Introduction
Breast cancer is one of the most common malignant
tumors in women worldwide (1). The
treatment generally includes surgery, radiation and chemotherapy.
Chemotherapy plays an important role in the multidisciplinary
treatment of breast cancer. A number of clinical trials have
demonstrated that adjuvant chemotherapy may improve survival among
women with breast cancer (2–4). Anthracyclines and taxanes are usually
included in the polychemotherapy used to treat breast cancer, but
their side effects, such as neutropenia, vomiting and diarrhea, may
be difficult for elderly women to tolerate. Due to these severe
side effects, several elderly patients refuse to receive standard
polychemotherapy intravenously. Patients occasionally prefer more
easily tolerated oral chemotherapeutic agents as an alternative to
standard adjuvant chemotherapy. Furthermore, elderly women with
breast cancer often suffer from comorbidities, such as
hypertension, diabetes and heart disease, and they are frequently
excluded from clinical trials. As a result, breast cancer in
elderly patients is not always treated according to the guidelines.
Oncologists occasionally administer oral chemotherapeutic agents to
elderly patients to replace the standard intravenous adjuvant
chemotherapy. Capecitabine, an orally administered fluoropyrimidine
carbamate, has a safe toxicity profile and is often administered to
elderly patients as single-agent adjuvant chemotherapy (5). Although the antitumor activity of
capecitabine in locally advanced or metastatic breast has been
reported, whether elderly patients with breast cancer benefit from
adjuvant capecitabine monotherapy remains unclear. The aim of the
present study was to retrospectively analyze clinical data from our
department to evaluate the clinical benefits of adjuvant
capecitabine monotherapy in elderly women with breast cancer.
Patients and methods
Patients
This retrospective cohort involved 251 primary
invasive breast cancer patients aged ≥60 years who underwent
surgery at the Department of Breast and Thyroid Surgery of the
Shandong Provincial Hospital Affiliated to Shandong University
(Jinan, China) between June 2001 and June 2013. A total of 147
patients received no chemotherapy and 104 patients received oral
capecitabine monotherapy (Xeloda®, Roche Pharma AG,
Grenzach-Wyhlen, Germany).
Patients who received any form of chemotherapy other
than capecitabine were not included, and patients who received
neoadjuvant chemotherapy or endocrine therapy were also excluded.
Treatment of radiotherapy or adjuvant endocrine therapy was not
considered as an exclusion criterion. No patients received adjuvant
trastuzumab therapy. All the patients were menopausal. The majority
of the patients underwent modified radical mastectomy. The clinical
and pathological characteristics of the patients are summarized in
Table I.
 | Table I.Clinicopathological characteristics of
the patients. |
Table I.
Clinicopathological characteristics of
the patients.
| Characteristics | No chemotherapy
(n=147) | Capecitabine
monotherapy (n=104) | P-value |
|---|
| Mean age (years) | 67.9 | 66.9 | 0.14 |
| Tumor size (cm) |
|
| 0.56 |
| ≤2 | 44 | 30 |
|
| >2 to
≤5 | 75 | 59 |
|
|
>5 | 28 | 15 |
|
| Grade |
|
| 0.85 |
| I | 9 | 8 |
|
| II | 55 | 40 |
|
| III | 73 | 49 |
|
| Not
available | 10 | 7 |
|
| Positive lymph nodes
(n) |
|
| 0.89 |
| 0 | 83 | 57 |
|
| 1–3 | 35 | 29 |
|
| 4–9 | 18 | 11 |
|
| ≥10 | 11 | 7 |
|
| ER status (n) |
|
| 0.31 |
|
Positive | 103 | 72 |
|
|
Negative | 44 | 32 |
|
| PR status (n) |
|
| 0.87 |
|
Positive | 92 | 64 |
|
|
Negative | 55 | 40 |
|
| HER2 status (n) |
|
| 0.74 |
|
Positive | 15 | 12 |
|
|
Negative | 132 | 92 |
|
| Surgery patterns
(n) |
|
| 0.97 |
| Modified
radical mastectomy | 132 | 94 |
|
| Simple
mastectomy | 11 | 7 |
|
|
Breastconserving surgery | 4 | 3 |
|
In the capecitabine monotherapy group, the patients
were treated with capecitabine 1,250 mg/m2 twice daily
for 14 days every 21 days as one cycle. Six cycles were planned.
Toxic reactions were assessed according to the Common Terminology
Criteria for Adverse Events, version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
All the patients with hormone receptor-positive tumors in both
groups received endocrine therapy regularly. The study protocol was
approved by the Ethics Committee of the Shandong Provincial
Hospital and written informed consent was obtained from the
participants for their clinical records to be used in this
study.
Follow-up
The adverse events of capecitabine monotherapy were
recorded every 8 weeks during outpatient visits. Follow-up
information regarding tumor recurrence and survival status after
chemotherapy was collected through personal contact with the
patients by routine letters, telephone visits, or a questionnaire,
conducted at the Shandong Provincial Hospital every 3 months during
the first 2 years after surgery, every 6 months during the next 2
years, and once a year thereafter.
Statistical analysis
A Chi-squared test was used to compare
clinicopathological characteristics between patients treated with
capecitabine monotherapy or without chemotherapy. Cancer-specific
survival was defined as the time from the first diagnosis of
primary breast cancer to death caused by the cancer, and
disease-free survival was defined as the time from the first
diagnosis of primary breast cancer to local recurrence or
metastasis. Cancer-specific and disease-free survival curves were
compared using log-rank tests. Survival curves were calculated
using the Kaplan-Meier method. Multivariate analyses were conducted
using Cox's proportional hazard regression model, which included
variables such as tumor size, lymph node status and nuclear grade
in the multivariate analysis model. Hazard ratios (HR) were
calculated with their 95% confidence intervals. P-values <0.05
were considered to indicate statistically significant differences.
Statistical analysis was performed using IBM SPSS software, version
20 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 251 patients met the inclusion criteria
of this retrospective study, with 147 patients assigned to the no
chemotherapy group and 104 to the capecitabine monotherapy group.
The clinical and pathological characteristics of all the patients
are summarized in Table I. There
were no significant differences in prognostic factors, such as age,
grade, number of positive lymph nodes and human epidermal growth
factor receptor (HER)2 status, between the capecitabine monotherapy
and the no chemotherapy groups (P>0.05). Estrogen receptor and
progesterone receptor status were also similar between the two
groups (P>0.05).
Cancer-specific and disease-free
survival
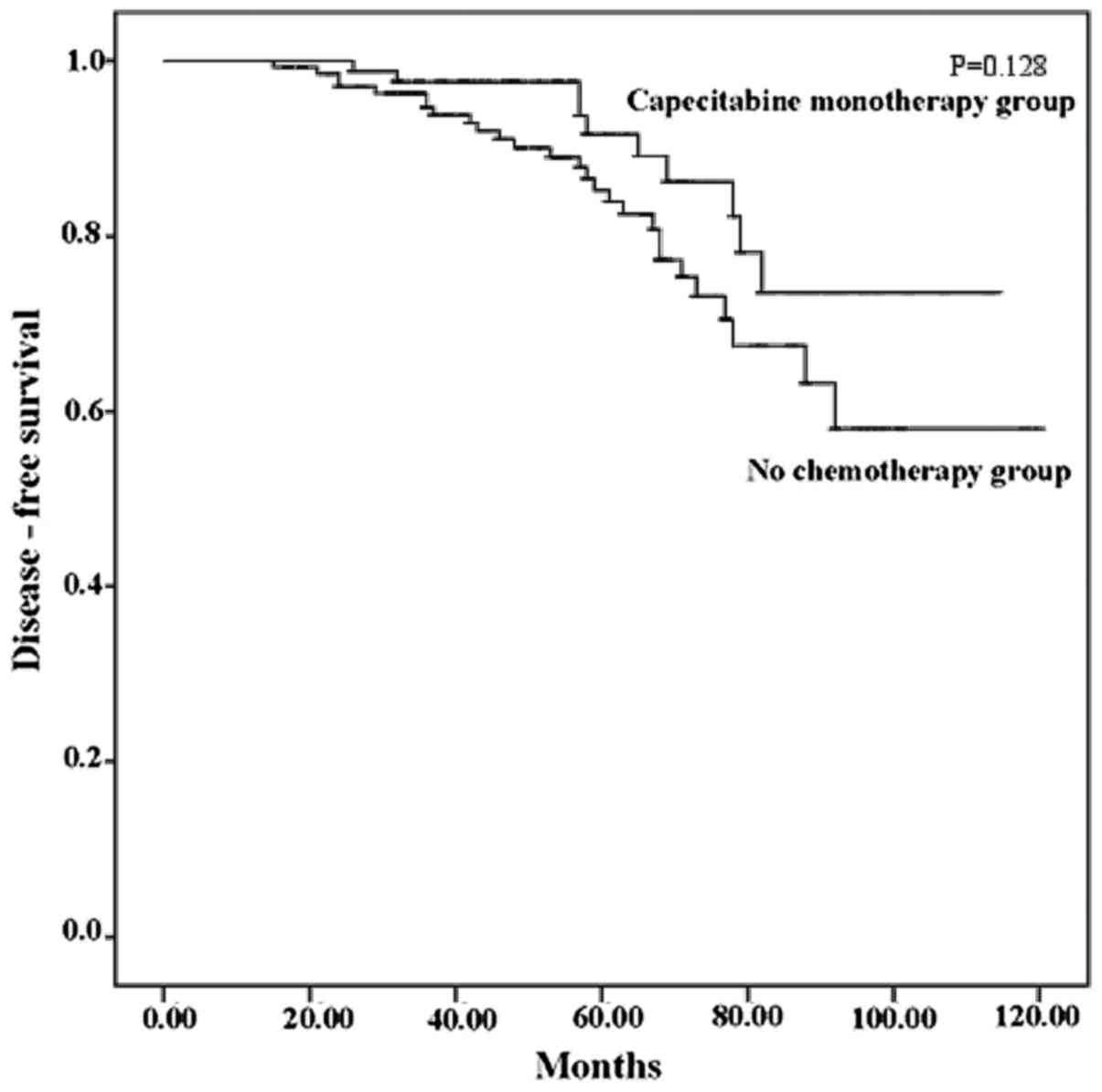
In the capecitabine monotherapy group, the breast
cancer-specific survival rate was 89.3%, with a median follow-up of
54 months (range, 13–114 months). The breast cancer-specific
survival rate in the no chemotherapy group was 81.3%, with a median
follow-up of 57 months (range, 10–120 months). One patient in the
capecitabine monotherapy group and 3 patients in the no
chemotherapy group succumbed to other causes. Although the
cancer-specific survival rate in the capecitabine monotherapy group
was higher, there were still no statistically significant
differences between the two groups in this respect (P=0.128). The
Kaplan-Meier curves for cancer specific survival are shown in
Fig. 1.
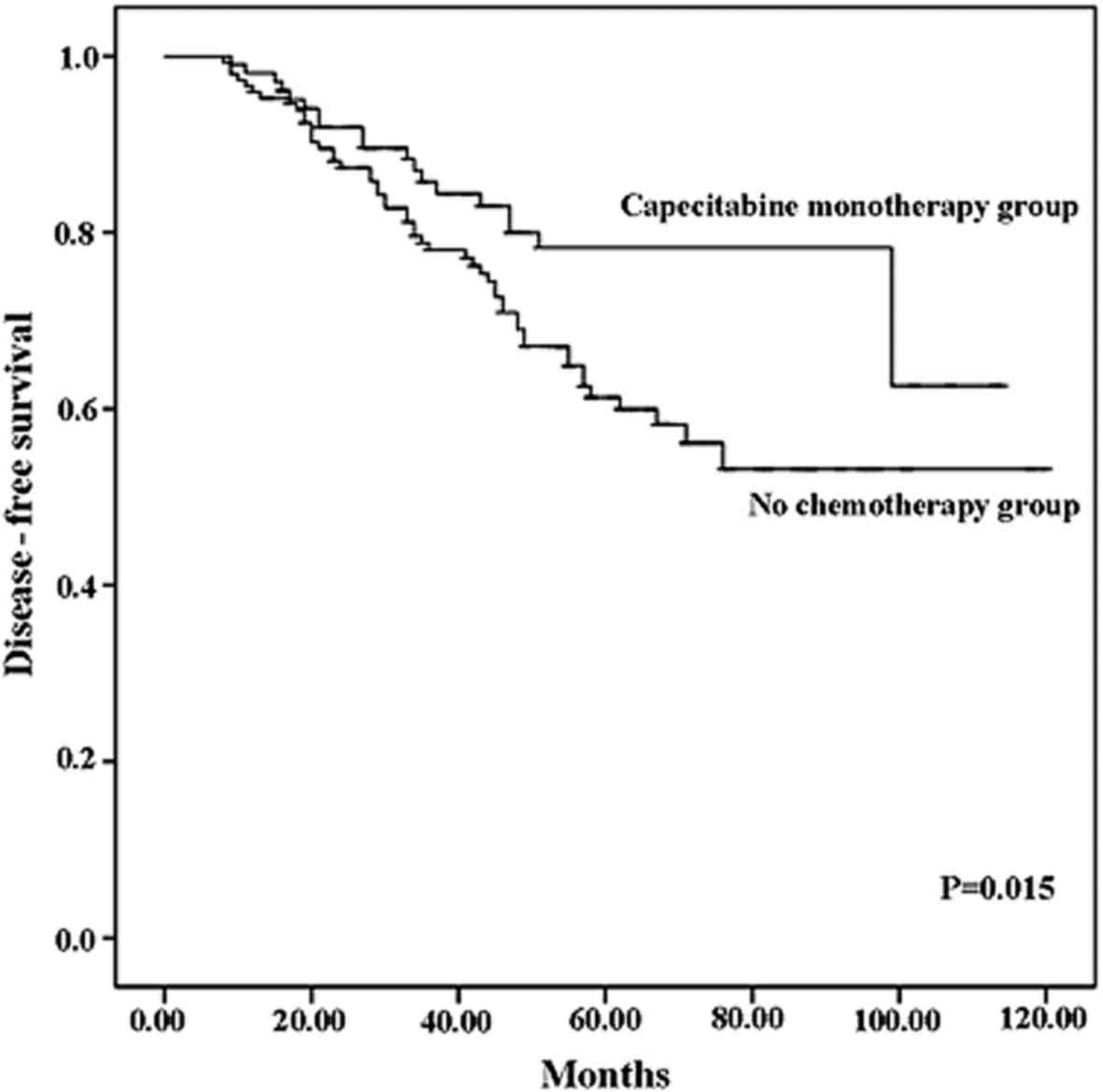
The disease-free survival rate was 81.7% in the
capecitabine monotherapy group and 65.3% in the no chemotherapy
group. Capecitabine monotherapy significantly reduced the
recurrence rate (P=0.015). The Kaplan-Meier curves for disease-free
survival are shown in Fig. 2.
Recurrence occurred in 19 patients in the capecitabine monotherapy
group and 51 in the no chemotherapy group. The details on
recurrence sites are presented in Table
II.
 | Table II.Details of recurrence sites in the two
groups. |
Table II.
Details of recurrence sites in the two
groups.
| Recurrence site | No chemotherapy
(n=51) | Capecitabine
monotherapy (n=19) |
|---|
| Locoregional | 21 | 8 |
| Lung | 19 | 7 |
| Liver | 12 | 5 |
| Bone | 16 | 6 |
| Others | 4 | 1 |
Multivariate survival analysis
Based on the Cox regression analysis, capecitabine
monotherapy was a significant independent predictive factor for
disease-free survival rate (P=0.014, HR=0.500, 95% CI: 0.288–0.867;
Table III), although it was not an
independent factor for cancerspecific survival rate (P=0.181,
Table IV).
 | Table III.Multivariate analysis of diseasefree
survival by Cox proportional hazards models. |
Table III.
Multivariate analysis of diseasefree
survival by Cox proportional hazards models.
| Variables | P-value | Hazard ratio (95%
CI) |
|---|
| Treatment
(capecitabine monotherapy vs. no chemotherapy) | 0.014 | 0.500
(0.288–0.867) |
| Tumor size (≤2 vs.
>2 cm) | 0.034 | 1.512
(1.032–2.215) |
| Nuclear grade (I or
II vs. III) | 0.233 | – |
| Axillary node
status (positive vs. negative) | 0.000 | 2.086
(1.605–2.713) |
| HER2 status
(positive vs. negative) | 0.000 | 3.807
(2.123–6.826) |
| ER status (positive
vs. negative) | 0.381 | – |
| PR status (positive
vs. negative) | 0.587 | – |
 | Table IV.Multivariate analysis of
cancerspecific survival by Cox proportional hazards models. |
Table IV.
Multivariate analysis of
cancerspecific survival by Cox proportional hazards models.
| Variables | P-value | Hazard ratio (95%
CI) |
|---|
| Treatment
(capecitabine monotherapy vs. no chemotherapy) | 0.181 | – |
| Tumor size (≤2 vs.
>2 cm) | 0.648 | – |
| Nuclear grade (I or
II vs. III) | 0.201 | – |
| Axilla status
(positive vs. negative) | 0.000 | 2.785
(1.924–4.031) |
| HER2 status
(positive vs. negative) | 0.030 | 2.412
(1.092–5.332) |
| ER status (positive
vs. negative) | 0.323 | – |
| PR status (positive
vs. negative) | 0.370 | – |
Adverse events and comorbidities
The adverse events of capecitabine monotherapy were
recorded. Hand-foot syndrome in the capecitabine monotherapy group
mainly occurred after 3 treatment cycles. Among the capecitabine
monotherapy group, 10 patients had grade ≥3 serious hand-foot
syndrome, among whom 2 aborted the chemotherapy after 3 and 4
cycles. Other adverse events, including anemia, neutropenia,
nausea, vomiting, diarrhea and liver injury, are summarized in
Table V. Therefore, capecitabine
appeared to be well-tolerated by elderly women with breast cancer
in the present study.
 | Table V.Grade 3, 4 or 5 adverse eventsa in
the capecitabine monotherapy group. |
Table V.
Grade 3, 4 or 5 adverse eventsa in
the capecitabine monotherapy group.
| Adverse events | No. of patients
(%) |
|---|
| Hematological |
|
|
Anemia | 2
(1.9) |
|
Neutropenia | 5
(4.8) |
| Febrile
neutropenia | 0
(0.0) |
| Gastrointestinal
reactions |
|
|
Nausea | 2
(1.9) |
|
Vomiting | 3
(2.9) |
|
Diarrhea | 3
(2.9) |
| Liver injury |
|
| ALT
increased | 2
(1.9) |
| AST
increased | 4
(3.8) |
|
Handfoot skin reaction | 10 (9.6) |
The patients' comorbidities are summarized in
Table VI. The incidence of
hypertension, cardiovascular disease and cerebral vascular disease
was higher in the no chemotherapy group compared with the
capecitabine monotherapy group. The percentage of patients with ≥2
comorbidities was also higher in the no chemotherapy group.
Comorbidities significantly affected the patients' decision to
receive chemotherapy.
 | Table VI.Comorbidities of patients in the two
groups. |
Table VI.
Comorbidities of patients in the two
groups.
| Comorbidities | No chemotherapy
(n=147) | Capecitabine
monotherapy (n=104) | P-value |
|---|
| Hypertension | 71 | 30 | 0.003 |
| Diabetes | 41 | 18 | 0.069 |
| Cardiovascular
disease | 38 | 12 | 0.006 |
| Cerebrovascular
disease | 27 | 7 | 0.008 |
| ≥2
comorbidities | 28 | 4 |
2.283×10−4 |
| No comorbidity | 19 | 41 |
2.085×10−6 |
Discussion
Capecitabine is an orally administered
chemotherapeutic agent that was licensed to be used as monotherapy
for the treatment of patients with locally advanced or metastatic
breast cancer following failure of previous anthracycline or
taxane-containing chemotherapy (6).
The efficacy of capecitabine in metastatic or locally advanced
breast cancer has been evaluated in various phase II/III clinical
trials. The response rate of metastatic breast cancer to
capecitabine monotherapy is ~30% (6,7).
Capecitabine clearly improved the survival rate in second- and
later-line salvage chemotherapy (6–10), and
its efficacy has been also proven in the first-line treatment of
metastatic breast cancer (11–14).
Kamal et al observed similar survival between capecitabine
monotherapy and single-agent taxane as a first-line therapy for
metastatic breast cancer (15).
Capecitabine has also been used was neoadjuvant
chemotherapy for breast cancer. Arowolo et al reported that
capecitabine monotherapy achieved good overall response rates with
manageable toxicity when administered as neoadjuvant chemotherapy
to 16 patients with locally advanced breast cancer (16). In a phase II study by Tolaney et
al, 3 patients exhibited a complete clinical response after 4
cycles of capecitabine monotherapy and 8 patients exhibited a
partial clinical response among 24 women with operable hormone
receptor-positive breast cancer (17). Li et al reviewed the
randomized controlled trials that included capecitabine in the
neoadjuvant chemotherapy for breast cancer and concluded that
adding capecitabine to neoadjuvant chemotherapy was unlikely to
improve the outcomes in breast cancer patients without distant
metastases (18).
The antitumor activity of capecitabine in metastatic
or locally advanced breast cancer makes it a potential alternative
to standard adjuvant chemotherapy for breast cancer. While
capecitabine demonstrated a poor efficacy as neoadjuvant therapy
for breast cancer, its efficacy in the adjuvant setting has not
been fully elucidated. In fact, it is not currently rare to use
adjuvant capecitabine monotherapy in elderly women with breast
cancer who are unable to undergo standard chemotherapy in China.
However, there is little evidence to support its effectiveness. A
meta-analysis by Jiang et al demonstrated that disease-free
survival significantly improved following addition of capecitabine
to an anthracycline-taxane-based adjuvant chemotherapy in patients
with high-risk early breast cancer (19). The final results of the FinXX trial
indicated that the addition of capecitabine significantly improved
breast cancer-specific survival, although not the recurrence-free
survival, in early breast cancer (20). A small retrospective study involving
elderly patients with stage IIa breast cancer by Hu et al
reported that the patients who underwent adjuvant capecitabine
monotherapy had a similar overall survival compared with those who
received traditional cyclophosphamide/epirubicin/5-fluorouracil
(CAF regimen) chemotherapy, but fewer adverse reactions (21). They compared the overall survival of
the two groups by Kaplan-Meier analysis, a univariate method;
however, multivariate survival analysis is required to investigate
whether capecitabine monotherapy is an independent factor that
affects survival. A randomized clinical trial by Muss et al
revealed that standard adjuvant chemotherapy was superior to
capecitabine monotherapy in elderly patients with early-stage
breast cancer (22). The standard
adjuvant chemotherapy included CMF cyclophosphamide, methotrexate
and fluorouracil (CMF regimen) and doxorubicin/cyclophosphamide (AC
regimen). The relapse-free survival rate was 68% in the
capecitabine monotherapy group vs. 85% in the standard chemotherapy
group (P<0.001), and the overall survival rate was 86 vs. 91%
(P=0.02). However, whether elderly women with breast cancer benefit
from adjuvant capecitabine monotherapy remains unclear.
To the best of our knowledge, the present
retrospective study was the first to demonstrate that adjuvant
capecitabine monotherapy significantly reduced recurrence or
metastasis in elderly patients with breast cancer. The disease-free
survival rate was 81.7% in the capecitabine monotherapy group and
65.3% in the no chemotherapy group (P=0.015). In the Kaplan-Meier
analysis, the disease-free survival curve of the capecitabine
monotherapy group was superior to that of the no chemotherapy group
(Fig. 1). The breast cancer-specific
survival rate of the capecitabine monotherapy group was 89.3% with
a median follow-up of 54 months, whereas it was 81.3% with a median
follow-up of 57 months in the no chemotherapy group. The
cancer-specific survival curve of the capecitabine monotherapy
group was superior to that of the no chemotherapy group (Fig. 2), although the difference was not
statistically significant (P=0.128). The results of the Cox
regression analysis demonstrated that capecitabine was an
independent predictive factor for disease-free survival, but not
for cancer-specific survival. Adjuvant capecitabine monotherapy did
not improve the cancer-specific survival rate. This finding may be
due to the short median follow-up duration. Positive results for
cancer-specific survival may be obtained in future analyses.
The present study included elderly patients aged ≥60
years, according to the definition of elderly individuals in the
Law of the People's Republic of China on Protection of the Rights
and Interests of the Elderly. Furthermore, only a limited number of
patients aged <60 years received adjuvant capecitabine
monotherapy in our department.
Adjuvant trastuzumab therapy was shown to
significantly improve disease-free and overall survival in patients
with HER2-positive early breast cancer (23). None of the 27 HER2-positive patients
in this retrospective cohort received adjuvant trastuzumab therapy,
as trastuzumab is not covered by health insurance in China, and the
majority of elderly patients cannot afford this treatment.
Capecitabine is a fluoropyrimidine carbamate that
achieves higher intratumoral levels with a lower toxicity compared
with 5-fluorouracil (24).
Capecitabine was considered as a chemotherapeutic agent that was
safe to use in patients with liver or renal disease. In the present
study, ~60% of patients in the capecitabine monotherapy group had
comorbidities, such as hypertension, diabetes, cerebrovascular
disease and cardiovascular disease. Severe adverse reactions were
rare, and the most common adverse event was hand-foot syndrome,
which occurred in 10 patients. Other adverse events, including
hematological adverse events, gastrointestinal reactions and liver
injury, were tolerable. None of the patients interrupted the
chemotherapy due to a severe adverse reaction. Thus, capecitabine
monotherapy was safe to for elderly patients, even those with
comorbidities. The comorbidities were compared between different
groups (Table VI) and were found to
be more frequent in the no chemotherapy group. Although
capecitabine monotherapy was safe, the comorbidities of the
patients affected their decision to receive this orally
administered chemotherapeutic agent.
The present study has some inevitable limitations
due to its retrospective nature. A prospective, randomized control
study has been designed and will be initiated in the near future.
Additionally, the effect of different endocrine therapy agents that
were administered to patients was not evaluated.
In summary, adjuvant capecitabine monotherapy was
found to be effective in elderly patients with breast cancer and
its side effects were manageable. Capecitabine monotherapy is a
good alternative for elderly breast cancer patients who refuse
standard adjuvant therapy. Our findings suggest that adjuvant
capecitabine monotherapy in elderly women with breast cancer was a
safe and effective treatment option.
Acknowledgements
The authors would like to thank the participating
patients for their willingness to cooperate. We also greatly
appreciate the assistance of American Journal Experts (AJE)
editors. This study was supported by the Key Research and
Development Program of Shandong Province (grant no. 2015GSF118023)
and the Projects of Medical and Health Technology Development
Program in Shandong Province (grant no. 2014WS0349).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joensuu H: Systemic chemotherapy for
cancer: From weapon to treatment. Lancet Oncol. 9:3042008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar
|
|
4
|
Berry DA, Cronin KA, Plevritis SK, Fryback
DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD and
Feuer EJ: Cancer Intervention and Surveillance Modeling Network
(CISNET) Collaborators: Effect of screening and adjuvant therapy on
mortality from breast cancer. N Engl J Med. 353:1784–1792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikhail SE, Sun JF and Marshall JL: Safety
of capecitabine: A review. Expert Opin Drug Saf. 9:831–841. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blum JL: The role of capecitabine, an
oral, enzymatically activated fluoropyrimidine, in the treatment of
metastatic breast cancer. Oncologist. 6:56–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blum JL, Jones SE, Buzdar AU, LoRusso PM,
Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS and Griffin T:
Multicenter phase II study of capecitabine in paclitaxel-refractory
metastatic breast cancer. J Clin Oncol. 17:485–493. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller KD, Chap LI, Holmes FA, Cobleigh
MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD,
Sing AP, et al: Randomized phase III trial of capecitabine compared
with bevacizumab plus capecitabine in patients with previously
treated metastatic breast cancer. J Clin Oncol. 23:792–799. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Talbot DC, Moiseyenko V, Van Belle S,
O'Reilly SM, Alba Conejo E, Ackland S, Eisenberg P, Melnychuk D,
Pienkowski T, Burger HU, et al: Randomised, phase II trial
comparing oral capecitabine (Xeloda) with paclitaxel in patients
with metastatic/advanced breast cancer pretreated with
anthracyclines. Br J Cancer. 86:1367–1372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schott AF, Barlow WE, Albain KS, Chew HK,
Wade JL III, Lanier KS, Lew DL, Hayes DF, Gralow JR, Livingston RB
and Hortobagyi GN: Phase II trial of simple oral therapy with
capecitabine and cyclophosphamide in patients with metastatic
breast cancer: SWOG S0430. Oncologist. 17:179–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stockler MR, Harvey VJ, Francis PA, Byrne
MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS,
Nowak AK, et al: Capecitabine versus classical cyclophosphamide,
methotrexate, and fluorouracil as first-line chemotherapy for
advanced breast cancer. J Clin Oncol. 29:4498–4504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaufmann M, Maass N, Costa SD, Schneeweiss
A, Loibl S, Sütterlin MW, Schrader I, Gerber B, Bauer W, Wiest W,
et al: First-line therapy with moderate dose capecitabine in
metastatic breast cancer is safe and active: Results of the MONICA
trial. Eur J Cancer. 46:3184–3191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oshaughnessy JA, Blum J, Moiseyenko V,
Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B,
Burger HU and Laws S: Randomized, open-label, phase II trial of
oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF
(cyclophosphamide, methotrexate and 5-fluorouracil) as first-line
therapy for advanced/metastatic breast cancer. Ann Oncol.
12:1247–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blum JL, Barrios CH, Feldman N, Verma S,
McKenna EF, Lee LF, Scotto N and Gralow J: Pooled analysis of
individual patient data from capecitabine monotherapy clinical
trials in locally advanced or metastatic breast cancer. Breast
Cancer Res Treat. 136:777–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamal AH, Camacho F, Anderson R, Wei W,
Balkrishnan R and Kimmick G: Similar survival with single-agent
capecitabine or taxane in first-line therapy for metastatic breast
cancer. Breast Cancer Res Treat. 134:371–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arowolo OA, Njiaju UO, Ogundiran TO,
Abidoye O, Lawal OO, Obajimi M, Adetiloye AV, Im HK, Akinkuolie AA,
Oluwasola A, et al: Neo-adjuvant capecitabine chemotherapy in women
with newly diagnosed locally advanced breast cancer in a
resource-poor setting (Nigeria): Efficacy and safety in a phase II
feasibility study. Breast J. 19:470–477. 2013.PubMed/NCBI
|
|
17
|
Tolaney SM, Jeong J, Guo H, Brock J,
Morganstern D, Come SE, Golshan M, Bellon J, Winer EP and Krop IE:
A phase II study of preoperative capecitabine in women with
operable hormone receptor positive breast cancer. Cancer Med.
3:293–299. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Jiang Y, Wei W, Yang H and Liu J:
Clinical efficacy of including capecitabine in neoadjuvant
chemotherapy for breast cancer: A systematic review and
meta-analysis of randomized controlled trials. PLoS One.
8:e534032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Yin W, Zhou L, Yan T, Zhou Q, Du
Y, Shen Z, Shao Z and Lu J: First efficacy results of capecitabine
with anthracycline- and taxane-based adjuvant therapy in high-risk
early breast cancer: A meta-analysis. PLoS One. 7:e324742012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joensuu H, Kellokumpu-Lehtinen PL,
Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, Ahlgren J,
Auvinen P, Paija O, Helle L, et al: Adjuvant capecitabine,
docetaxel, cyclophosphamide, and epirubicin for early breast
cancer: Final analysis of the randomized FinXX trial. J Clin Oncol.
30:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu W, Shi J, Sheng Y, Li L, Su D and Wang
CK: Clinical study of adjuvant capecitabine monotherapy in Chinese
elderly patients (aged 55–70) with stage IIa breast cancer.
Onkologie. 33:433–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muss HB, Berry DA, Cirrincione CT,
Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG,
Cohen HJ, Becker HP, et al: Adjuvant chemotherapy in older women
with early-stage breast cancer. N Engl J Med. 360:2055–2065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gianni L, Dafni U, Gelber RD, Azambuja E,
Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch
C, et al: Treatment with trastuzumab for 1 year after adjuvant
chemotherapy in patients with HER2-positive early breast cancer: A
4-year follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nole F, Catania C, Munzone E, Rocca A,
Verri E, Sanna G, Ascione G, Adamoli L, Zampino MG, Minchella I and
Goldhirsch A: Capecitabine/vinorelbine: An effective and
well-tolerated regimen for women with pretreated advanced-stage
breast cancer. Clin Breast Cancer. 6:518–524. 2006. View Article : Google Scholar : PubMed/NCBI
|