Introduction
Chemotherapy-induced nausea and vomiting (CINV)
causes patients considerable emotional and physical distress,
greatly diminishing quality of life (1). Japan has developed clinical practice
guidelines that support and aid the implementation of
evidence-based practices (2),
covering usage of aprepitant, a selective neurokinin 1 receptor
antagonist, as well as first-generation granisetron and
second-generation palonosetron [5-hydroxytryptamine type 3 (5-HT3)
receptor antagonists]. Previous studies have made significant
progress in the development of supportive therapies for patients
receiving cisplatin and doxorubicin + cyclophosphamide; this
includes anti-emetic drugs for highly emetogenic chemotherapy (HEC)
regimens (3,4). Anti-emetic treatment recommendations
for patients receiving HEC (5,6) and
moderately emetogenic chemotherapy (7–9) for the
treatment of solid tumors are now well established, and evidence
suggests that palonosetron is fairly effective (10).
CINV comprises two of the most unpleasant
chemotherapy-associated side effects for patients with cancer, and
numerous studies (3–9) have been conducted since 2012 to assess
the effectiveness of a growing list of anticancer drug options that
are able to reduce CINV. However, balanced against the
effectiveness of the treatment as a supportive drug, the cost
burden remains a major concern.
Findings from the TRIPLE TEST clinical trial
(11), published in 2013 revealed
that palonosetron markedly improved the complete recovery (CR) rate
during the delayed phase compared with other drug options. The
TRIPLE TEST was originally used to assess the advanced emetic risk
of cisplatin regimens, but acute and delayed phases overlap in
remission induction therapy and other daily continuous
administration regimens. This study demonstrated that palonosetron
was remarkably effective for controlling acute vomiting.
Patients with hematological tumors (as opposed to
solid tumors) are generally younger and have greater risk of CINV
due to highly emetogenic treatments and other psychological
factors. Unfortunately, there are currently no collective
international guidelines for combating CINV in these patients.
The current study is a retrospective comparative
analysis of the anti-emetic effects of granisetron and palonosetron
when used during remission induction therapy and consolidation
therapy of patients with acute myeloid leukemia.
Patients and methods
Study participants
The present study included a total of 83 patients
diagnosed with acute myeloid leukemia treated between August 2011
and June 2013 with remission induction therapy and consolidation
therapy, with a 5-day or 7-day course of cytarabine (100–200
mg/m2) and a three-day course of anthracycline
(idarubicin, 12 mg/m2; daunorubicin, 50
mg/m2; aclarubicin, 20 mg/m2; mitoxantrone, 7
mg/m2). These patients were selected by the Department
of Hematology and Outpatient Chemotherapy Center, Hakodate
Municipal Hospital, Hokkaido, Japan.
The study protocol was approved by the Hakodate
Municipal Hospital Institutional Review Board (Hakodate, Japan).
Based on the Declaration of Helsinki, written informed consent was
obtained from all participating patients.
Study methodology
Granisetron or palonosetron were administered at the
judgment of the physician in Japanese-approved dosages (3 mg
granisetron once per day for 5 or 7 days, or one dose of 0.75 mg
palonosetron). An analysis was performed the rates of complete
control (CC) during acute-phase and delayed-phase vomiting of
patients receiving the two drugs. The effects of each drug were
evaluated as CC, if the following conditions were met: i) No
vomiting or dry vomiting; ii) no other anti-emetic treatments; and
iii) no moderate or severe nausea. The data were obtained from the
doctors' and nurses' records. The daily dosage of aprepitant was as
follows: Day 1, 125 mg; days 2 and 3, 80 mg. Aprepitant is approved
in Japan for the use during the early stages of chemotherapy.
Statistical analysis
Fisher's exact test was used to analyze the data and
statistical analysis was performed with StatMate series version 5
software (ATMS Co., Ltd., Tokyo, Japan). P<0.05 was considered
to indicate a statistically significant difference.
Results
Age and gender of patients
Patients in the granisetron group ranged in age from
30–80 years, with a median age of 65 years. The palonosetron group
ranged in age from 40–80 years, with a median age of 62 years. The
granisetron group consisted of 13 female (29%) and 32 male (71%)
patients, whereas the palonosetron group consisted of 17 female
(45%) and 21 male (55%) patients (Table
I). There were no significant differences in the patients' age
and gender.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Anti-emetic
received |
|---|
|
|
|
|---|
| Characteristic | Granisetron | Palonosetron |
|---|
| Median age,
years | 65 | 62 |
| Minimum age,
years | 30 | 40 |
| Maximum age,
years | 80 | 80 |
| Gender, n (%) |
|
|
|
Female | 13 (29) | 17 (45) |
| Male | 32 (71) | 21 (55) |
Treatments
A detailed breakdown of the treatments analyzed is
presented in Table II. The study
included 23 cases of granisetron treatment alone, 22 cases of
granisetron combined with aprepitant, 23 cases of palonosetron
alone and 15 cases of palonosetron treatment combined with
aprepitant.
 | Table II.Patient treatment groups. |
Table II.
Patient treatment groups.
|
| Granisetron, n | Palonosetron, n |
|---|
| Total no. of
cases | 45 | 38 |
| IDA-AraC | 14 | 13 |
| ACR-AraC | 6 | 4 |
| DNR-AraC | 7 | 9 |
| MIT AraC | 10 | 6 |
| MEC | 8 | 6 |
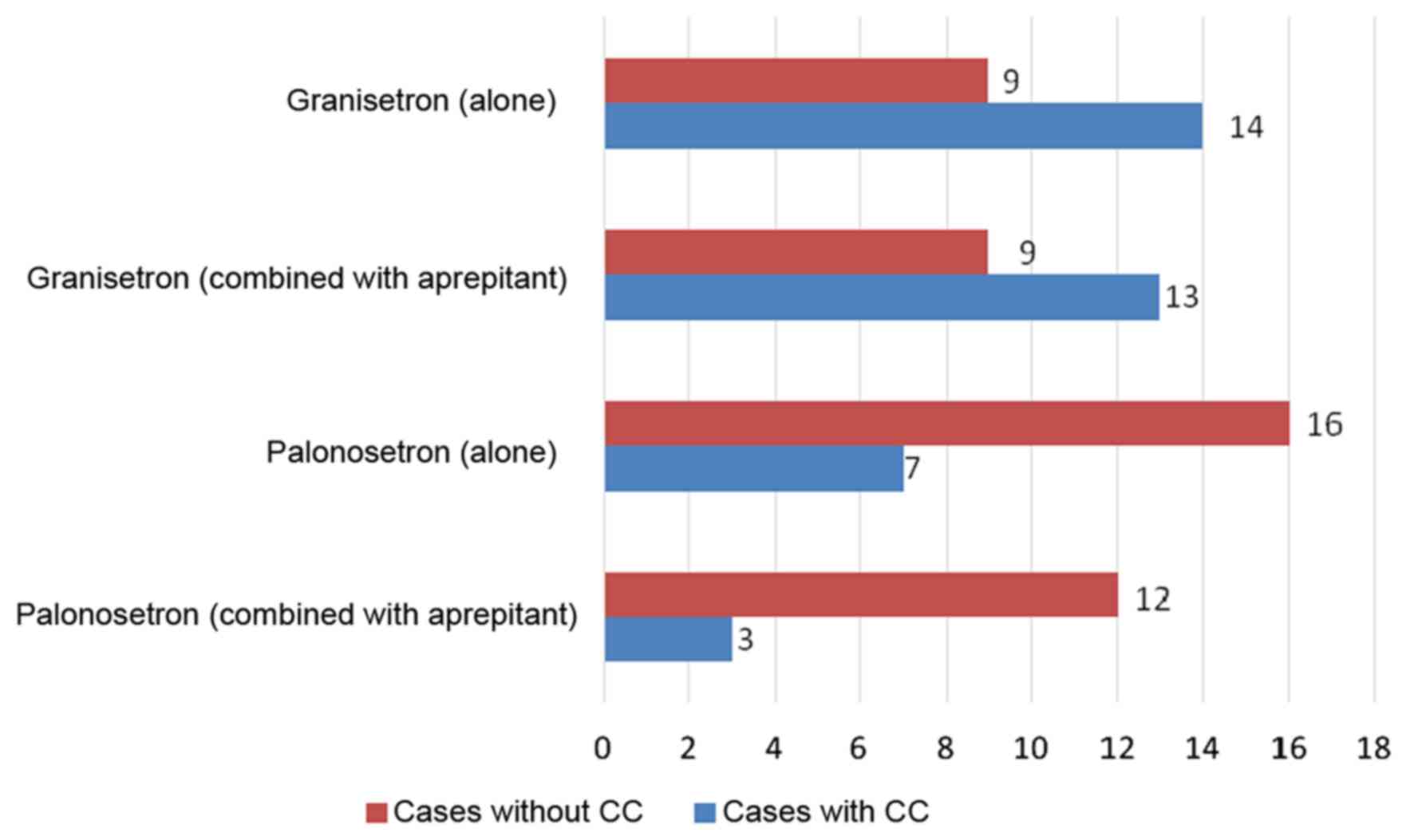
CC of acute vomiting was achieved in 39% of the
granisetron alone group, 41% of the combined granisetron +
aprepitant group, 70% of the palonosetron alone group and 80% of
the combined palonosetron + aprepitant group. Significant
differences were observed between the palonosetron alone and
granisetron alone groups (P=0.0458) and the combined granisetron +
aprepitant and palonosetron + aprepitant groups (P=0.0409);
palonosetron was demonstrated to be the more effective treatment
for the CC of vomiting (Fig. 1).
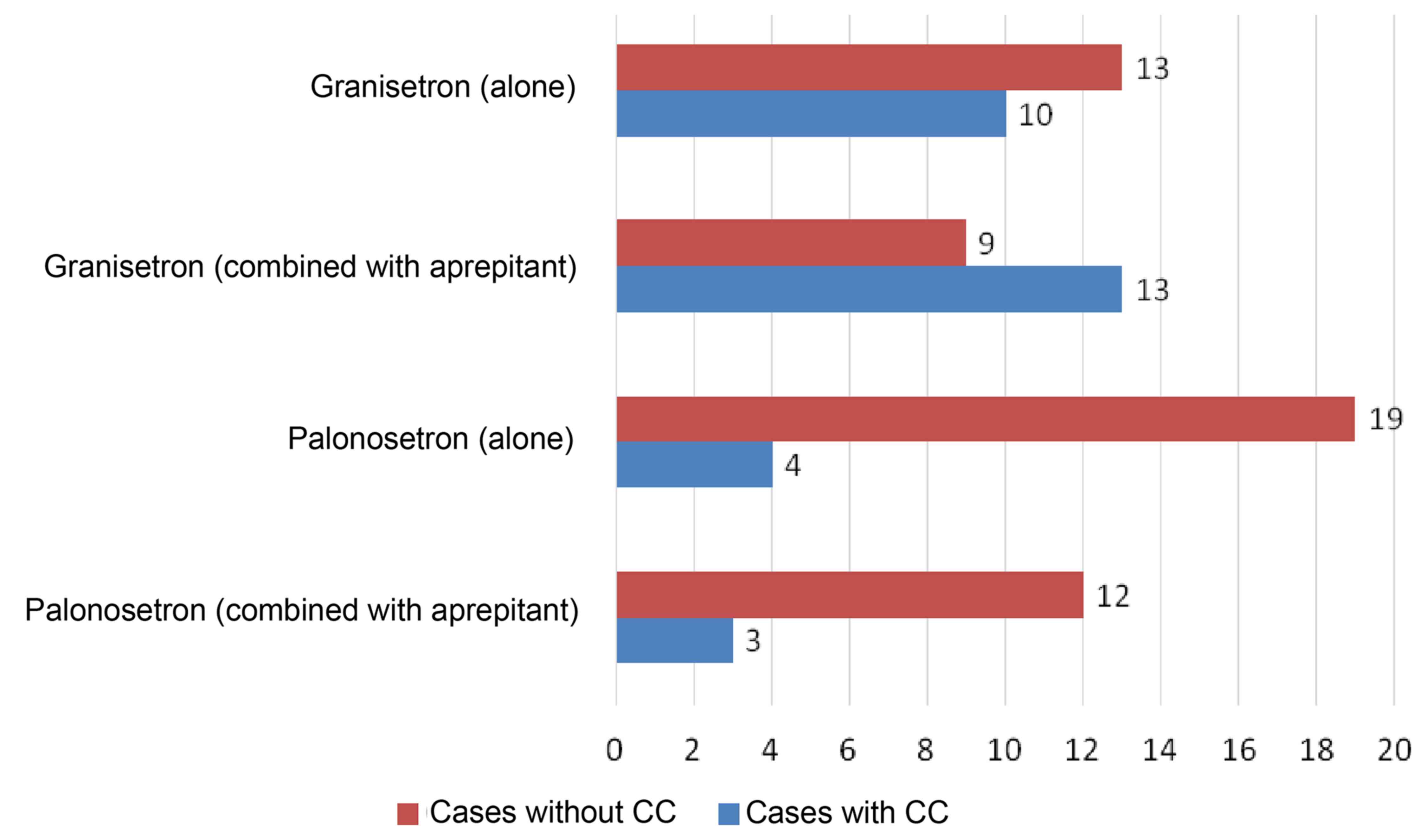
CC of delayed vomiting was achieved in 57% of the
granisetron alone group, 41% of the combined granisetron +
aprepitant group, 83% of the palonosetron alone group and 80% of
the combined palonosetron + aprepitant group. No significant
differences were observed between the granisetron alone and
palonosetron alone groups (P=0.0653); however, a somewhat improved
performance was observed for the palonosetron group. Comparing the
combined granisetron + aprepitant and palonosetron + aprepitant
groups yielded a significant difference (P=0.0409), thus, the
combined palonosetron + aprepitant group was effective for the CC
of delayed vomiting (Fig. 2).
In monetary terms, granisetron costs $23.36 per 3
mg, whereas palonosetron costs $118.00 for 0.75 mg of the drug.
Using the 5-HT3 receptor antagonists for a regimen lasting several
days, granisetron would cost $116.80 if taken once per day for 5
days, $163.50 if taken once per day for 7 days, $233.60 if taken
twice per day for 5 days and $327.10 if taken twice per day for 7
days. By contrast, palonosetron is administered once per week;
therefore, whether or not palonosetron is prescribed for a single
day or as a multi-day treatment, the cost remains the same at
$118.00. Thus, if a 5-HT3 receptor antagonist is administered for
longer than 5 days, palonosetron is less expensive than granisetron
(Table III).
 | Table III.Costs of anti-emetics in dollars. |
Table III.
Costs of anti-emetics in dollars.
|
| Treatment
duration |
|---|
|
|
|
|---|
| Anti-emetic | 1 day | 5 days | 7 days |
|---|
| Granisetron | 23.36 | 116.8 | 163.5 |
| Palonosetron | 118.0 | 118.0 | 118.0 |
Discussion
The efficacy of palonosetron may be attributed to
its long plasma elimination half-life and high receptor binding
affinity (12) compared with other
5-HT3 receptor antagonists. The superior anti-emetic efficacy of
palonosetron compared with other 5-HT3 receptor antagonists may
also be attributed to allosteric interactions and a positive
cooperative effect of palonosetron, prolonged structural changes of
the receptor and internalization (13–15), in
addition to other factors.
Several investigators reported that palonosetron
provides more effective CINV control through the entire
chemotherapy process (including the delayed phase) than any other
5-HT3 receptor to date (16,17). The present results similarly observed
that palonosetron is effective for controlling acute- and
delayed-phase nausea through the entire treatment period.
Compared with the short half-life of granisetron,
3.14±1.20 h (17), palonosetron is
eliminated from the body very slowly and has a markedly longer
half-life of 41.6±13.1 h (18).
Hothersall et al (19)
reported that palonosetron acts as a 5-HT3 receptor over a
prolonged period or even as an irreversible antagonist for at least
4 days. Allosteric receptor-receptor interactions may play a
significant role in this phenomenon (19). The key objective of the present study
was to identify a continuous daily regimen of anti-emetic drugs
that may be used during remission induction therapy. The results
revealed that the anti-emetic effects of granisetron last only a
relatively short time when the drug is administered once or even
twice per day; however, the effects of palonosetron persist over
its long plasma elimination half-life, which facilitates continuous
supportive therapy of a daily regimen following nausea.
Furthermore, comparing the relative costs in the treatment of bone
and soft tissue tumors, Kimura et al (20) demonstrated that a single dose of
palonosetron was in no way inferior to continuous administration of
multiple doses of granisetron. In the current analysis,
palonosetron is also more cost-effective and is not inferior to
granisetron when used in continuous administration regimens.
These results suggest that palonosetron is a highly
effective 5-HT3 receptor antagonist anti-emetic for use with the
daily administration of an antineoplastic agent, in terms of
medical efficacy and cost-effectiveness.
It must be noted that, due to the retrospective
study design, the current results may be skewed by the fact that
the attending physicians decided whether granisetron or
palonosetron were administered alone or in combination with
aprepitant. This is an issue that may be addressed in a future
study.
Guidelines developed by the Japanese Society of
Medical Oncology note that ‘When a clinician underestimates the
occurrence of nausea, this indicates that vomiting is even less
well controlled’ (21). Therefore,
there is a requirement for further investigation of additional
effective anti-emetic therapies in the future.
In conclusion, the results of the present study
suggest that, in the treatment of hematopoietic malignancies,
palonosetron is an effective regimen to be administered alongside
more than 5 continuous days of anti-cancer agents. Furthermore, the
combination of palonosetron and aprepitant was revealed to be the
optimal regimen.
References
|
1
|
Cohen L, de Moor CA, Eisenberg P, Ming EE
and Hu H: Chemotherapy-induced nausea and vomiting: Incidence and
impact on patient quality of life at community oncology settings.
Support Care Cancer. 15:497–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hori K, Kobayashi N, Atsumi H, Nagayama A,
Kondoh M, Noge I, Kimura M, Utsugi H, Iwasaki T, Nakamura M, et al:
Changes in compliance with Japanese antiemetic guideline for
chemotherapy-induced nausea and vomiting: A nationwide survey using
a distributed research network. Support Care Cancer. 22:969–977.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rojia F, Herrstedt J, Aapro M, Gralla RJ,
Einhorn LH, Ballatori E, et al: Guideline update for MASCC and ESMO
in the prevention of chemotherapy-and radiotherapy-induced nausea
and vomiting:results of the Perugia consensus conference. Ann
Oncol. 21 Suppl 5:232–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kris MG, Hesketh PJ, Somerfield MR, Feyer
P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ,
Gralla RJ, et al: American Society of Clinical Oncology: American
Society of Clinical Oncology guideline for antiemetics in oncology:
Update 2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aapro MS, Grunberg SM, Manikhas GM,
Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B,
Lordick F, et al: A phase III, double-blind, randomized trial of
palonosetron compared with ondansetron in preventing
chemotherapy-induced nausea and vomiting following highly
emetogenic chemotherapy. Ann Oncol. 17:1441–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kris MG, Tonato M, Bria E, Ballatori E,
Espersen B, Herrstedt J, Rittenberg C, Einhorn LH, Grunberg S,
Saito M, et al: Consensus recommendations for the prevention of
vomiting and nausea following high-emetic-risk chemotherapy.
Support Care Cancer. 19:S25–S32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botrel TEA, Clark OAC, Clark L, Paladini
L, Faleiros E and Pegoretti B: Efficacy of palonosetron (PAL)
compared to other serotonin inhibitors (5-HT3R) in preventing
chemotherapy-induced nausea and vomiting (CINV) in patients
receiving moderately or highly emetogenic (MoHE) treatment:
Systematic review and meta-analysis. Support Care Cancer.
19:823–832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenberg P, Figueroa-Vadillo J, Zamora R,
Charu V, et al: Improved prevention of moderately emetogenic
chemotherapy-induced nausea and vomiting with Plonosetron, a
pharmacollogically novel 5-HT3 receptor antagonist. Cancer.
1:2473–2482. 2003. View Article : Google Scholar
|
|
9
|
Yang LPH and Scott LJ: Palonosetron: In
the prevention of nausea and vomiting. Drugs. 69:2257–2278. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rojas C, Stathis M, Thomas AG, Massuda EB,
Alt J, Zhang J, Rubenstein E, Sebastiani S, Cantoreggi S, Snyder
SH, et al: Palonosetron exhibits unique molecular interactions with
the 5-HT3 receptor. Anesth Analg. 107:469–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki K, Yamanaka T, Hashimoto H, Shimada
Y, Arata K, Matsui R, Goto K, Takiguchi T, Ohyanagi F, Kogure Y, et
al: Randomized, double-blind, phase III trial of palonosetron
versus granisetron in the triplet regimen for preventing
chemotherapy-induced nausea and vomiting after highly emetogenic
chemotherapy: TRIPLE study. Ann Oncol. 27:1601–1606. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Navari RM: Pharmacological management of
chemotherapy-induced nausea and vomiting: Focus on recent
developments. Drugs. 69:515–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rojas C, Thomas AG, Alt J, Stathis M,
Zhang J, Rubenstein EB, Sebastiani S, Cantoreggi S and Slusher BS:
Palonosetron triggers 5-HT(3) receptor internalization and causes
prolonged inhibition of receptor function. Eur J Pharmacol.
626:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito M and Tsukuda M: Review of
palonosetron: Emerging data distinguishing it as a novel 5-HT(3)
receptor antagonist for chemotherapy-induced nausea and vomiting.
Expert Opin Pharmacother. 11:1003–1014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito M, Aogi K, Sekine I, Yoshizawa H,
Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T and Mitsuhashi S:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: A double-blind, double-dummy, randomised, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartzberg L, Barbour SY, Morrow GR,
Ballinari G, Thorn MD and Cox D: Pooled analysis of phase III
clinical studies of palonosetron versus ondansetron, dolasetron,
and granisetron in the prevention of chemotherapy-induced nausea
and vomiting (CINV). Support Care Cancer. 22:469–477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phase I clinical trial of
granisetron(part2)-Pharmacokinetics at the time of single and
repeated intravenous administration. Rinsho Iyaku Jpn. 6:(suppl-5).
25–34. 1990.
|
|
18
|
Maemoto M, Tsukuda M, et al: A
PhaseIIstudy of palonosetron combined with dexamethasone to prevent
nausea and vomiting induced by highly emetogenic chemotherapy. Ann
Oncol. 20:1874–1880. 2009.PubMed/NCBI
|
|
19
|
Hothersall JD, Moffat C and Connolly CN:
Prolonged inhibition of 5-HT receptors by palonosetron results from
surface receptor inhibition rather than inducing receptor
internalization. Br J Pharmacol. 169:1252–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kimura H, Yamamoto N, Shirai T, Nishida H,
Hayashi K, Tanzawa Y, Takeuchi A, Igarashi K, Inatani H, Shimozaki
S, et al: Efficacy of triplet regimen antiemetic therapy for
chemotherapy-induced nausea and vomiting (CINV) in bone and soft
tissue sarcoma patients receiving highly emetogenic chemotherapy,
and an efficacy comparison of single-shot palonosetron and
consecutive-day granisetron for CINV in a randomized,
single-blinded crossover study. Cancer Med. 4:333–341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basch E, Prestrud AA, Hesketh PJ, Kris MG,
Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM,
Freundlich B, et al: American Society of Clinical Oncology:
Antiemetics: American Society of Clinical Oncology clinical
practice guideline update. J Clin Oncol. 29:4189–4198. 2011.
View Article : Google Scholar : PubMed/NCBI
|