Introduction
Burkitt lymphoma is a highly aggressive type of
B-cell non-Hodgkin lymphoma (NHL) and is characterized by a
translocation involving the c-MYC oncogene. Burkitt lymphoma is
internationally recognized to comprise three clinical variants,
namely endemic, sporadic and immunodeficiency-related. The endemic
type is associated with Epstein-Barr virus infection and is most
prevalent in Africa and the Middle East; the sporadic type occurs
throughout the rest of the world and constitutes 1–2% of adult and
30–40% of childhood NHL cases in Europe and North America; the
immunodeficiency-related type is associated with infection by the
human immunodeficiency virus (1,2). Due to
its chemosensitive nature, specific regimens of intensive
chemotherapy have been developed for the treatment of Burkitt
lymphoma, and a regimen of cyclophosphamide, vincristine,
doxorubicin, high-dose methotrexate ifosfamide, etoposide and
high-dose cytarabine (CODOX-M/IVAC) has been established as the
standard therapy with a relatively high event-free survival (EFS)
and overall survival (OS), particularly in patients aged <65
years (2). Moreover, several studies
have reported that adding anti-CD20 monoclonal antibody (rituximab)
to the therapy may achieve superior outcomes compared with
controls. The CODOX-M/IVAC regimen has been reported to be
associated with 2-year OS rates of 64–82% and an EFS rate of 92%
(2,3).
Intestinal atresia affects neonates in the majority
of the cases. Primary small intestinal atresia mostly affects the
duodenum (>50%), and is usually associated with chromosomal
anomalies, such as trisomy 21, as well as other factors, including
race, maternal age and birthweight (4). Secondary atresia rarely occurs after
necrotizing enteritis, intussusception, meconium peritonitis and
abdominal surgeries (5). Although
chemotherapy-induced intestinal mucositis has been broadly
reported, and intestinal hemorrhage and spontaneous perforation
have been considered as complications of NHL following intensive
chemotherapy in children, intestinal atresia secondary to
chemotherapy is uncommon (6–9). We herein describe a case of NHL in a
young adult who developed extreme intestinal stenosis, likely
triggered by chemotherapy, but presenting as superior mesenteric
artery syndrome (SMAS).
Case report
A 22-year-old male patient presented to a community
hospital with a 4-month history of fatigue, paleness and
intermittent melena in July 2015. The patient reported suffering
from anorexia for 1 month prior to the onset of the abovementioned
symptoms. The patient had no history of hair coloring and no
exposure to radioactive substances or oil-based paints. The white
blood cell count was 17.5×109/l (normal range,
4.0–10.0×109/l) and the hemoglobin level was 55 g/l
(normal range, 120–160 g/l). Baseline abdominal computed tomography
(CT) detected multiple intestinal lesions in the epigastric and
umbilical region, with extensive retroperitoneal lymphadenopathy.
Biopsy of the descending part of the duodenum revealed the presence
of diffuse large B-cell lymphoma. One month later, the patient
presented with cramping pain and abdominal bloating, with edema of
the bilateral lower limbs, and was transferred to a tertiary care
hospital for further assessment. On physical examination, severe
anemic appearance was noted. No superficial lymph nodes were
palpated, and the findings of the cardiovascular and pulmonary
examinations were unremarkable. There was a slight protuberance
over the abdomen, but there was no tenderness or organomegaly.
The patient received one course of chemotherapy with
rituximab, vincristine, epirubicin, etoposide, dexamethasone and
cyclophosphamide (R-EPOCH regimen). The definitive diagnosis after
the first course was Burkitt lymphoma and the patient received
cyclophosphamide, doxorubicin, rituximab and vincristine, with
intrathecal injection of cytarabine and methotrexate (modified
CODOX-M regimen with rituximab). During the period of chemotherapy,
the patient presented with repeated vomiting, which was relieved by
administration of antiemetic drugs. On January 4, 2016, sudden
onset of aggravated vomiting ensued, with palpitations and weakness
of the limbs, which could not be relieved by antiemetic therapy.
The patient reported no epigastric pain, abdominal distension or
headache, but on physical examination, tenderness over the
epigastric region and positive succussion splash were noted. The
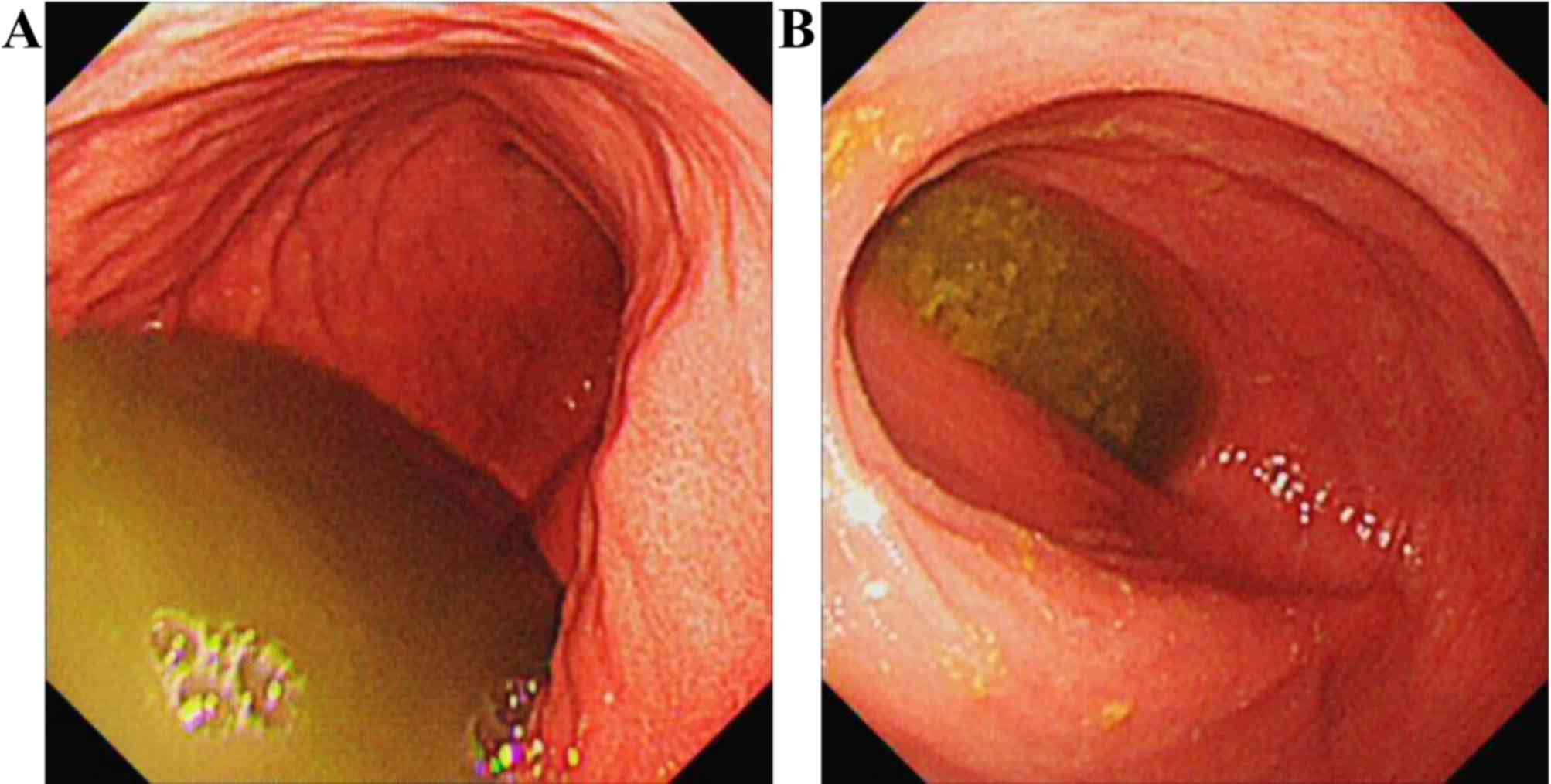
subsequent esophagogastroduodenoscopy (EGD) detected a large amount
of fluid retention in the gastric cavity and the duodenum. There
was no residual lymphoma or stenosis observed in the descending
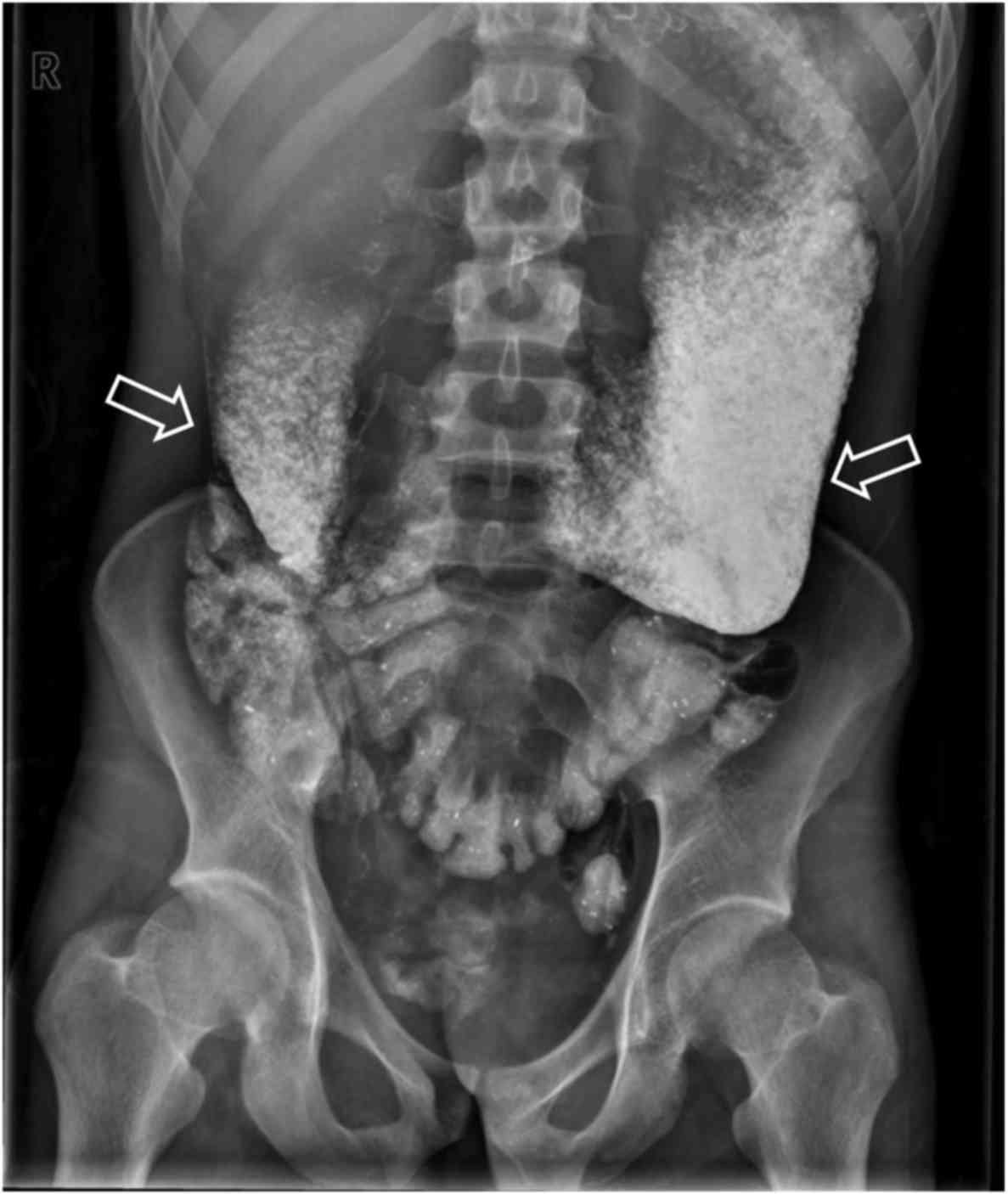
part of the duodenum detected on EGD (Fig. 1). Abdominal plain X-ray and
alimentary tract radiography with barium revealed duodenal
obstruction, raising the suspicion of superior mesenteric artery
syndrome (Figs. 2 and 3). Ultrasound revealed a narrow angle of
10° between the superior mesenteric artery and the abdominal aorta.
The patient was primarily diagnosed with superior mesenteric artery
syndrome and treated with proton pump inhibitors, somatostatin,
nasogastric drainage and sufficient parenteral nutritional supply,
but the symptoms continued. On February 21, 2016, the patient was
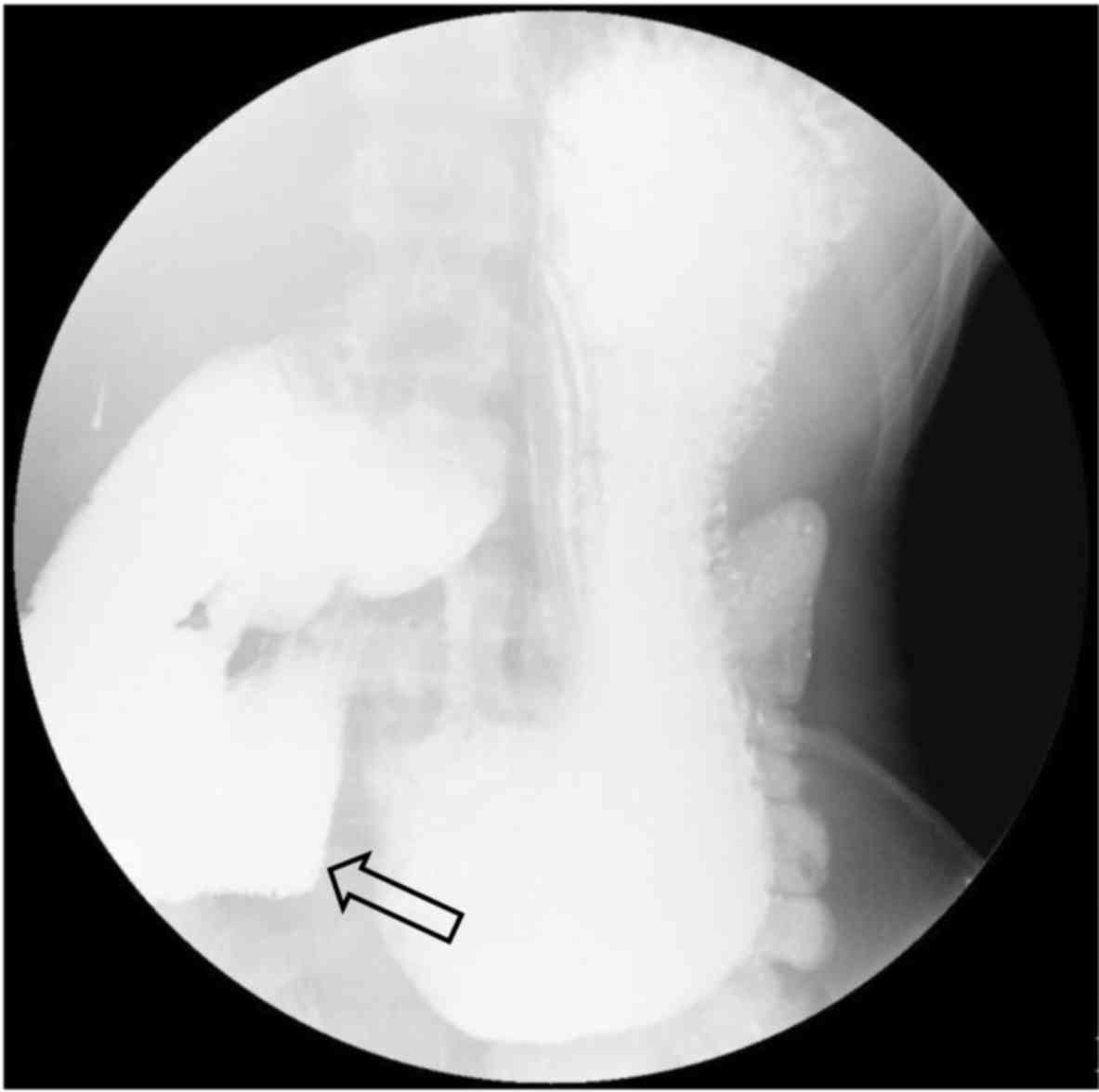
transferred to the Department of Surgery for exploratory
laparotomy. During surgery, a focal ‘band-like’ narrowing was
observed at the junction between the ascending part of the duodenum
and the proximal part of the jejunum, with a gross appearance of
occluded intestinal lumen at the site of lesion. Of note, mild
dilation was observed in the part of the duodenum posterior to the
superior mesenteric artery (Fig. 4).
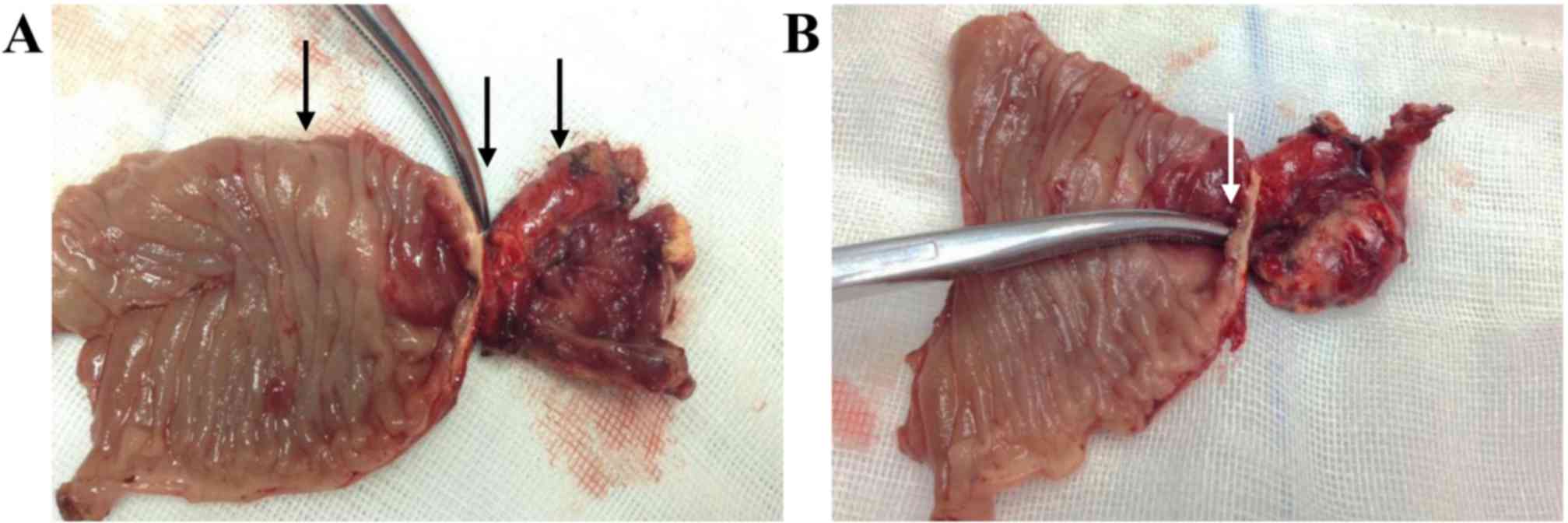
Duodenojejunectomy and end-to-side anastomosis were performed
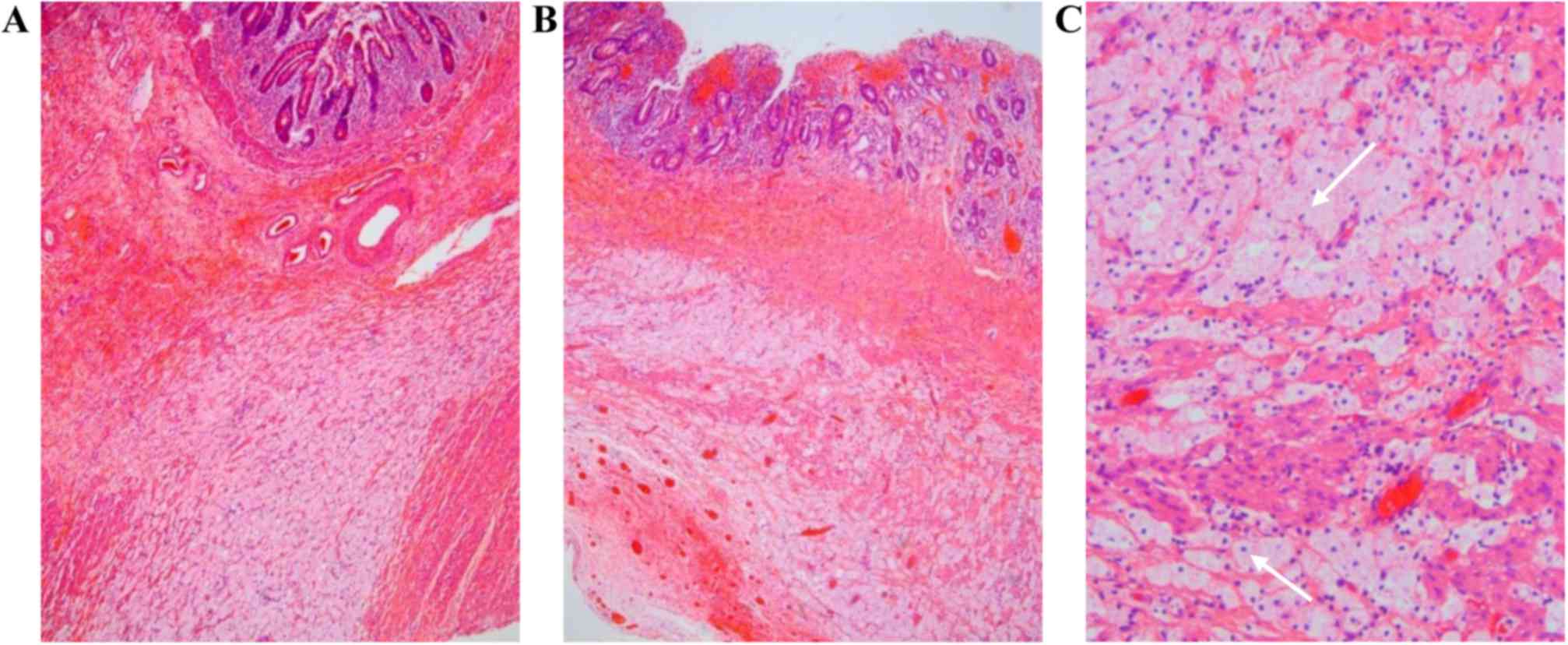
during the surgery. The histopathology of the resected specimen
revealed atrophy of the epithelium and severe structural
disturbance of the submucosa and muscularis propria at the site of
atresia (Fig. 5).
The patient recovered well from the procedure, with
resolution of the symptoms, and further chemotherapies were
well-tolerated.
Written informed consent was obtained from the
patient authorizing use and disclosure of his protected health
information.
Discussion
We herein describe a case of secondary intestinal
atresia, possibly induced by chemotherapy, in a young adult
diagnosed with Burkitt lymphoma. The case is interesting, as the
onset and progression of the symptoms were highly suggestive of
SMAS. Although the present case included lymphomatous involvement
of the duodenum prior to treatment, the microscopic examination of
the surgical specimen detected no signs of neoplasia after
chemotherapy.
Nausea, vomiting and diarrhea are most common
gastrointestinal complications of cancer chemotherapy, and
mucositis due to the damage of the proliferating cells at the base
of the mucosal squamous epithelia or in the intestinal crypts
usually involves the gastrointestinal tract (10). Hemorrhage and perforation of the
small intestine have been reported in the previous literature as
complications associated with chemotherapy for NHL; however, in the
majority of the cases, evidence of the tumor is found on
histological examination of the specimen. Coward et al
reported a case of suspected chemotherapy-induced bowel obstruction
in small-cell lung cancer, in which pathological examination of the
excised bowel specimen revealed severe ulceration, transmural
necrosis and extensive fibrosis (6).
Kehoe et al reported that >100 patients developed
intestinal obstruction following intraperitoneal chemotherapy and
concluded that the majority of the obstructions were associated
with the progression of the primary malignancy, whereas only 12% of
the patients developed mechanical obstruction due to adhesions. The
adhesions were described as ‘coating or encasing the bowels’
intraoperatively, but no apparent stenosis of the intestine was
observed (7).
Several factors may contribute to the intestinal
obstruction after chemotherapy. The administration of antiemetic
drugs of 5-HT receptor antagonists may greatly decrease peristalsis
and result in paralytic ileus; furthermore, constipation may
occasionally develop secondary to prolonged bed rest. However,
tumor cell necrosis under chemotherapy is hypothesized to be a
prominent factor in the development of intestinal obstruction.
Microtubular toxins, such as vincristine, are commonly associated
with autonomic bowel dysfunction, and etoposide, as a
podophyllotoxin derivative that interferes with microtubular
binding, may cause paralytic ileus (6). Burkitt lymphoma as a highly
proliferative hemotological malignancy usually involving several
organs. The tumor cells infiltrating the wall of the small
intestine are destroyed during chemotherapy, causing inflammation,
necrosis, fibrosis of the adjacent tissue, and even perforation and
hemorrhage (9,11,12). In
addition, it is widely known that chemotherapy may interrupt the
DNA synthesis of intestinal mucosal cells and cause atrophy of
epithelia (13). The transmural
necrosis and fibrosis were not prominent on histological
examination in the present case, but extensive tissue degeneration
was observed in the submucosa and muscularis propria, with an
extensive tissue cell response. The necrosis of lymphoma cells
occurred in the submucosa and muscularis propria, and the space was
replaced by large numbers of foam-like cells, which were likely
derived from macrophages. Therefore, it is possible that the
intestinal atresia observed in this case is attributable to the
altered structure of the intestinal wall.
The diagnosis of SMAS is primarily based on clinical
suspicion, with additional upper gastrointestinal barium
examination, ultrosonography and/or CT angiography. The diagnosis
is most probable when radiography detects a decreased
aortomesenteric angle (<25°) and an aortomesenteric distance of
<10 mm (14,15). The case was misdiagnosed prior to
surgery, since the clinical manifestations with acute weight loss,
and the supportive findings on barium contrast examination, with an
extremely narrow angle between the superior mesenteric artery and
abdominal aorta, constituted strong evidence supporting the
diagnosis of SMAS. Furthermore, the short distance between the
predicted compression site of the superior mesenteric artery and
the actual site of obstruction, also contributed to the
misdiagnosis. Unfortunately, abdominal CT scan, which may have
enabled identification of the exact obstruction site prior to
surgery, was not performed in this case. However, regardless of
intestinal necrosis, atresia or perforation, surgical intervention
is considered to be a necessary intervention.
In summary, the present study describes a case of
NHL, with post-chemotherapeutic intestinal atresia originally
misdiagnosed as SMAS. This case indicates the importance of an
abdominal CT scan in confirming the cause of the intestinal
obstruction, and may also remind physicians to be aware of rare
bowel complications associated with polychemotherapeutic regimens,
and take measures for the protection of the intestinal mucosa
during chemotherapy.
References
|
1
|
Molyneux EM, Rochford R, Griffin B, Newton
R, Jackson G, Menon G, Harrison CJ, Israels T and Bailey S:
Burkitt's lymphoma. Lancet. 379:1234–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casulo C and Friedberg J: Treating Burkitt
lymphoma in adults. Curr Hematol Malig Rep. 10:266–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasterlid T, Brown PN, Hagberg O, Hagberg
H, Pedersen LM, D'Amore F and Jerkeman M: Impact of chemotherapy
regimen and rituximab in adult Burkitt lymphoma: A retrospective
population-based study from the Nordic Lymphoma Group. Ann Oncol.
24:1879–1886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi D, Hiroma T, Takamizawa S and
Nakamura T: Population-based study of esophageal and small
intestinal atresia/stenosis. Pediatr Int. 56:838–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu KC, Wu XJ, Feng JX and Wei MF:
Experienes of managing children with acquired intestinal stenosis
and atresia. Chin J Pediatr Surg. 36:211–214. 2015.(In
Chinese).
|
|
6
|
Coward JI, Ding NL, Feakins R, Kocher H,
Popat S and Szlosarek PW: Chemotherapy-induced bowel obstruction in
small cell lung cancer: A case report. Med Oncol. 29:2623–2625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kehoe SM, Williams NL, Yakubu R, Levine
DA, Chi DS, Sabbatini PJ, Aghajanian CA, Barakat RR and Abu-Rustum
NR: Incidence of intestinal obstruction following intraperitoneal
chemotherapy for ovarian tubal and peritoneal malignancies. Gynecol
Oncol. 113:228–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hata S, Pietsch J and Shankar S:
Intestinal complications in children undergoing chemotherapy for
mediastinal non-Hodgkin's lymphoma. Pediatr Hematol Oncol.
21:707–710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fallon SC, Redell MS, El-Bietar J, Lopez
ME, Vasudevan SA and Brandt ML: Intestinal perforation after
treatment of Burkitt's lymphoma: Case report and review of the
literature. J Pediatr Surg. 48:436–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sausville EA and Longo DL: Principles of
cancer treatment. In: Harrison's principles of internal medicine.
Longo DL and Fauci AS: McGraw-Hill Companies. (New York, NY).
708–709. 2011.
|
|
11
|
Howarth GS, Tooley KL, Davidson GP and
Butler RN: A non-invasive method for detection of intestinal
mucositis induced by different classes of chemotherapy drugs in the
rat. Cancer Biol Ther. 5:1189–1195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ara C, Coban S, Kayaalp C, Yilmaz S and
Kirimlioglu V: Spontaneous intestinal perforation due to
non-Hodgkin's lymphoma: Evaluation of eight cases. Dig Dis Sci.
52:1752–1756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abel E, Ekman T, Warnhammar E, Hultborn R,
Jennische E and Lange S: Early disturbance of microvascular
function precedes chemotherapy-induced intestinal injury. Dig Dis
Sci. 50:1729–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pottorf BJ, Husain FA, Hollis HJ and Lin
E: Laparoscopic management of duodenal obstruction resulting from
superior mesenteric artery syndrome. JAMA Surg. 149:1319–1322.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merrett ND, Wilson RB, Cosman P and
Biankin AV: Superior mesenteric artery syndrome: Diagnosis and
treatment strategies. J Gastrointest Surg. 13:287–292. 2009.
View Article : Google Scholar : PubMed/NCBI
|