Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
adult cancers and represents the third most common urological
malignancy in Europe (1). At
diagnosis, one-third of patients present with locally advanced or
metastatic disease, and one-third of patients undergoing
nephrectomy will eventually develop metastasis (2,3).
Previously, immunotherapy agents, such as interleukin-2 and
interferon (IFN)-α, were the only treatments available and achieved
response rates of ~10–22% (4–9). In
recent years, however, the strategy for treating metastatic RCC
(mRCC) has changed from immunotherapy to the administration of
molecular-targeted therapies, such as multitargeted inhibitors of
tyrosine kinases, and mammalian target of rapamycin. Therefore, the
establishment of a tool for predicting the effect of targeted
agents for mRCC is of critical importance.
One of the most well-established classification
systems for patients with mRCC is the Memorial Sloan Kettering
Cancer Center (MSKCC) system reported by Motzer et al in
1999 (10) and modified in 2002
(11). This model was independently
validated by investigators at the Cleveland Clinic and has been
used for the study and interpretation of cytokine and targeted drug
therapies (12). In this era of
molecular-targeted therapy, prognostic factors for mRCC other than
the MSKCC risk classification system have been identified, such as
the serum C-reactive protein (CRP) level (13,14),
metastatic status (15,16) and tumor shrinkage (17). However, there have been few reports
on independent prognostic factors.
To further investigate the association between
clinical parameters and overall survival (OS) in mRCC, a
retrospective analysis of consecutive patients treated with
molecular-targeted therapy at the Kyushu Cancer Center (Fukuoka,
Japan) was performed.
Patients and methods
Patients and survival
A total of 59 patients undergoing molecular-targeted
therapy for mRCC at the Kyushu Cancer Center (Fukuoka, Japan)
between May 2008 and September 2015 were retrospectively
investigated.
Progression-free survival (PFS) was assessed and
defined as the time from the initiation of first-line
molecular-targeted therapy to the day tumor progression was proven
or death occurred. The patients were censored at the date of the
last follow-up. The OS was investigated from the initiation of
first-line molecular-targeted therapy to the time of death as a
result of any cause or censored at the date of the last
follow-up.
Pre- and post-treatment factors
The evaluated pretreatment factors included age,
gender, pre-treatment therapy, histological type, number of
metastatic sites, low Eastern Cooperative Oncology Group
performance status, low hemoglobin levels (men <13.5 g/dl and
women <11.5 g/dl), high serum lactate dehydrogenase levels (LDH;
>1.5-fold the upper limit of normal), high corrected serum
calcium levels (>10 mg/dl), short time from diagnosis to therapy
(<1 year), MSKCC risk classification, and pre-treatment serum
CRP level (normal, <0.3 mg/dl). The post-treatment factors
included best response to first-line treatment, worst adverse event
with first-line treatment, PFS of first-line molecular-targeted
agents and the number of lines of molecular-targeted agents.
Toxicity and response to
treatment
Decisions regarding adverse events were made based
on the Common Terminology Criteria for Adverse Events, version 4.0
(18). Tumor response was evaluated
as the best response according to the Response Evaluation Criteria
In Solid Tumors, version 1.1 (19).
Ethical considerations
All the patients provided their written informed
consent to participate in this study, and the study protocol was
approved by the Ethics Committee of the Kyushu Cancer Center
(Fukuoka, Japan).
Statistical analysis
The statistical analyses were performed using the
JMP Pro software package, version 11.0.0 (SAS Institute, Inc.,
Cary, NC, USA). PFS and OS were determined using the Kaplan-Meier
method, and the log-rank test was used to determine the differences
between the MSKCC risk groups and the PFS of first-line treatment
groups. The significance of the clinicopathological parameters
associated with OS was assessed using the Cox proportional hazards
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The study cohort comprised 59 patients who underwent
molecular-targeted therapy for mRCC, the characteristics of whom
are listed in Table I. Of these 59
patients, 10 were not treated by nephrectomy, but instead underwent
needle biopsies of either the primary or metastatic tumor to
determine the histological subtype. Therefore, all the included
patients were pathologically diagnosed with primary RCC. The
majority of the patients were diagnosed with mRCC of clear cell
histology. According to the MSKCC criteria, the favorable-,
intermediate- and high-risk groups comprised 17 (28.8%), 34 (57.6%)
and 8 (13.6%) patients, respectively.
 | Table I.Patient characteristics (n=59). |
Table I.
Patient characteristics (n=59).
| Characteristics | No. |
|---|
| Age, years |
|
| Median
(range) | 67 (38–82) |
| Gender |
|
| Male | 42 |
|
Female | 17 |
| Histological
type |
|
| Clear
cell renal cell carcinoma | 49 |
| Papillary
renal cell carcinoma | 4 |
| Carcinoma
of the collecting ducts of Bellini | 3 |
| Renal
cell carcinoma, unclassified | 3 |
| Pre-treatment |
|
|
Nephrectomy | 49 |
|
Interferon-α | 9 |
|
Interleukin-2 | 2 |
| Metastatic sites |
|
| Lung | 40 |
| Lymph
node | 20 |
| Bone | 16 |
|
Pancreas | 5 |
|
Liver | 5 |
|
Brain | 4 |
| Adrenal
glands | 4 |
|
Others | 9 |
| No. of metastatic
sites |
|
| 1 | 26 |
| 2 | 25 |
| 3 | 5 |
| ≥4 | 3 |
| ECOG PS |
|
| 0 | 44 |
| 1 | 13 |
| 2 | 1 |
| 3 | 0 |
| 4 | 1 |
| High lactate
dehydrogenase |
|
|
Yes | 5 |
| No | 54 |
| Low serum
hemoglobin |
|
|
Yes | 26 |
| No | 33 |
| High corrected
serum calcium |
|
|
Yes | 6 |
| No | 53 |
| Time from diagnosis
to therapy <1 year |
|
|
Yes | 38 |
| No | 21 |
| MSKCC risk
classification |
|
|
Favorable | 17 |
|
Intermediate | 34 |
|
Poor | 8 |
| High C-reactive
protein |
|
|
Yes | 28 |
| No | 31 |
OS and profile of molecular-targeted
therapy for mRCC
The median OS for all the patients was 23.7 months
[95% confidence interval (CI): 17.9–30 months; Fig. 1 and Table
II], and the median duration of first-line treatment was 5.1
months (95% CI: 2.1–8.1 months). A total of 44 patients (74.6%)
were treated with sunitinib as first-line treatment. Regarding the
response to first-line treatment, 13 patients (22.1%) achieved
objective tumor remission (complete or partial response), 32
patients (54.2%) had stable disease, and 14 patients (23.7%) had
progressive disease. Regarding the number of lines of
molecular-targeted agents, 22 patients (37.3%) received 1, 15
(25.4%) received 2, and 22 (37.3%) received ≥3 lines of
treatment.
 | Table II.Overall survival and profile of
molecular-targeted therapy for mRCC (n=59). |
Table II.
Overall survival and profile of
molecular-targeted therapy for mRCC (n=59).
| Variables | No. |
|---|
| Overall survival,
months |
|
| Median
(range) | 23.7
(1.2–70.1) |
| Duration of
first-line treatment, months |
|
| Median
(range) | 5.1 (0.2–55.2) |
| First-line
treatment |
|
|
Sunitinib | 44 |
|
Sorafenib | 10 |
|
Axitinib | 2 |
|
Temsirolimus | 2 |
|
Pazopanib | 1 |
|
Everolimus | 0 |
| Best response to
first-line treatment |
|
| CR or
PR | 13 |
| SD | 32 |
| PD | 14 |
| Adverse event to
first-line treatment |
|
| Grade
<3 | 21 |
| Grade
≥3 | 28 |
| No. of lines of
molecular-targeted agents |
|
| 1 | 22 |
| 2 | 15 |
| 3 | 11 |
| 4 | 8 |
| 5 | 1 |
| 6 | 2 |
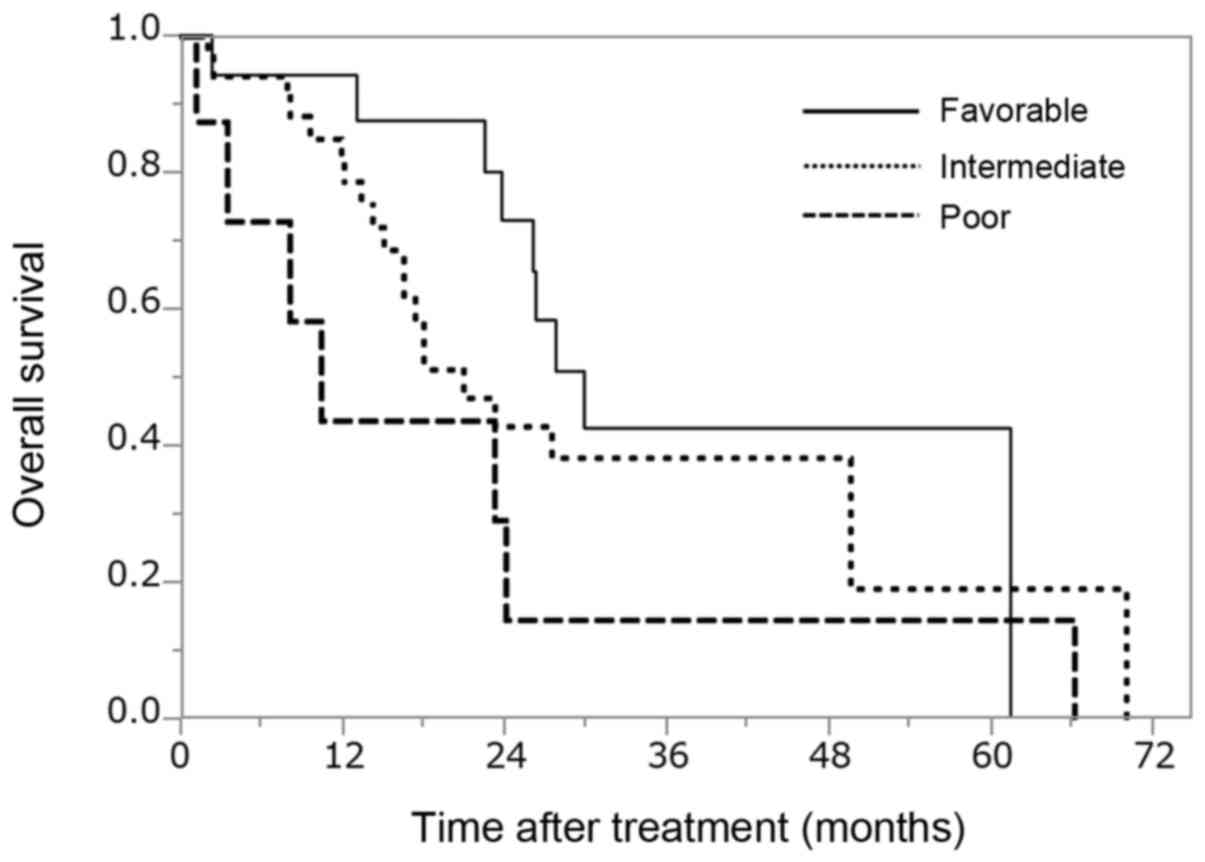
OS for all patients with mRCC
stratified using the MSKCC risk classification
The median OS stratified by MSKCC risk
classification was 28.5, 20.9 and 8.1 months for the favorable-,
intermediate- and poor-risk groups, respectively (Fig. 2, P=0.137; degree of freedom: 2). No
significant difference in the OS was observed between the
favorable- and intermediate-risk (P=0.271), the favorable- and
poor-risk (P=0.066), or the intermediate- and poor-risk groups
(P=0.143).
Univariate and multivariate analyses
of the association between various factors and OS
To identify the prognostic factors associated with
OS, univariate and multivariate analyses were performed using the
Cox proportional hazards model (Table
III). Univariate analyses for various factors identified prior
nephrectomy, number of metastatic sites, anemia, best response to
first-line treatment and PFS with first-line treatment as
prognostic variables. Multivariate analyses identified the number
of metastatic sites (2: HR=3.351, 95% CI: 1.460–8.201, P=0.004; ≥3:
HR=6.397, 95% CI: 1.939–20.209, P=0.003), time from diagnosis to
therapy (≥1 year: HR=0.334, 95% CI: 0.137–0.755, P=0.008), PFS with
first-line treatment (≥5.1 months: HR=0.353, 95% CI: 0.156–0.766,
P=0.008) and number of lines of molecular-targeted agents (≥3:
HR=0.248, 95% CI: 0.091–0.664, P=0.006) as independent prognostic
factors.
 | Table III.Univariate and multivariate analyses
of the association between various factors and overall
survival. |
Table III.
Univariate and multivariate analyses
of the association between various factors and overall
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factors | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
<67 | 1 |
|
|
|
|
≥67 | 0.726
(0.365–1.432) | 0.354 |
|
|
| Sex |
|
|
|
|
|
Men | 1 |
|
|
|
|
Women | 0.610
(0.257–1.300) | 0.208 |
|
|
| Prior
nephrectomy |
|
|
|
|
|
Yes | 1 |
| 1 |
|
| No | 2.951
(1.160–6.611) | 0.025 | 1.671
(0.573–4.569) | 0.335 |
| Histological
type |
|
|
|
|
| Clear
cell | 1 |
|
|
|
|
Non-clear cell | 1.354
(0.566–2.894) | 0.472 |
|
|
| No. of metastatic
sites |
|
|
|
|
| 1 | 1 |
| 1 |
|
| 2 | 2.752
(1.256–6.475) | 0.011 | 3.351
(1.460–8.201) | 0.004 |
| ≥3 | 4.603
(1.526–12.966) | 0.008 | 6.397
(1.939–20.209) | 0.003 |
| ECOG performance
status |
|
|
|
|
| 0 | 1 |
|
|
|
| ≥1 | 1.795
(0.815–3.674) | 0.139 |
|
|
| Anemia |
|
|
|
|
| No | 1 |
| 1 |
|
|
Yes | 2.066
(1.043–4.160) | 0.037 | 1.152
(0.479–2.963) | 0.761 |
| Elevated serum
lactate dehydrogenase |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 1.321
(0.293–4.072) | 0.679 |
|
|
| High corrected
serum calcium |
|
|
|
|
| No | 1 |
|
|
|
|
Yes | 1.563
(0.462–3.997) | 0.431 |
|
|
| Time from diagnosis
to therapy, years |
|
|
|
|
|
<1 | 1 |
| 1 |
|
| ≥1 | 0.545
(0.248–1.113) | 0.097 | 0.334
(0.137–0.755) | 0.008 |
| Pre-treatment
C-reactive protein level, mg/dl |
|
|
|
|
|
<0.3 | 1 |
|
|
|
|
≥0.3 | 1.631
(0.838–3.217) | 0.149 |
|
|
| Best response to
first-line treatment |
|
|
|
|
|
Progressive disease | 1 |
| 1 |
|
| Stable
disease | 0.391
(0.179–0.867) | 0.022 | 0.589
(0.249–1.396) | 0.226 |
|
Complete or partial
response | 0.211
(0.074–0.553) | 0.002 | 0.536
(0.162–1.666) | 0.285 |
| Worst adverse event
to first-line treatment |
|
|
|
|
| Grade
<3 | 1 |
|
|
|
| Grade
≥3 | 0.792
(0.401–1.626) | 0.514 |
|
|
| PFS with first-line
treatment, months |
|
|
|
|
|
<5.1 | 1 |
| 1 |
|
|
≥5.1 | 0.479
(0.237–0.954) | 0.036 | 0.353
(0.156–0.766) | 0.008 |
| No. of lines of
molecular-targeted agents |
|
|
|
|
| 1 | 1 |
| 1 |
|
| 2 | 1.066
(0.453–2.511) | 0.882 | 0.977
(0.397–2.398) | 0.959 |
| ≥3 | 0.437
(0.186–1.031) | 0.059 | 0.248
(0.091–0.664) | 0.006 |
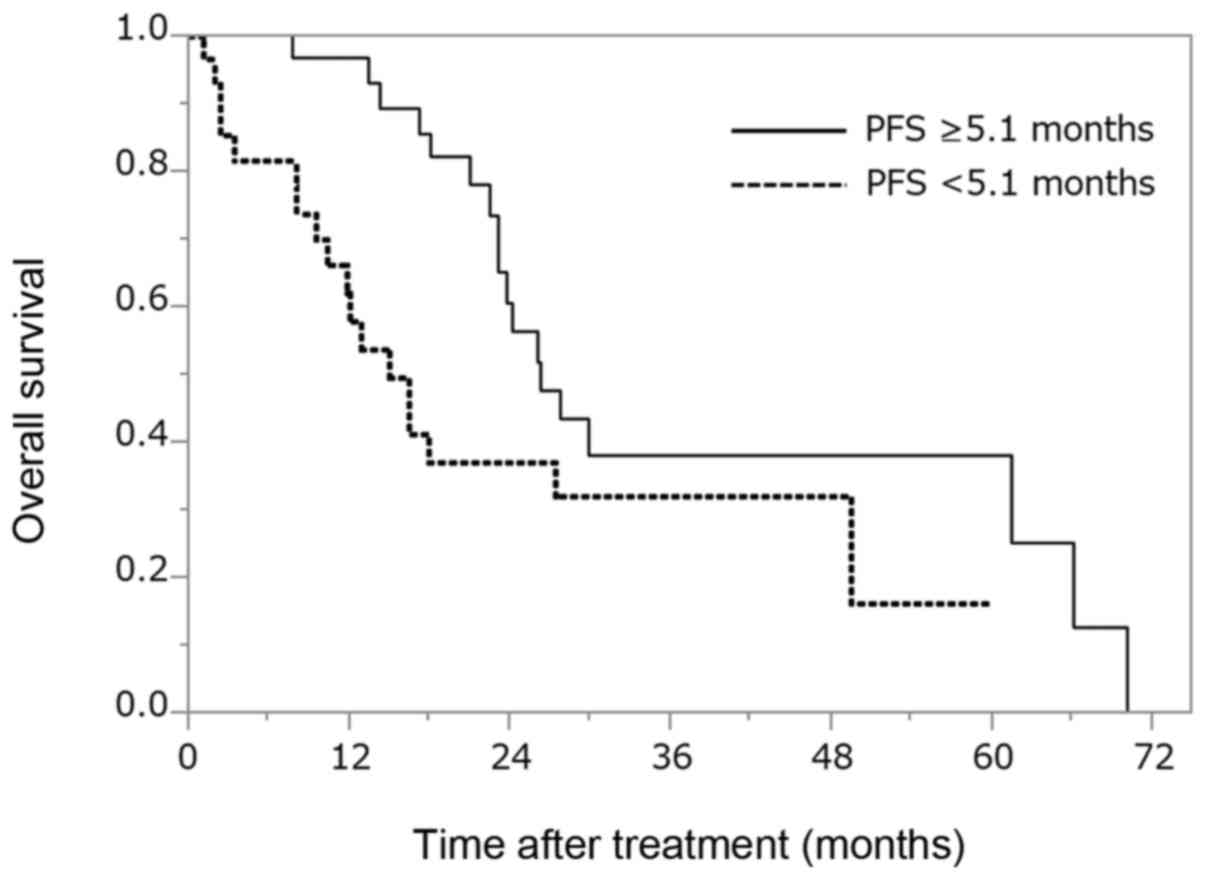
OS for all patients with mRCC
according to PFS with first-line treatment
First-line PFS was analyzed to determine its
association with OS (Fig. 3).
Patients with PFS ≥5.1 months had a significantly longer OS (26.3
months) compared with those with PFS <5.1 months (OS: 15.1
months) (P=0.032; degree of freedom: 1).
Discussion
Molecular-targeted therapy has markedly changed the
treatment strategy for mRCC, and several recent studies have
investigated the clinical prognostic factors. At present, the most
widely used system is the MSKCC classification, which may
facilitate prognostic individualization in mRCC patients who
received systemic therapy (11).
However, despite being validated in the era of molecular-targeted
therapy (20), this model was
developed based on data from patients treated with IFN-α in a
clinical trial. Therefore, there is a need for a system that allows
for a more precise assessment of the prognostic risk in patients
with mRCC receiving molecular-targeted therapy, since such a tool
would be useful for counseling patients, evaluating therapeutic
options and planning treatment. However, only few reports have
identified any independent prognostic factors. Therefore, the aim
of the present study was to retrospectively investigate the
prognostic factors for patients with mRCC treated with
molecular-targeted agents.
The current estimates for the median OS in patients
with mRCC range from 22.9 to 26.4 months (21,22). The
OS of 23.7 months in the present study falls within this range
(Fig. 1), indicating that
molecular-targeted agents have been administered appropriately at
our institution. The median OS stratified by the MSKCC risk
classification was 28.5, 20.9 and 8.1 months for the favorable-,
intermediate- and poor-risk groups, respectively (P=0.137; degree
of freedom: 2). While the OS of the favorable-risk group was longer
compared with that of the intermediate- and poor-risk groups, no
significant difference in the OS was found between the favorable-
and intermediate-risk (P=0.2713), the favorable- and poor-risk
(P=0.0664), or the intermediate- and poor-risk groups (P=0.1426).
Therefore, univariate and multivariate analyses were
retrospectively performed using the Cox proportional hazards model
in consecutive patients treated with molecular-targeted therapy, to
determine the association between the OS and clinical parameters,
including five risk factors of the MSKCC risk classification
(Table III). Univariate analyses
for various factors identified prior nephrectomy, number of
metastatic sites, anemia, best response to first-line treatment and
PFS with first-line treatment as prognostic variables. Furthermore,
multivariate analyses identified the following as independent
prognostic factors: Number of metastatic sites (2: HR=3.351, 95%
CI: 1.460–8.201, P=0.004; ≥3: HR=6.397, 95% CI: 1.939–20.209,
P=0.003) and time from diagnosis to therapy (≥1 year: HR=0.334, 95%
CI: 0.137–0.755, P=0.008) as pre-treatment factors, and PFS with
first-line treatment (≥5.1 months: HR=0.353, 95% CI: 0.156–0.766,
P=0.008) and number of lines of molecular-targeted agents (≥3:
HR=0.248, 95% CI: 0.091–0.664, P=0.006) as post-treatment
factors.
The pre-treatment factor ‘time from diagnosis to
therapy’ is included in the MSKCC system, and subsequent studies
conducted to validate the prognostic factors for RCC have evaluated
the time from diagnosis to the initiation of systemic therapy
(23–25). However, a survival analysis
stratified by the number of disease sites is not often performed in
clinical trials, although it may represent an additional prognostic
factor of outcome. Grassi et al reported that the presence
of >2 disease sites was associated with a statistically
significantly shortened PFS and OS (26). The number of metastatic sites may
thus be a surrogate for the tumor burden, which may be easily
evaluated, although it does not include the spread of
metastases.
Currently, the most widely used prognostic factor
model is based on the MSKCC (11).
Adverse prognostic factors in a multivariable analysis included a
low Karnofsky performance status (<80%), high LDH (>1.5 times
the upper limit of normal), low serum hemoglobin levels, high
corrected serum calcium levels (>10 mg/dl), and a time from
initial diagnosis to treatment of <1 year. Based on these five
risk factors, each patient was assigned to one of three risk
groups: Favorable risk (0 risk factors), intermediate risk (1–2
risk factors) and poor risk (≥3 risk factors). This means that the
MSKCC risk is classified based only on the number of factors
present, not each risk factor or any combination. Therefore, the
breadth of cases included, particularly in the intermediate- and
poor-risk groups, is wide, and variations naturally exist among
patients, even within each risk group. As such, urological
oncologists recognize that even patients in the same risk group may
not achieve the same results. From this standpoint, it may be
argued that post-treatment as well as pre-treatment variables are
important as prognostic factors.
In the present study, a significant difference was
observed, not in the best response to first-line treatment, but in
PFS with first-line treatment (≥5.1 months: HR=0.353, 95% CI:
0.156–0.766, P=0.008) in the multivariate analysis, and these
results were naturally obtained after molecular-targeted agent
administration. Recent analyses have demonstrated that patients
with insufficient response to first-line treatment have a dismal
prognosis (27,28). Siedal et al suggested that
early tumor shrinkage is a prognostic tool, and superior tumor
shrinkage is associated with a favorable prognosis (17). The patients in that analysis were
stratified into five groups according to the change in the tumor
size at the first-treatment evaluation, whereas the present study
stratified patients into three groups according to the best
response to first-line treatment. However, the best response to
first-line treatment was not found to be an independent prognostic
factor in the present study. This result suggested that the
clinical response rate may not reflect the survival time. In
another study, Seidal et al suggested that a PFS with
first-line treatment of >6 months was an independent prognostic
marker (29). Heng et al also
mentioned that a PFS of 6 months under first-line vascular
endothelial growth factor-targeted treatment was applied as a
cut-off marker and proved capable of significantly differentiating
patients with favorable and poor prognosis (30). These descriptions are consistent with
our observations in the present study, although the duration of PFS
with the first-line treatment was slightly different in the
previous study. The longer PFS with first-line treatment may thus
be associated with the better prognosis observed in patients with
mRCC. These results appear to be important, not only for urological
oncologists, but also for patients in clinical practice; thus, even
patients in the same risk group may achieve a different OS compared
with what was expected prior to treatment administration. Given
that urological oncologists naturally interact with patients from
pre-treatment to post-treatment, they have several opportunities to
explain to their patients their condition and prognosis. Urological
oncologists may therefore be able to discuss a patient's prognosis
with greater specificity after treatment administration. This
information may greatly help patients make important decisions.
In addition, multivariate analyses also identified
the number of lines of molecular-targeted agents (≥3: HR=0.243, 95%
CI: 0.089–0.654, P=0.005) to be an independent prognostic factor.
Ko et al demonstrated that patients with mRCC who were able
to receive more lines of molecular-targeted therapy lived longer,
with longer PFS (31). These results
suggest that sequential therapy with molecular-targeted agents may
prolong the survival of patients with mRCC.
Multiple candidate predictive and prognostic
biomarkers have been evaluated (32–38).
However, the association between the OS and these biomarkers was
not examined in these previous studies. At present, no available
biomarkers are superior to clinical parameters, such as those used
for the MSKCC score.
The limitations of such an analysis are its
retrospective nature and the small number of patients enrolled.
However, our experience from everyday clinical practice has
highlighted the potential use of such information on the prognostic
role of PFS with first-line treatment with molecular-targeted
therapy for mRCC. The results of the present study indicate that
the PFS of first-line treatment may be a meaningful intermediate
endpoint for OS in mRCC patients treated with molecular-targeted
therapy.
References
|
1
|
Herrmann E, Bierer S and Wülfing C: Update
on systemic therapies of metastatic renal cell carcinoma. World J
Urol. 28:303–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Athar U and Gentile TC: Treatment options
for metastatic renal cell carcinoma: A review. Can J Urol.
15:3954–3966. 2008.PubMed/NCBI
|
|
3
|
Lam JS, Leppert JT, Belldegrun AS and
Figlin RA: Novel approaches in the therapy of metastatic renal cell
carcinoma. World J Urol. 23:202–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McDermott DF, Regan MM, Clark JI, Flaherty
LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff
MS, et al: Randomized phase III trial of high-dose interleukin-2
versus subcutaneous interleukin-2 and interferon in patients with
metastatic renal cell carcinoma. J Clin Oncol. 23:133–141. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JC, Sherry RM, Steinberg SM, Topalian
SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton
KE, White DE, et al: Randomized study of high-dose and low-dose
interleukin-2 in patients with metastatic renal cancer. J Clin
Oncol. 21:3127–3132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Negrier S, Escudier B, Lasset C, Douillard
JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau
M, et al: Recombinant human interleukin-2, recombinant human
interferon alfa-2a, or both in metastatic renal-cell carcinoma.
Groupe Français d'Immunothérapie. N Engl J Med. 338:1272–1278.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Negrier S, Perol D, Ravaud A, Chevreau C,
Bay JO, Delva R, Sevin E, Caty A and Escudier B: French
Immunotherapy Intergroup: Medroxyprogesterone, interferon alfa-2a,
interleukin 2, or combination of both cytokines in patients with
metastatic renal carcinoma of intermediate prognosis: Results of a
randomized controlled trial. Cancer. 110:2468–2477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coppin C, Porzsolt F, Awa A, Kumpf J,
Coldman A and Wilt T: Immunotherapy for advanced renal cell cancer.
Cochrane Database Syst Rev. CD0014252005.PubMed/NCBI
|
|
9
|
Coppin C, Le L, Porzsolt F and Wilt T:
Targeted therapy for advanced renal cell carcinoma. Cochrane
Database Syst Rev: CD006017. 2008. View Article : Google Scholar
|
|
10
|
Motzer RJ, Mazumdar M, Bacik J, Berg W,
Amsterdam A and Ferrara J: Survival and prognostic stratification
of 670 patients with advanced renal cell carcinoma. J Clin Oncol.
17:2530–2540. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mekhail TM, Abou-Jawde RM, Boumerhi G,
Malhi S, Wood L, Elson P and Bukowski R: Validation and extension
of the Memorial Sloan-Kettering prognostic factors model for
survival in patients with previously untreated metastatic renal
cell carcinoma. J Clin Oncol. 23:832–841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teishima J, Kobatake K, Hayashi T, Seno Y,
Ikeda K, Nagamatsu H, Hieda K, Shoji K, Miyamoto K, Inoue S, et al:
Prognostic significance of C-reactive protein in patients with
intermediate-risk metastatic renal cell carcinoma treated with
molecular targeted therapy. Oncol Lett. 8:881–885. 2014.PubMed/NCBI
|
|
14
|
Yasuda Y, Saito K, Yuasa T, Kitsukawa S,
Urakami S, Yamamoto S, Yonese J, Takahashi S and Fukui I:
Prognostic impact of pretreatment C-reactive protein for patients
with metastatic renal cell carcinoma treated with tyrosine kinase
inhibitors. Int J Clin Oncol. 18:884–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patil S, Figlin RA, Hutson TE, Michaelson
MD, Négrier S, Kim ST, Huang X and Motzer RJ: Prognostic factors
for progression-free and overall survival with sunitinib targeted
therapy and with cytokine as first-line therapy in patients with
metastatic renal cell carcinoma. Ann Oncol. 22:295–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beuselinck B, Oudard S, Rixe O, Wolter P,
Blesius A, Ayllon J, Elaidi R, Schöffski P, Barrascout E, Morel A,
et al: Negative impact of bone metastasis on outcome in clear-cell
renal cell carcinoma treated with sunitinib. Ann Oncol. 22:794–800.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seidel C, Busch J, Weikert S, Steffens S,
Bokemeyer C and Grünwald V: Tumour shrinkage measured with first
treatment evaluation under VEGF-targeted therapy as prognostic
marker in metastatic renal cell carcinoma (mRCC). Br J Cancer.
109:2998–3004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon WA, Cho IC, Yu A, Nam BH, Joung JY,
Seo HK, Lee KH and Chung J: Validation of the MSKCC and Heng risk
criteria models for predicting survival in patients with metastatic
renal cell carcinoma treated with sunitinib. Ann Surg Oncol.
20:4397–4404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choueiri TK, Garcia JA, Elson P, Khasawneh
M, Usman S, Golshayan AR, Baz RC, Wood L, Rini BI and Bukowski RM:
Clinical factors associated with outcome in patients with
metastatic clear-cell renal cell carcinoma treated with vascular
endothelial growth factor-targeted therapy. Cancer. 110:543–550.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manola J, Royston P, Elson P, McCormack
JB, Mazumdar M, Négrier S, Escudier B, Eisen T, Dutcher J, Atkins
M, et al: Prognostic model for survival in patients with metastatic
renal cell carcinoma: Results from the international kidney cancer
working group. Clin Cancer Res. 17:5443–5450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grassi P, Verzoni E, Porcu L, Testa I,
Iacovelli R, Torri V, Braud Fd and Procopio G: Targeted therapies
in advanced renal cell carcinoma: The role of metastatic sites as a
prognostic factor. Future Oncol. 10:1361–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Busch J, Seidel C, Weikert S, Wolff I,
Kempkensteffen C, Weinkauf L, Hinz S, Magheli A, Miller K and
Grünwald V: Intrinsic resistance to tyrosine kinase inhibitors is
associated with poor clinical outcome in metastatic renal cell
carcinoma. BMC Cancer. 11:2952011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heng DY, Mackenzie MJ, Vaishampayan UN,
Bjarnason GA, Knox JJ, Tan MH, Wood L, Wang Y, Kollmannsberger C,
North S, et al: Primary anti-vascular endothelial growth factor
(VEGF)-refractory metastatic renal cell carcinoma: Clinical
characteristics, risk factors, and subsequent therapy. Ann Oncol.
23:1549–1555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seidel C, Busch J, Weikert S, Steffens S,
Fenner M, Ganser A and Grünwald V: Progression free survival of
first line vascular endothelial growth factor-targeted therapy is
an important prognostic parameter in patients with metastatic renal
cell carcinoma. Eur J Cancer. 48:1023–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heng DY, Xie W, Bjarnason GA, Vaishampayan
U, Tan MH, Knox J, Donskov F, Wood L, Kollmannsberger C, Rini BI
and Choueiri TK: Progression-free survival as a predictor of
overall survival in metastatic renal cell carcinoma treated with
contemporary targeted therapy. Cancer. 117:2637–2642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ko JJ, Choueiri TK, Rini BI, Lee JL,
Kroeger N, Srinivas S, Harshman LC, Knox JJ, Bjarnason GA,
MacKenzie MJ, et al: First-, second-, third-line therapy for mRCC:
Benchmarks for trial design from the IMDC. Br J Cancer.
110:1917–1922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peña C, Lathia C, Shan M, Escudier B and
Bukowski RM: Biomarkers predicting outcome in patients with
advanced renal cell carcinoma: Results from sorafenib phase III
treatment approaches in renal cancer global evaluation trial. Clin
Cancer Res. 16:4853–4863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zurita AJ, Jonasch E, Wang X, Khajavi M,
Yan S, Du DZ, Xu L, Herynk MH, McKee KS, Tran HT, et al: A cytokine
and angiogenic factor (CAF) analysis in plasma for selection of
sorafenib therapy in patients with metastatic renal cell carcinoma.
Ann Oncol. 23:46–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tran HT, Liu Y, Zurita AJ, Lin Y,
Baker-Neblett KL, Martin AM, Figlin RA, Hutson TE, Sternberg CN,
Amado RG, et al: Prognostic or predictive plasma cytokines and
angiogenic factors for patients treated with pazopanib for
metastatic renal-cell cancer: A retrospective analysis of phase 2
and phase 3 trials. Lancet Oncol. 13:827–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hegde PS, Jubb AM, Chen D, Li NF, Meng YG,
Bernaards C, Elliott R, Scherer SJ and Chen DS: Predictive impact
of circulating vascular endothelial growth factor in four phase III
trials evaluating bevacizumab. Clin Cancer Res. 19:929–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsavachidou-Fenner D, Tannir N, Tamboli P,
Liu W, Petillo D, Teh B, Mills GB and Jonasch E: Gene and protein
expression markers of response to combined antiangiogenic and
epidermal growth factor targeted therapy in renal cell carcinoma.
Ann Oncol. 21:1599–1606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muriel L, ópez C, Esteban E, Astudillo A,
Pardo P, Berros JP, Izquierdo M, Crespo G, Fonseca PJ, Sanmamed M
and Martínez-Camblor P: Predictive factors for response to
treatment in patients with advanced renal cell carcinoma. Invest
New Drugs. 30:2443–2449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jonasch E, Corn P, Pagliaro LC, Warneke
CL, Johnson MM, Tamboli P, Ng C, Aparicio A, Ashe RG, Wright JJ and
Tannir NM: Upfront, randomized, phase 2 trial of sorafenib versus
sorafenib and low-dose interferon alfa in patients with advanced
renal cell carcinoma: Clinical and biomarker analysis. Cancer.
116:57–65. 2010.PubMed/NCBI
|