Introduction
Tegafur-gimeracil-oteracil potassium (S-1) adjuvant
chemotherapy is administered for stage II/III gastric cancer based
on the results of the Adjuvant Chemotherapy Trial of TS-1 for
Gastric Cancer (ACTS-GC) (1). In the
stratified survival analysis of the ACTS-GC trial, S-1
administration for 1 year at >70% of the planned dose resulted
in improved survival, demonstrating the effectiveness of this
treatment in patients with gastric cancer (1). Therefore, the dosage and duration of
S-1 treatment may influence patient prognosis, suggesting that
medication adherence and continuity of treatment are crucial.
However, S-1 continuation is sometimes challenging due to adverse
events (AEs) such as leukopenia, neutropenia, anorexia, nausea,
vomiting, diarrhea and stomatitis (2–4). In
particular, AEs such as malaise and anorexia may reduce patient
quality of life (QOL), which may significantly reduce medication
adherence, leading to subsequent medication rejection (4).
S-1 adjuvant chemotherapy for gastric cancer is
currently used as an outpatient treatment (5). Outpatients manage their own medications
and any associated AEs (5,6). Therefore, it is necessary to establish
a support system for cancer outpatients to provide safe and secure
drug therapy.
Patients with gastric cancer with serum albumin
levels <3.5 g/dl and creatinine clearance <80 ml/min
typically require a reduced dose or discontinuation of S-1 adjuvant
chemotherapy (3). In addition,
patients who discontinued S-1 or required dosage reductions due to
AEs exhibited weight loss, which was due to decreased postoperative
oral intake of food (3). Kawabata
et al (7) reported that
decreases in body mass index (BMI) during treatment may affect
treatment continuity. Therefore, decreased BMI is a useful
indicator for designing timely and appropriate patient guidance and
prescription design support systems during S-1 adjuvant
chemotherapy.
For cancer chemotherapy, hospital pharmacists in
medical teams are part of a pharmacist outpatient service, and such
services have improved the quality of medical care and patient QOL
(8–15). However, few facilities have offered
pharmacist outpatient services to patients undergoing monotherapy
with oral anti-cancer agents prior to examination by a physician.
Therefore, reports on the usefulness of these services are limited
(10,14).
A pharmacist outpatient service (pharmacist
interviews before medical examinations) for patients undergoing S-1
adjuvant chemotherapy was launched at Ogaki Municipal Hospital
(Ogaki-shi, Japan) in November 2014. In the present study, the
effect of this service on the persistence rate of S-1 adjuvant
chemotherapy over the course of a year was assessed.
Patients and methods
Study population
Between September 2012 and March 2016, the records
of patients undergoing S-1 adjuvant chemotherapy for gastric cancer
were evaluated. Initially, 146 subjects were included. The
pharmacist outpatient service group (PG) included 40 subjects who
attended the pharmacist outpatient service for S-1 adjuvant
chemotherapy for gastric cancer between November 2014 and March
2016, whose records were examined retrospectively. As a control
group (CG), the doctor and pharmacy service records of 94 subjects
who received S-1 adjuvant chemotherapy for gastric cancer between
September 2012 and October 2015 were examined retrospectively. For
the CG, pharmacist guidance was not performed. In the present
study, the CG and PG targeted subjects who started and completed
S-1 postoperative adjuvant chemotherapy between the respective
periods, and patients for whom outpatient pharmacists were
introduced partway through their treatment were excluded from the
study. Additionally, those who were transferred to another hospital
during the treatment period were excluded. Oncology pharmacists
were responsible for validation of patients' conditions. Patient
characteristics of the two groups are presented in Table I. Personal information was protected
in the aggregated data. This study received approval from the
Institutional Review Board of Ogaki Municipal Hospital (no.
20150303-5), and all patients provided informed consent.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | CG | PG | P-value |
|---|
| Number of
patients | 94 | 40 |
|
| Age, years |
|
|
|
| Median
(range) | 68 (37–83) | 71 (47–83) | 0.1997 |
| Sex, n |
|
|
|
|
Male:female | 63:31 | 29:11 | 0.3398 |
| Body surface area,
m2 |
|
|
|
|
<1.25 | 7 | 2 | 0.8549 |
|
1.25–1.50 | 44 | 20 |
|
|
≥1.50 | 43 | 18 |
|
| Disease stage (number
of patients) |
|
|
|
| Ib | 1 | 0 | 0.7529 |
| Ia | 15 | 4 |
|
| IIb | 15 | 10 |
|
| IIIa | 18 | 6 |
|
| IIIb | 20 | 9 |
|
| IIIc | 25 | 11 |
|
| CrCl, ml/min |
|
|
|
| Median
(range) | 70.3 (31.2–111) | 78.7 (31.5–114) | 0.2260 |
| BMI |
|
|
|
| Median
(range) | 20.2 (14.1–26.3) | 19.0 (16.0–27.5) | 0.8595 |
| Alb, g/dl |
|
|
|
| Median
(range) | 4.1 (2.9–4.6) | 4.1 (2.9–4.5) | 0.8962 |
Pharmacist outpatient service
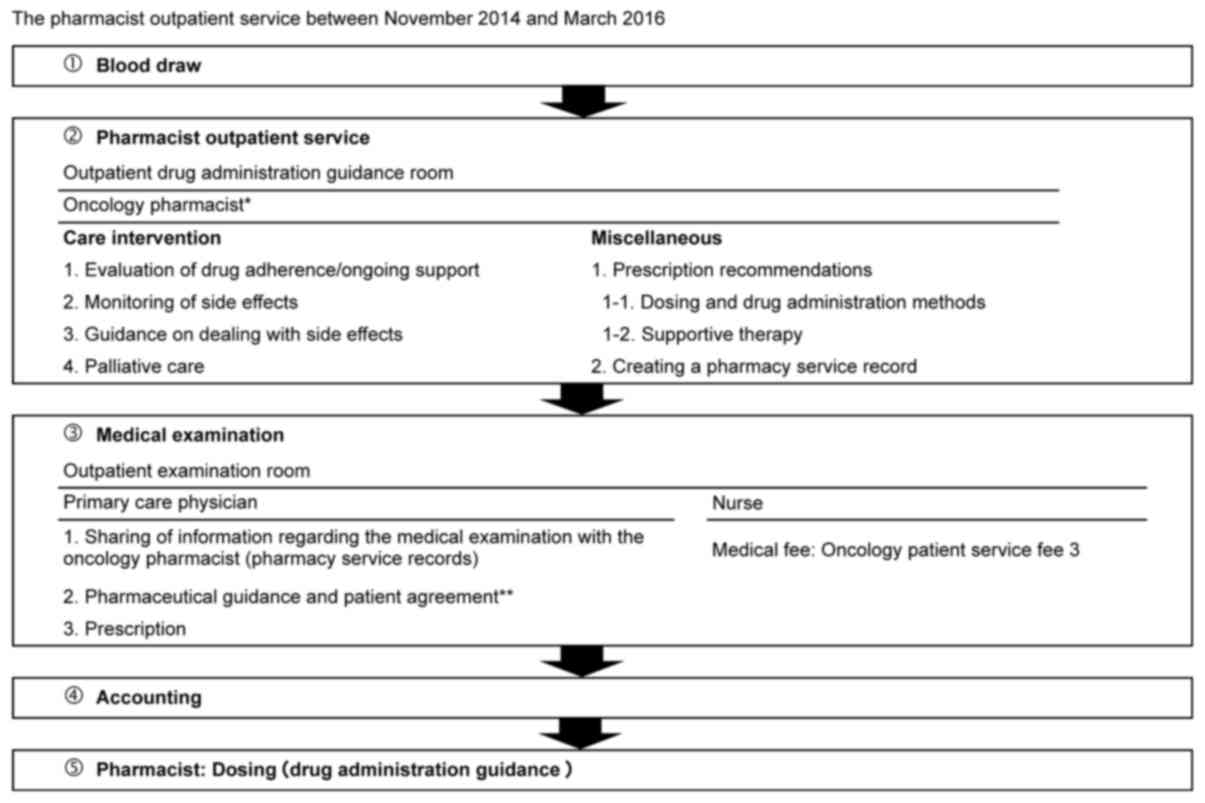
A flowchart of the pharmacist outpatient service is
presented in Fig. 1. The pharmacist
outpatient service was practiced on weekdays between the h of 08:15
and 17:15 between November 2014 and March 2016. On the first visit,
oncology pharmacists, certified by the Japanese Society of
Pharmaceutical Health Care and Sciences, provided guidance to
patients after a treatment plan including the therapeutic agent and
dose was formulated. If treatment plan changes were desired, then
inquiries with the treating physician were required. From the
second visit onwards, following blood collection, pharmacists
interviewed patients in the outpatient drug administration guidance
room. During these interviews, adherence status was ascertained,
and AEs resulting from the S-1 therapy were monitored (16). Additionally, pharmacy service records
were created, prescription recommendations including those related
to dosage and drug administration were issued, and supportive care
(drug therapy for AEs such as nausea and diarrhea) was provided. An
S-1 management table template was used to monitor AEs as described
previously (16).
During medical examinations, physicians utilized the
pharmacy service records (prescription proposal and AE monitoring
results). While at home, patients were able to seek medical advice
from pharmacists via telephone consultation during the pharmacist
outpatient service opening times.
S-1 adjuvant chemotherapy
The minimum S-1 dose prior to attending the
outpatient service was 60 mg/day. The therapy protocol during the
outpatient service was as follows: For 1 cycle of therapy, S-1 was
administered twice daily, after morning and evening meals, for 4
weeks [80 mg/day for <1.25 m2 body surface area
(BSA); 100 mg/day for 1.25–1.5 m2 BSA; and 120 mg/day
for ≥1.5 m2 BSA], followed by a 2-week washout period.
This administration regimen was continued for a year. BSA was
calculated as follows:
BSA (m2)=body weight0.444 (kg)
× height0.663 (cm) ×0.008883
Data collection
Data on prescription recommendations (prescription
dose, administration interval and supportive therapy) were
retrieved from the drug management guidance records of the PG. In
addition, the prescription proposal acceptance rate was calculated.
The persistence rate of S-1 adjuvant chemotherapy over the course
of a year and the reasons for discontinuation due to AEs were
extracted from electronic charts. The AEs were retrospectively
compared between the CG and PG. Data regarding specific AEs were
extracted from the electronic charts and pharmacy service records.
The severity of AEs was classified according to the Common
Terminology Criteria for Adverse Events, version 4.0 (17). For subjects who completed S-1
adjuvant chemotherapy, the relative dose intensities (RDIs) of the
CG and PG were compared. RDI is an index for evaluating the
therapeutic intensity of the actual dose against the standard dose
as follows:
RDI (%)=dose intensity (DI) calculated from the
actual dose/DI of the standard dose ×100.
DI represents the chemotherapy drug dose per unit
time (week) as follows:
DI (mg/m2/week)=total dose
(mg/m2)/time of treatment (week).
Statistical analysis
An F test was performed to compare the PG and CG.
The Student's t or Welch's t-tests were used to analyze patient
characteristics (Table I). A
χ2 test was used to compare rates between the groups
(data depicted in the PG and CG). One-way analysis of variance was
conducted to determine the significance of differences in RDIs
between the groups. All statistical analyses were performed using
JMP 8 software (SAS Institute Inc., Cary, NC, USA). For all
statistical tests, P<0.05 was considered to indicate a
statistically significant difference.
Results
Operational performance of the
pharmacist outpatient service (prescription recommendations)
The patient characteristics are summarized in
Table I. Details of the prescription
recommendations issued by the pharmacist outpatient service as well
as the prescription proposal acceptance rates, between November
2014 and March 2016, are presented in Table II. Supportive therapy was
recommended when there was a need to reduce AEs in order to
continue S-1 treatment. The majority of prescription
recommendations for supportive care included nutritional
supplements, anti-diarrheal, stomatitis remedies and anti-emetics.
Telephone consultations regarding skin disorders and diarrhea were
performed in 44 cases. The prescription proposal acceptance rate
was 92.5% (198/214 cases).
 | Table II.Interventions and prescription
recommendation details. |
Table II.
Interventions and prescription
recommendation details.
| A, Intervention
performance |
|---|
|
|---|
| Factor | Value |
|---|
| Number of
intervention cases | 40 |
| Number of
consultations | 644 |
| Number of
prescription recommendationsa | 214 |
| Number of cases
reflected by these recommendationsb | 198 |
| Prescription
recommendation adoption percentage | 92.5 |
|
| B, Prescription
recommendation details |
|
| Factor | n |
|
| Prescription
dose | 62 |
| Administration
interval (rest days) | 15 |
| Supportive
therapy | 132 |
| Others | 5 |
Persistence rate and reasons for
discontinuation of S-1 adjuvant chemotherapy
The persistence rate of S-1 adjuvant chemotherapy
over the course of a year and the reasons for discontinuation are
presented in Table III. The
persistence rate of S-1 adjuvant chemotherapy in the PG was
significantly higher compared with that in the CG (P<0.0001). In
addition, the rate of discontinuation due to AEs was significantly
lower in the PG compared with the CG (P=0.0015). The specific AEs
resulting in discontinuation of S-1 adjuvant chemotherapy are
listed in Table IV. The most common
reasons for AE-associated discontinuations in the CG were malaise,
anorexia, diarrhea and myelosuppression. The most common reasons
for AE-associated discontinuations in the PG were malaise,
myelosuppression, diarrhea and stomatitis.
 | Table III.Persistence rate of S-1 adjuvant
chemotherapy over the course of a year. |
Table III.
Persistence rate of S-1 adjuvant
chemotherapy over the course of a year.
|
| n (%) |
|
|---|
|
|
|
|
|---|
| Type | CG (n=94) | PG (n=40) | P-value |
|---|
|
Completea | 37 (39.4) | 33 (82.5) |
<0.0001b |
| Recurrence | 24 (25.5) | 4 (10.0) | 0.0322b |
| Adverse events | 30 (31.9) | 3 (7.5) | 0.0015b |
| Others | 3 (3.2) | 0 (0.0) | 0.3418 |
| Complete/(complete
+ adverse events) | 37/67 (55.2) | 33/36 (91.7) |
<0.0001b |
 | Table IV.Adverse events associated with
discontinuations. CG, control group; PG, pharmacist outpatient
clinic group. |
Table IV.
Adverse events associated with
discontinuations. CG, control group; PG, pharmacist outpatient
clinic group.
|
| Number of
instances |
|---|
|
|
|
|---|
| Adverse event | CG (n=30) | PG (n=3) |
|---|
| Malaise | 13 | 1 |
| Anorexia | 8 | 0 |
| Dehydration | 1 | 0 |
| Rash | 3 | 0 |
|
Myelosuppression | 5 | 1 |
| Diarrhea | 6 | 1 |
| Lung infection | 1 | 0 |
| Hyperkalemia | 1 | 0 |
| Stomatitis | 1 | 1 |
| Nausea | 2 | 0 |
| Eye disorders | 2 | 0 |
| Fever | 1 | 0 |
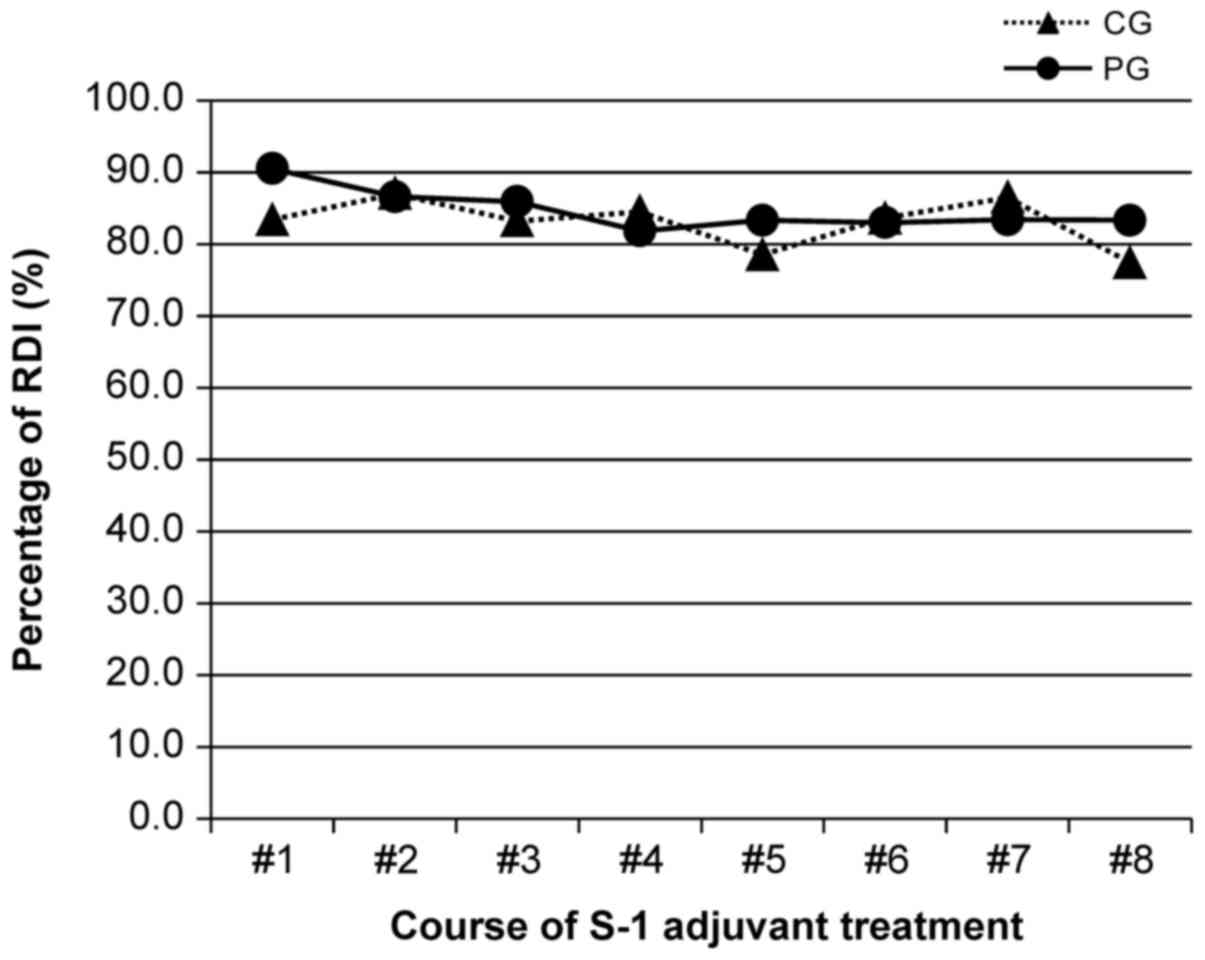
The pattern of RDIs
In subjects who completed S-1 adjuvant chemotherapy,
the RDIs for the CG and PG were not significantly different at 82.9
and 84.7%, respectively (P=0.2942; Fig.
2).
Frequency of AEs
The frequencies of AEs in all patients are presented
in Table V. There was a significant
difference in the incidence of neutropenia, with that in the CG
being greater than that in the PG (P=0.0462).
 | Table V.Adverse events in all patients who
underwent treatment. |
Table V.
Adverse events in all patients who
underwent treatment.
|
| CG (n=94) | PG (n=40) |
|
|---|
|
|
|
|
|
|---|
|
| Grade |
| Grade |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Adverse event | 1 | 2 | 3 | 4 | All grades (%) | 1 | 2 | 3 | 4 | All grades (%) | P-value |
|---|
| Leukopenia | 19 | 16 | 6 | 0 | 41 (43.6) | 11 | 6 | 0 | 0 | 17 (42.5) | 0.5297 |
| Neutropenia | 14 | 20 | 13 | 0 | 47 (50.0) | 1 | 10 | 2 | 0 | 13 (32.5) | 0.0462a |
| Anemia | 23 | 17 | 1 | 0 | 41 (43.6) | 6 | 12 | 1 | 0 | 19 (47.5) | 0.7273 |
|
Thrombocytopenia | 4 | 2 | 0 | 0 | 6 (6.4) | 2 | 1 | 0 | 0 | 3 (7.5) | 0.7386 |
| AST/ALT
increase | 15 | 1 | 0 | 0 | 16 (17.0) | 4 | 0 | 0 | 0 | 4 (10.0) | 0.2215 |
| T-Bil increase | 13 | 3 | 0 | 0 | 16 (17.0) | 5 | 2 | 0 | 0 | 7 (17.5) | 0.6319 |
| Diarrhea | 22 | 3 | 0 | 0 | 25 (26.6) | 12 | 1 | 0 | 0 | 13 (32.5) | 0.8174 |
| Nausea | 14 | 1 | 0 | 0 | 15 (16.0) | 3 | 2 | 0 | 0 | 5 (12.5) | 0.4114 |
| Vomiting | 9 | 0 | 0 | 0 | 9 (9.6) | 2 | 0 | 0 | 0 | 2 (5.0) | 0.3066 |
| Anorexia | 30 | 6 | 2 | 0 | 38 (40.4) | 12 | 3 | 0 | 0 | 15 (37.5) | 0.4529 |
| Stomatitis | 11 | 0 | 0 | 0 | 11 (11.7) | 10 | 0 | 0 | 0 | 10 (25.0) | 0.9837 |
| Constipation | 3 | 0 | 0 | 0 | 3 (3.2) | 3 | 0 | 0 | 0 | 3 (7.5) | 0.2493 |
| Malaise | 20 | 10 | 1 | 0 | 31 (33.0) | 10 | 4 | 1 | 0 | 15 (37.5) | 0.7602 |
| Itching | 5 | 0 | 0 | NA | 5 (5.3) | 5 | 0 | 0 | NA | 5 (12.5) | 0.1390 |
| Taste
alteration | 5 | 1 | NA | NA | 6 (6.4) | 5 | 0 | NA | NA | 5 (12.5) | 0.9321 |
| Hyperkalemia | 9 | 0 | 0 | 0 | 9 (9.6) | 2 | 0 | 0 | 0 | 2 (5.0) | 0.3066 |
|
Hyperpigmentation | 6 | 0 | NA | NA | 6 (6.4) | 7 | 0 | NA | NA | 7 (17.5) | 0.0514 |
| Watering eyes | 10 | 1 | 0 | NA | 11 (11.7) | 7 | 1 | 0 | NA | 8 (20.0) | 0.9127 |
| Hand-foot
syndrome | 3 | 0 | 0 | 0 | 3 (3.2) | 4 | 0 | 0 | 0 | 4 (10.0) | 0.1181 |
| Rash | 10 | 0 | 0 | 0 | 10 (10.6) | 9 | 0 | 0 | 0 | 9 (22.5) | 0.9781 |
| Fever | 3 | 0 | 0 | 0 | 3 (3.2) | 2 | 0 | 0 | 0 | 2 (5.0) | 0.8429 |
| Others | 16 | 0 | 0 | 0 | 16 (17.0) | 11 | 0 | 0 | 0 | 11 (27.5) | 0.9448 |
The frequencies of AEs in patients who completed the
treatment are presented in Table
VI. The incidences of leukopenia, neutropenia, aspartate and
alanine transaminase increases and total bilirubin increases were
greater in the CG compared with the PG (P=0.0311, <0.0001,
0.0025 and 0.0219, respectively). By contrast, the incidences of
rash and stomatitis were greater in the PG compared with the CG
(P=0.0271 and 0.0085, respectively).
 | Table VI.Adverse events in patients who
completed the treatment. |
Table VI.
Adverse events in patients who
completed the treatment.
|
| CG (n=37) | PG (n=33) |
|
|---|
|
|
|
|
|
|---|
|
| Grade |
| Grade |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Adverse events | 1 | 2 | 3 | 4 | All grades (%) | 1 | 2 | 3 | 4 | All grades (%) | P-value |
|---|
| Leukopenia | 10 | 13 | 3 | 0 | 26 (70.3) | 10 | 5 | 0 | 0 | 15 (45.5) |
0.0311a |
| Neutropenia | 9 | 15 | 7 | 0 | 31 (83.8) | 1 | 9 | 2 | 0 | 12 (36.4) |
<0.0001a |
| Anemia | 12 | 11 | 1 | 0 | 24 (64.9) | 5 | 12 | 1 | 0 | 18 (54.5) | 0.2626 |
|
Thrombocytopenia | 4 | 2 | 0 | 0 | 6 (16.2) | 2 | 1 | 0 | 0 | 3 (9.1) | 0.3001 |
| AST/ALT
increase | 14 | 1 | 0 | 0 | 15 (40.5) | 3 | 0 | 0 | 0 | 3 (9.1) |
0.0025a |
| T-Bil increase | 13 | 3 | 0 | 0 | 16 (43.2) | 5 | 1 | 0 | 0 | 6 (18.2) | 0.0219 |
| Diarrhea | 13 | 0 | 0 | 0 | 13 (35.1) | 8 | 1 | 0 | 0 | 9 (27.3) | 0.3274 |
| Nausea | 9 | 0 | 0 | 0 | 9 (24.3) | 2 | 1 | 0 | 0 | 3 (9.1) | 0.0839 |
| Vomiting | 6 | 0 | 0 | 0 | 6 (16.2) | 1 | 0 | 0 | 0 | 1 (3.0) | 0.0726 |
| Anorexia | 12 | 3 | 1 | 0 | 16 (43.2) | 10 | 3 | 0 | 0 | 13 (39.4) | 0.4671 |
| Stomatitis | 2 | 0 | 0 | 0 | 2 (5.4) | 8 | 0 | 0 | 0 | 8 (24.2) |
0.0271a |
| Constipation | 3 | 0 | 0 | 0 | 3 (8.1) | 3 | 0 | 0 | 0 | 3 (9.1) | 0.6066 |
| Malaise | 11 | 4 | 0 | 0 | 15 (40.5) | 10 | 4 | 1 | 0 | 15 (45.5) | 0.4312 |
| Itching | 1 | 0 | 0 | NA | 1 (2.7) | 4 | 0 | 0 | NA | 4 (12.1) | 0.1447 |
| Taste
alteration | 2 | 0 | NA | NA | 2 (5.4) | 5 | 0 | NA | NA | 5 (15.2) | 0.1696 |
| Hyperkalemia | 7 | 0 | 0 | 0 | 7 (18.9) | 2 | 0 | 0 | 0 | 2 (6.1) | 0.1051 |
|
Hyperpigmentation | 4 | 0 | NA | NA | 4 (10.8) | 7 | 0 | NA | NA | 7 (21.2) | 0.1938 |
| Watering eyes | 3 | 1 | 0 | NA | 5 (10.8) | 6 | 1 | 0 | NA | 7 (21.2) | 0.1938 |
| Hand-foot
syndrome | 1 | 0 | 0 | 0 | 1 (2.7) | 4 | 0 | 0 | 0 | 4 (12.1) | 0.1447 |
| Rash | 1 | 0 | 0 | 0 | 2 (2.7) | 8 | 0 | 0 | 0 | 8 (24.2) |
0.0085a |
| Fever | 2 | 0 | 0 | 0 | 2 (5.4) | 1 | 0 | 0 | 0 | 1 (3.0) | 0.5434 |
| Others | 11 | 0 | 0 | 0 | 11 (29.7) | 11 | 0 | 0 | 0 | 11 (33.3) | 0.4729 |
Discussion
In the present study, continued pharmaceutical
intervention resulted in a high persistence rate of S-1 adjuvant
chemotherapy over the course of a year and a high completion rate.
The increase in persistence and completion rates in the PG may be
considered the result of a reduction in discontinuation due to AEs.
Prior to intervention, treatment withdrawal due to malaise,
anorexia, diarrhea, stomatitis and nausea was observed. However,
following intervention, withdrawal due to AEs occurred in only 3
cases. However, in patients who completed S-1 adjuvant treatment,
the incidence of rash and stomatitis were greater in the PG
compared with the CG. The present study therefore considers that
patients in the PG were able to continue S-1 adjuvant treatment
with the appropriate therapy support from the pharmacist outpatient
service.
In previous studies, in patients who underwent
adjuvant chemotherapy following complete tumor resection, patients
discontinued adjuvant chemotherapy due to non-hematological
toxicities (7). In addition, in a
previous case, 1 patient experienced anorexia, fatigue and
diarrhea, and decided they no longer wished to undergo S-1 therapy
(4). The findings of these previous
studies and the present study suggest that patient guidance is
paramount for adherence and continuation to S-1 therapy.
In a previous study, the associations between AEs
and blood levels of fluorouracil (5-FU) at the time of S-1
administration were evaluated (18).
In addition, Van Groeningen et al (19) and Findlay et al (20) reported that the blood levels of 5-FU
differed between patients with esophageal and gastric cancer.
Patients with gastric cancer who are administered S-1 adjuvant
chemotherapy have undergone gastric surgery, and therefore, may
have difficulties with food intake, as has been previously reported
(3). Therefore, in the present study
it was considered that absorption abnormalities may affect the
blood concentration of the drug (3,8). In
addition, weight loss due to a decrease in postoperative oral
intake is likely to reduce muscle mass, which may also affect blood
drug levels (21–24). Therefore, the concentration of drug
in the blood would exceed the therapeutic index resulting in an
increase in AEs, which may lead to subsequent non-adherence. In
such cases, dose reductions have the potential to improve
adherence. Therefore, in the current study, the pharmacist
outpatient service was utilized during the initial administration
of S-1 to identify any patients requiring dose reductions due to
possible eating difficulties or absorption problems.
During chemotherapy with oral anti-cancer drugs,
medication adherence is important. Direct associations between
adherence in patients undergoing chemotherapy with oral anti-cancer
agents and pharmacist-mediated provision of education on correct
drug use and the avoidance of AEs have been reported (6,10). In
the present study, the total prescription proposal acceptance rate
was 92.5% (198/214 cases). Such a high rate indicates that
pharmacists are able to appropriately evaluate the association
between medication-related AEs and prescription recommendations. It
is necessary to consider symptoms when assessing AEs associated
with chemotherapeutic agents; however, a physician may not be able
to provide adequate support during complicated outpatient
examinations (8). Pharmacists
frequently have varying perspectives on the outpatient situation
when proposing supportive care and evaluating therapeutic effects
and AEs. Pharmacists interview patients about their lifestyle,
physical condition and drug intake, obtaining a more holistic
understanding of an individual's requirements. In addition, as
medication experts, pharmacists have a greater understanding of
medication adherence and AEs. Therefore, the present study
hypothesizes that a pharmacist may be able to intervene in patient
treatment, as they have a different viewpoint to that of the
physician.
With regard to the results of the ACTS-GC study, the
prognosis was superior in patients who continued S-1 adjuvant
chemotherapy for at least 1 year (1). Furthermore, the prognosis was improved
when the RDI was >70% (1). In the
present study, patients who were able to complete S-1 adjuvant
chemotherapy following intervention by the pharmacist outpatient
service had an RDI of >70%. Therefore, it is considered that
treatment efficacy may be improved by a pharmacist outpatient
service.
In the present study, the completion rate prior to
intervention by the pharmacist outpatient service was very low
(39.4%). However, continued pharmaceutical intervention (pharmacist
outpatient service) resulted in a high persistence rate of S-1
adjuvant chemotherapy over the course of a year (82.5%), which was
higher than those reported in previous studies, i.e., 64.2%
(3) and 65.8% (1). These results indicate the efficiency of
the pharmacist outpatient service. Until recently, there was no
guidance for S-1 treatment in Ogaki Municipal Hospital, and
treatment was conducted at the discretion of the treating
physician. In addition, efforts to improve the continuity of S-1
have been conducted in other facilities. Tatematsu et al
(25) reported that the persistence
rate of S-1 adjuvant chemotherapy over the course of a year
improved from 75% (33/44 cases) to 92.3% (12/13 cases) during
cooperation with out-of-hospital pharmacies. In addition, Kishimoto
et al (26) reported that the
persistence rate of S-1 adjuvant chemotherapy over the course of a
year was 87.5% (14/16 cases) following liaisons in the clinical
pathway. Therefore, the present study considers that AE monitoring
and outpatient counseling were adversely affected in the previous
studies that had lower persistence rates due to the lack of a
pharmacist outpatient service.
The present study had several limitations, such as
the small number of cases and short follow-up time. However, to
validate these findings, more cases are currently being accumulated
with extended follow-up times.
In conclusion, the continued pharmaceutical
intervention for S-1 adjuvant chemotherapy in patients with gastric
cancer led to a reduction in AEs. Further, by feeding back
information such as the occurrence of AEs to the physician, the
persistence rate of S-1 adjuvant chemotherapy over the course of a
year was significantly increased.
References
|
1
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimura M, Usami E, Yoshimura T, Yasuda T,
Kaneoka Y, Teramachi H, Sugiyama T and Tsuchiya T: Pharmaceutical
care for patients undergoing S-1 plus cisplatin therapy for
unresectable recurrent gastric cancer. J Pharm Pract. 26:409–414.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimura M, Morihata K, Ito D, Iwai M, Okada
K, Usami E, Nakao T, Yoshimura T and Yasuda T: Continuous
administration and safety of S-1 in adjuvant chemotherapy for
gastric cancer. Gan To Kagaku Ryoho. 37:829–834. 2010.(In
Japanese). PubMed/NCBI
|
|
4
|
Iwai M, Kimura M, Yoshimura T and Yasuda
T: The necessity of adverse effect monitoring in patients using
TS-1: A survey of reasons for treatment withdrawal or switching. J
Jpn Soc Hosp Pharm. 44:1386–1388. 2008.(In Japanese).
|
|
5
|
Kimura M, Usami E, Iwai M, Nakao T,
Yoshimura T, Mori H, Sugiyama T and Teramachi H: Oral anticancer
agent medication adherence by outpatients. Oncol Lett. 8:2318–2324.
2014.PubMed/NCBI
|
|
6
|
Kimura M, Nakashima K, Usami E, Iwai M,
Nakao T, Yoshimura T, Mori H and Teramachi H: Adherence and
awareness of the therapeutic intent of oral anticancer agents in an
outpatient setting. Oncol Lett. 9:2341–2346. 2015.PubMed/NCBI
|
|
7
|
Kawabata R, Imamura H, Kishimoto T,
Hachino Y, Yasui Y, Fujino M, Fujii C, Fukunaga M, Ohzato H and
Furukawa H: Examination of factors influencing continuity of S-1
adjuvant chemotherapy for gastric cancer patients. Gan To Kagaku
Ryoho. 39:1205–1208. 2012.(In Japanese). PubMed/NCBI
|
|
8
|
Imamura M, Nakura H and Takemoto C:
Evaluation of usefulness of pharmaceutical outpatient clinic for
cancer patients. Jpn J Pharm Health Care Sci. 36:85–98. 2010.(In
Japanese). View Article : Google Scholar
|
|
9
|
Nakashima K, Mano Y, Ohuchi K, Sato D,
Iwata K, Higuchi A, Ebara K, Kato Y, Hirosawa I, Tjima M, et al:
Role of pharmaceutical outpatient clinic in cancer patients and
evaluation. Jpn J Pharm Health Care Sci. 38:599–608. 2012.(In
Japanese). View Article : Google Scholar
|
|
10
|
Aimono Y, Nemoto M, Sato W, Saito Y,
Aoyama Y, Joko F, Maruyama T and Kamoshida T: Examination of the
usefulness of the pharmacists' outpatient clinic for treatment with
oral molecule-targeting drugs. Gan To Kagaku Ryoho. 40:901–905.
2013.(In Japanese). PubMed/NCBI
|
|
11
|
MacLeod A, Branch A, Cassidy J, McDonald
A, Mohammed N and MacDonald L: A nurse-/pharmacy-led capecitabine
clinic for colorectal cancer: Results of a prospective audit and
retrospective survey of patient experiences. Eur J Oncol Nurs.
11:247–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong SF, Bounthavong M, Nguyen C,
Bechtoldt K and Hernandez E: Implementation and preliminary
outcomes of a comprehensive oral chemotherapy management clinic. Am
J Health Syst Pharm. 71:960–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simons S, Ringsdorf S, Braun M, Mey UJ,
Schwindt PF, Ko YD, Schmidt-Wolf I, Kuhn W and Jaehde U: Enhancing
adherence to capecitabine chemotherapy by means of
multidisciplinary pharmaceutical care. Support Care Cancer.
19:1009–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arakawa-Todo M, Yoshizawa T, Zennami K,
Nishikawa G, Kato Y, Kobayashi I, Kajikawa K, Yamada Y, Matsuura K,
Tsukiyama I, et al: Management of adverse events in patients with
metastatic renal cell carcinoma treated with sunitinib and clinical
outcomes. Anticancer Res. 33:5043–5050. 2013.PubMed/NCBI
|
|
15
|
Shah NN, Casella E, Capozzi D, McGettigan
S, Gangadhar TC, Schuchter L and Myers JS: Improving the safety of
oral chemotherapy at an academic medical center. J Oncol Pract.
12:e71–e77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura M, Go M, Iwai M, Usami E, Teramachi
H and Yoshimura T: Evaluation of the role and usefulness of a
pharmacist outpatient service for patients undergoing monotherapy
with oral anti-cancer agents. J Oncol Pharm Pract. Jun
20–2016.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
US Department Of Health And Human
Services: Common terminology criteria for adverse events (CTCAE)
version 4.0. United States, National Cancer Institute, .
2009.http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5X7.pdf
|
|
18
|
Matsumoto H, Hirai T, Hirabayashi Y,
Murakami H, Higashida M, Kawabe Y, Fuchimoto M, Fujikura H, Hato S,
Urakami A, et al: Pharmacokinetics of 5-FU after S-1 oral
administration for adjuvant chemotherapy in gastric cancer
patients. Gan To Kagaku Ryoho. 34:869–873. 2007.(In Japanese).
PubMed/NCBI
|
|
19
|
van Groeningen CJ, Pinedo HM, Heddes J,
Kok RM, de Jong AP, Wattel E, Peters GJ and Lankelma J:
Pharmacokinetics of 5-fluouracil assessed with a sensitive mass
spectrometric method in patient on a dose escalation schedule.
Cancer Res. 48:6956–6961. 1988.PubMed/NCBI
|
|
20
|
Findlay MP, Raynaud F, Cunningham D,
Iveson A, Collins DJ and Leach MO: Measurement of plasma
5-fluorouracil by high-performance liquid chromatography with
comparison of results to tissue drug levels observed using in vivo
19F magnetic resonance spectroscopy in patients on a protracted
venous infusion with or without interferon-alpha. Ann Oncol.
7:47–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aslani A, Smith RC, Allen BJ, Pavlakis N
and Levi JA: The predictive value of body protein for
chemotherapy-induced toxicity. Cancer. 88:796–803. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gusella M, Toso S, Ferrazzi E, Ferrari M
and Padrini R: Relationships between body composition parameters
and fluorouracil pharmacokinetics. Br J Clin Pharmacol. 54:131–139.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prado CM, Baracos VE, McCargar LJ,
Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG and Sawyer
MB: Body composition as an independent determinant of
5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res.
13:3264–3268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prado CM, Baracos VE, McCargar LJ, Reiman
T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E and
Sawyer MB: Sarcopenia as a determinant of chemotherapy toxicity and
time to tumor progression in metastatic breast cancer patients
receiving capecitabine treatment. Clin Cancer Res. 15:2920–2926.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tatematsu M, Kuroki R, Hata T, Hata T and
Muro K: Outcomes of support for ambulatory cancer patients through
a collaborative alliance of doctors, nurses, hospital pharmacists
and community pharmacists. Jpn J Pharm Palliat Care Sei. 7:13–19.
2014.(In Japanese).
|
|
26
|
Kishimoto T, Imamura H, Kawabata R, Kimura
Y, Fukunaga M and Ohzato H: Feasibility and outcome of S-1 adjuvant
chemotherapy for patients with gastric cancer treated based on the
liaison-clinical pathway. Gan To Kagaku Ryoho. 40:489–492. 2013.(In
Japanese). PubMed/NCBI
|