2= | 3.111 |
|
|
|
| χ2 test
P-value= | 0.0006 |
|
|
| χ2 test
P-value= | 0.07776 |
|
|
|
|
| Not Obey |
|
|
|
| Obey |
|
Association between MDM2-SNP309 and
p53R72P and the risk of the bladder cancer
To investigate the association between MDM2-SNP309
and p53R72P polymorphisms and the risk of bladder cancer in
Mongolian patients, a multivariate logistic regression model was
used to calculate the ORs of heterozygotes and homozygotes relative
to the wild-type genotype. For MDM2-SNP309, the crude OR of the
homozygous genotype (G/G) was 1.629 (95% CI=0.622–4.2663) compared
with the wild-type genotype (T/T). On the contrary, the OR of the
heterozygous genotype (T/G) was 0.944 (95% CI=0.4031–2.2111). The
confounders-adjusted ORs of the G/G and T/G genotypes were 1.306
(95% CI=0.3938–4.2204) and 0.993 (95% CI=0.3246–3.0403),
respectively. Notably, the crude OR and adjusted OR for the
homozygous genotype of p53R72P were 0.445 (95% CI=0.1727–1.147) and
0.491 (95% CI=0.1487–1.6199), respectively. However, the ORs of
MDM2-SNP309 and p53R72P were not significant (Table III). Thus, the current analysis
showed that the homozygous genotype (G/G) of MDM2-SNP309 tended to
increase the risk of bladder cancer in Mongolian populations, but
this effect was not observed for p53R72P.
 | Table III.Risk evaluation of the MDM2 SNP309
and p53R72P genotypes and the development of bladder cancer in the
Mongolian population. |
Table III.
Risk evaluation of the MDM2 SNP309
and p53R72P genotypes and the development of bladder cancer in the
Mongolian population.
| Genotypes | OR (95%
CI)a | P-value | OR (95%
CI)b | P-value |
|---|
| MDM2-SNP309 |
|
|
|
|
| TT | 1 (reference) |
| 1 (reference) |
|
| TG | 0.944 (0.4031 to
2.2111) | 0.8945 | 0.993 (0.3246 to
3.0403) | 0.9908 |
| GG | 1.629 (0.622 to
4.2663) | 0.3206 | 1.306 (0.3938 to
4.3304) | 0.6626 |
|
TG+GG | 1.135 (0.5072 to
2.5396) | 0.7581 | 1.074 (0.3869 to
2.9789) | 0.8915 |
| p53R72P |
|
|
|
|
| RR
(GG)c | 1 (reference) |
| 1 (reference) |
|
| PR
(GC) | 0.919 (0.4313 to
1.9593) | 0.8274 | 0.598 (0.2128 to
1.6791) | 0.3288 |
| PP
(CC) | 0.445 (0.1727 to
1.147) | 0.0937 | 0.491 (0.1487 to
1.6199) | 0.3503 |
|
PP+PR | 0.705 (0.3624 to
1.3704) | 0.3024 | 0.566 (0.2409 to
1.3302) | 0.1918 |
Risk evaluation of MDM2-SNP309 and
p53R72P genotypes and bladder cancer development
MDM2-SNP309 and p53R72P were examined by stratifying
according to the following potential confounding factors: Smoking,
alcohol drinking and history of chronic urinary tract diseases, as
stated in Table I. The G/G genotype
of MDM2-SNP309 had a higher risk in the smoking group (OR, 2.3704;
95% CI=0.4308–13.0436) than the non-smoker group (OR, 1; 95%
CI=0.2077–4.8138). Furthermore, the G/G and T/G genotypes of
MDM2-SNP309 were associated with the risk of bladder cancer
following stratification by history of chronic urinary tract
diseases. For alcohol drinking, the G/G genotype of MDM2-SNP309 had
a protective effect on the risk of bladder cancer (OR, 0.6944; 95%
CI=0.1296–3.7203), whereas the T/G genotype increased the risk of
bladder cancer (OR, 1.5625; 95% CI, 0.3612–6.7587). The P/R
genotype of the p53R72P was also associated with increased risk
(OR, 1.875; 95% CI=0.4415–7.9631) of bladder cancer following
stratification by alcohol drinking, but not the P/P genotype (OR,
0.5; 95% CI=0.108–2.3142). Although the ORs were altered after
stratification, the results of the statistical analysis were not
significant (Table IV). Therefore,
the adjusted ORs of MDM2-SNP309 and p53R72P genotypes revealed no
risk association between these SNPs and bladder cancer in Mongolian
patients.
 | Table IV.Stratification of smoking, history of
CUTD and alcohol drinking to evaluate the risk of the MDM2 SNP309
and p53R72P genotypes regarding the incidence of bladder cancer in
the Mongolian population. |
Table IV.
Stratification of smoking, history of
CUTD and alcohol drinking to evaluate the risk of the MDM2 SNP309
and p53R72P genotypes regarding the incidence of bladder cancer in
the Mongolian population.
|
| Smoking | Non-smoking | History of
CUTD | Non-history of
CUTD | Alcohol
drinking | Non-alcohol
drinking |
|---|
|
|
|
|
|
|
|
|
|---|
| Stratifying
factorsa | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| MDM2-SNP309 |
|
|
|
|
|
|
|
|
|
|
|
|
| TT | 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
|
| TG | 0.7407 | 0.6695 | 1.0937 | 0.894 | 5 | 0.0759 | 0.4695 | 0.1757 | 1.5625 | 0.5503 | 0.7738 | 0.6473 |
|
|
(0.1867–2.9391) |
|
(0.2926–4.0891) |
|
(0.8442–30.4088) |
|
(0.1572–1.4025) |
|
(0.3612–6.7587) |
|
(0.2579–2.3216) |
|
| GG | 2.3704 | 0.3212 | 1 | 1 | 1.55 | 0.3546 | 1.17 | 0.595 | 0.6944 | 0.6702 | 2.5325 | 0.1304 |
|
|
(0.4308–13.0436) |
|
(0.2077–4.8138) |
|
(0.6132–3.9140) |
|
(0.6520–2.1090) |
|
(0.1296–3.7203) |
|
(0.7597–8.4419) |
|
|
TG+GG | 1.0667 | 0.9238 | 1.0652 | 0.9218 | 3.733 | 0.1029 | 0.7 | 0.4843 | 1.1905 | 0.8029 | 1.1854 | 0.7454 |
|
|
(0.2841–4.0046) |
|
(0.3015–3.7630) |
|
(0.7666–18.1813) |
|
(0.2576–1.9019) |
|
(0.3027–4.6818) |
|
(0.4246–3.3094) |
|
| p53R72P |
|
|
|
|
|
|
|
|
|
|
|
|
| RR
(GG) | 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
| 1 (reference) |
|
| PR
(GC) | 0.56 | 0.3407 | 0.8835 | 0.708 | 1.11 | 0.8977 | 0.7529 | 0.5815 | 1.875 | 0.3943 | 0.6407 | 0.3552 |
|
|
(0.1699–1.8459) |
|
(0.4621–1.6893) |
|
(0.2228–5.5420) |
|
(0.2745–2.0654) |
|
(0.4415–7.9631) |
|
(0.2494–1.6463) |
|
| PP
(CC) | 0.85 | 0.4051 | 0.9264 | 0.7348 | 0.6455 | 0.4015 | 0.7441 | 0.3168 | 0.5 | 0.3753 | 0.3746 | 0.1234 |
|
|
(0.5798–1.2461) |
|
(0.5951–1.4421) |
|
(0.2322–1.7948) |
|
(0.4171–1.3274) |
| (0.108–2.3142) |
|
(0.1074–1.3062) |
|
|
PP+PR | 0.7048 | 0.3024 | 0.8 | 0.6795 | 0.8333 | 0.8008 | 0.6614 | 0.3479 | 1.05 | 0.9357 | 0.532 | 0.1396 |
|
|
(0.3624–1.3704) |
|
(0.2776–2.3057) |
|
(0.2022–3.4352) |
|
(0.2789–1.5682) |
|
(0.3209–3.4361) |
|
(0.2309–1.2286) |
|
Evaluation of the effect of
MDM2-SNP309 on the risk of bladder cancer after stratification by
p53R72P
To investigate whether MDM2-SNP309 and p53R72P
interact to influence the risk of bladder cancer, the genotypes of
p53R72P were stratified by MDM2-SNP309 genotype. Based on this
dataset, the results revealed no significant effects regarding the
homozygous genotype (G/G) of MDM2-SNP309 and the risk of bladder
cancer in Mongolian patients in the RR group of the p53R72P SNP
(OR, 3.3554; 95% CI=0.3914–28.7657). For heterozygous (R/P)
genotypes of p53R72P, both genotypes of MDM2-SNP309 showed no risk
association with bladder cancer. All of these results are
summarized in Table V.
 | Table V.The risk evaluation of MDM2-SNP309 on
bladder cancer stratified for p53 R72P. |
Table V.
The risk evaluation of MDM2-SNP309 on
bladder cancer stratified for p53 R72P.
| p53R72P | Cases (n, %) | Control (n, %) | OR (95% CI) | P-value |
|---|
| RR |
|
|
|
|
| MDM2-SNP309 |
|
|
|
|
| TT | 6,17.1 | 8,22.2 | 1 |
|
| TG | 16,45.7 | 21,58.3 | 1.2925
(0.2750–6.0754) | 0.7452 |
| GG | 13,37.2 | 7,19.5 | 3.3554
(0.3914–28.7657) | 0.2695 |
|
TG+GG | 29,82.9 | 28,77.8 | 1.8557
(0.4455–7.7291) | 0.3957 |
| RP |
|
|
|
|
| MDM2-SNP309 |
|
|
|
|
| TT | 6,30 | 3,13.6 | 1 |
|
| TG | 9,45 | 13,59.1 | 0.1011
(0.0067–1.5284) | 0.0982 |
| GG | 5,25 | 6,27.3 | 0.1289
(0.0066–2.5307) | 0.1774 |
|
TG+GG | 14,70 | 19,86.4 | 0.1611
(0.0174–1.4937) | 0.2334 |
| PP |
|
|
|
|
| MDM2-SNP309 |
|
|
|
|
| TT | 1,12.5 | 6,31.6 | 1 |
|
| TG | 5,62.5 | 9,47.4 | 0.3607
(0.0177–7.3437) | 0.5072 |
| GG | 2,25 | 4,21 | 47.692
(0.0015–1512560.1357) | 0.4649 |
|
TG+GG | 7,87.5 | 13,68.4 | 3.0848
(0.1990–47.8194) | 0.4205 |
Comparison of different genotypes of
MDM2-SNP309 and p53R72P and the age at diagnosis of bladder
cancer
The median age at diagnosis of bladder cancer in
Mongolian patients was compared among different genotypes of
MDM2-SNP309 and p53R72P. The results were obtained from 84 patients
with bladder cancer with registered age at diagnosis. For
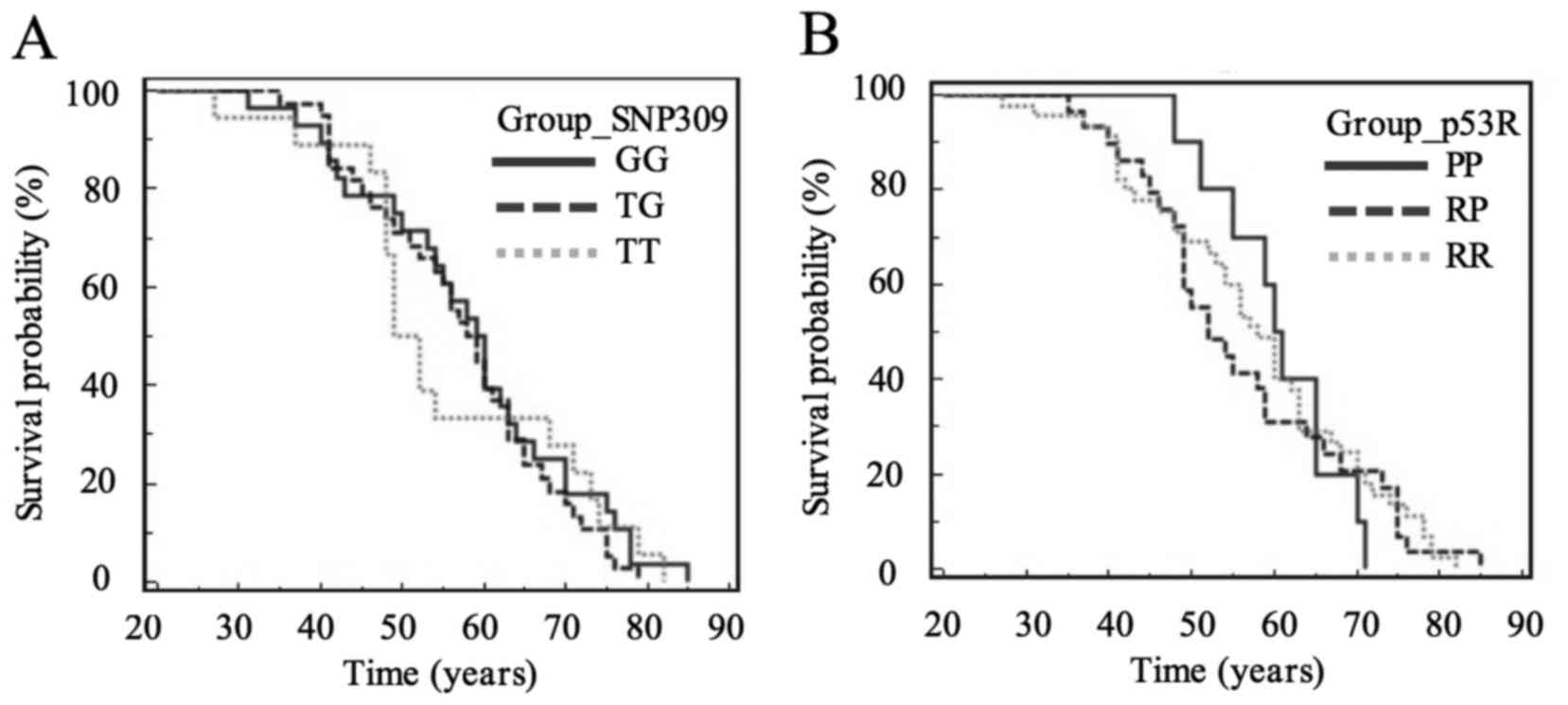
MDM2-SNP309, the median ages of G/G (60 years) and T/G (59 years)
genotypes were older than that of wild-type (T/T) genotype (50.5
years) (Table VI). However, there
was no significant difference among these three genotypes of
MDM2-SNP309 (Fig. 1A). For p53R72P,
the median patients age at diagnosis for the R/P genotype (52
years) was lower than for the homozygous (RR, 58; PP, 61) genotypes
(Table VII); however, among the
p53R72P genotypes no significant differences were identified
(Fig. 1B).
 | Table VI.Comparison of age at diagnosis of
bladder cancer in Mongolian patients according to MDM2-SNP309
genotype. |
Table VI.
Comparison of age at diagnosis of
bladder cancer in Mongolian patients according to MDM2-SNP309
genotype.
| MDM2-SNP309 | GG | TG | TT |
|---|
| Sample size | 28 | 38 | 18 |
| Median age
(years) | 60 | 59 | 50.5 |
| Mean age ± SD | 52.35±8.9 | 53±13.77 | 55.46±10.14 |
 | Table VII.Comparison of age at diagnosis
bladder cancer in Mongolian patients according to p53R72P
genotype. |
Table VII.
Comparison of age at diagnosis
bladder cancer in Mongolian patients according to p53R72P
genotype.
| p53R72P | RR | RP | PP |
|---|
| Sample size | 45 | 29 | 10 |
| Median age
(years) | 58 | 52 | 61 |
| Mean age ± SD | 53.81±8.86 | 51.73±12.49 | 54.73±16.78 |
Discussion
The current study investigated the association
between the MDM2 SNP309 and p53R72P polymorphisms and bladder
cancer risk in Mongolian populations. According to the results, the
homozygous (G/G) genotype of MDM2 SNP309 is associated with
increased risk of bladder cancer in Mongolian populations, but not
significantly so. This result is similar to the result of a
previous Turkish study, which indicated that the G/G genotype was a
potential genetic susceptibility factor for bladder cancer
(OR=2.68; 95% CI=1.34–5.40) (17).
Furthermore, it is partially consistent with previous studies
reporting that the G allele of MDM2-SNP309 is associated with the
development of an aggressive tumor phenotype but not overall risk
of bladder cancer in Caucasian Japanese populations (16,19).
In the present study, several risk factors for
bladder cancer including smoking, drinking alcohol and history of
urinary tract infections exhibited significant differences between
cases and controls, as determined via structured questionnaires
(21). Smoking and history of
chronic urinary tract disease increased the risk association of the
G/G genotype of MDM2-SNP309 with bladder cancer, whereas the T/G
genotype of MDM2-SNP309 may increase the risk following
stratification by history of urinary tract disease and drinking
alcohol, but not smoking.
In the current study, for p53 Arg72Pro genotypes,
each genotype individually was not associated with the risk of
bladder cancer in Mongolian populations. These results were similar
to the results of studies conducted among Japanese and north Indian
populations (19,22). Notably, according to a meta-analysis
of 15 publications, p53R72P is associated with the risk of bladder
cancer in Asian but not Caucasian patients (9). However, the north Indian population was
categorized as Caucasian in this previous meta-analysis (11). Furthermore, patients with the P/P
genotype of p53R72P increased the risk (OR=3.02; 95% CI=1.42–6.40)
of the development of bladder cancer in Bangladeshi populations
(23). Another meta-analysis
reported that, in Caucasians, the wild-type (R/R) genotype of
p53R72P increases the risk (OR=1.64; 95% CI=1.18–2.28), but the
heterozygous (R/P) genotype reduces the risk (OR=0.62; 95%
CI=0.44–0.99) of bladder cancer (24).
In the current study, when the genotypes of p53R72P
were stratified by the confounding factors, only the R/P genotype
increased the risk of bladder cancer in patients drinking alcohol.
Furthermore, the combination of the R/R genotype of p53R72P with
the G/G genotype of MDM2-SNP309 was associated with increased risk
of bladder cancer in the present study (Table V). It is speculated that the G/G
genotype of MDM2-SNP309 may suppress the level of wild-type (R/R)
p53 and increase the risk of cancer development accordingly. The
present results found no association between these two SNPs and
early age onset of bladder cancer. However, the sample size was too
small to determine the interaction between these two SNPs.
The current study has several limitations that may
have influenced the results. A major potential limitation of the
study was the small patient sample size; the study involved 79
controls and 63 patients with bladder cancer who participated
fully, although 129 controls and 141 patients were initially
enrolled in the research. This may be due to the population
density, large geographical area and lack of specialized cancer
registry system. However, the number of volunteers in the present
study may be standard compared with similar studies in regards to
population (16,17,19,22). For
Mongolians, lifestyle and diet (primarily red meat and dairy) are
not the critical risk factors for bladder cancer development in the
present study. Regarding sex, males were 3.5 times more affected by
bladder cancer than females in the current study. This result is
similar to a previous study in that the incidence, staging and
prognosis of bladder cancer was associated with sex (25).
In conclusion, the current data suggest no
significant association between MDM2-SNP309 and p53R72P and the
risk of bladder cancer in Mongolian populations. Patients who smoke
or have urinary tract disease have an enhanced bladder cancer risk
effect associated with MDM2-SNP309 but not p53R72P. The present
study failed to demonstrate any association between early age at
onset of MDM2-SNP309 and p53R72P and the development of bladder
cancer. As these statistical results were based on small sample
sizes of cases and controls, enlargement of the volunteer pool may
be important to further elucidate the effect of MDM-SNP309 and
p53R72P on the risk of Mongolian bladder cancer.
Acknowledgements
The present study was supported by The National
Science Council (now Minister of Science and Technology), Taiwan
(NSC 101-2923-B-010-001-MOST 105-2628-B-010-013-MY3) and The
Mongolian Foundation for Science and Technology (NSC-MECS2012004).
The authors would also like to thank Miss Odgerel, Miss B.
Chuluuntsetseg, Miss D. Odgerel, Miss Chun-Yuan Chang and Miss
Hsian-Wen Lins' for technical support.
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandagdorj T, Sanjaajamts E, Tudev U,
Oyunchimeg D, Ochir C and Roder D: Cancer incidence and mortality
in Mongolia-national registry data. Asian Pac J Cancer Prev.
11:1509–1514. 2010.PubMed/NCBI
|
|
3
|
Bolortuyaa B, Bayarmaa E and Galtsog L:
Bladder cancer is diagnosed by pathology. Mongolian Med Sci.
2:12–15. 2010.
|
|
4
|
La Vecchia C and Airoldi L: Human bladder
cancer: Epidemiological, pathological and mechanistic aspects. IARC
Sci Publ. 1–157. 1999.
|
|
5
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bond GL and Levine AJ: A single nucleotide
polymorphism in the p53 pathway interacts with gender,
environmental stresses and tumor genetics to influence cancer in
humans. Oncogene. 26:1317–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hrstka R, Coates PJ and Vojtesek B:
Polymorphisms in p53 and the p53 pathway: Roles in cancer
susceptibility and response to treatment. J Cell Mol Med.
13:440–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pietsch EC, Humbey O and Murphy ME:
Polymorphisms in the p53 pathway. Oncogene. 25:1602–1611. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas M, Kalita A, Labrecque S, Pim D,
Banks L and Matlashewski G: Two polymorphic variants of wild-type
p53 differ biochemically and biologically. Mol Cell Biol.
19:1092–1100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu T, Xu ZC, Zou Q, Yu B and Huang XE: P53
Arg72Pro polymorphism and bladder cancer risk-meta-analysis
evidence for a link in Asians but not Caucasians. Asian Pac J
Cancer Prev. 13:2349–2354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Nie S, Zhu H, Wu X, Jia S, Luo Y
and Tang W: Association of p53 Arg72Pro polymorphism with bladder
cancer: A meta-analysis. Gene. 512:408–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al: A
single nucleotide polymorphism in the MDM2 promoter attenuates the
p53 tumor suppressor pathway and accelerates tumor formation in
humans. Cell. 119:591–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freedman DA and Levine AJ: Regulation of
the p53 protein by the MDM2 oncoprotein-thirty-eighth G.H.A. Clowes
Memorial Award Lecture. Cancer Res. 59:1–7. 1999.PubMed/NCBI
|
|
15
|
Knappskog S and Lønning PE: Effects of the
MDM2 promoter SNP285 and SNP309 on Sp1 transcription factor binding
and cancer risk. Transcription. 2:207–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hitzenbichler F, Stoehr CG, Rogenhofer M,
Wieland WF, Ruemmele P, Hartmann A and Stoehr R: Mdm2 SNP309
G-variant is associated with invasive growth of human urinary
bladder cancer. Pathobiology. 81:53–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Onat OE, Tez M, Ozçelik T and Törüner GA:
MDM2 T309G polymorphism is associated with bladder cancer.
Anticancer Res. 26:3473–3475. 2006.PubMed/NCBI
|
|
18
|
Sanchez-Carbayo M, Socci ND, Kirchoff T,
Erill N, Offit K, Bochner BH and Cordon-Cardo C: A polymorphism in
HDM2 (SNP309) associates with early onset in superficial tumors,
TP53 mutations, and poor outcome in invasive bladder cancer. Clin
Cancer Res. 13:3215–3220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horikawa Y, Nadaoka J, Saito M, Kumazawa
T, Inoue T, Yuasa T, Tsuchiya N, Nishiyama H, Ogawa O and Habuchi
T: Clinical implications of the MDM2 SNP309 and p53 Arg72Pro
polymorphisms in transitional cell carcinoma of the bladder. Oncol
Rep. 20:49–55. 2008.PubMed/NCBI
|
|
20
|
Leu JD, Wang CY, Tsai HY, Lin IF, Chen RC
and Lee YJ: Involvement of p53 R72P polymorphism in the association
of MDM2-SNP309 with breast cancer. Oncol Rep. 25:1755–1763.
2011.PubMed/NCBI
|
|
21
|
Janković S and Radosavljević V: Risk
factors for bladder cancer. Tumori. 93:4–12. 2007.PubMed/NCBI
|
|
22
|
Srivastava P, Jaiswal PK, Singh V and
Mittal RD: Role of p53 gene polymorphism and bladder cancer
predisposition in northern India. Cancer Biomark. 8:21–28.
2010–2011. View Article : Google Scholar
|
|
23
|
Hosen MB, Salam MA, Islam MF, Hossain A,
Hawlader MZ and Kabir Y: Association of TP53 gene polymorphisms
with susceptibility of bladder cancer in Bangladeshi population.
Tumour Biol. 36:6369–6374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li DB, Wei X, Jiang LH, Wang Y and Xu F:
Meta-analysis of epidemiological studies of association of P53
codon 72 polymorphism with bladder cancer. Genet Mol Res.
9:1599–1605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fajkovic H, Halpern JA, Cha EK, Bahadori
A, Chromecki TF, Karakiewicz PI, Breinl E, Merseburger AS and
Shariat SF: Impact of gender on bladder cancer incidence, staging,
and prognosis. World J Urol. 29:457–463. 2011. View Article : Google Scholar : PubMed/NCBI
|