Introduction
Bile duct hamartomas (BDH), also referred to as von
Meyenburg complex (VMC), were first described in 1918 (1), with a reported incidence of 5.6% in
adults and 0.9% in children (2). An
increasing number of patients with BDH may be diagnosed with the
development of imaging modalities. In the past, when the diagnosis
of BDH relied on pathological examination following liver biopsy,
it was considered to be a benign disease, managed by regular
observation rather than surgery. However, in recent years, an
increasing number of cases demonstrated that there is a possible
association between BDH and intrahepatic cholangiocarcinoma
(3–5). Therefore, BDH should be resected when
possible. Thus far, whether BDH patients should undergo surgical
treatment remains controversial. The aim of the present study was
to retrospectively investigate 9 BDH cases in our hospital to
highlight the importance of surgical treatment for BDH.
Patients and methods
Study design
A retrospective study on patients who were diagnosed
with BDH between June 2007 and December 2015 at the Eastern
Hepatobiliary Surgery Hospital (Shanghai, China) was conducted. The
medical records of these patients, including clinical, imaging and
pathological characteristics, were retrieved. The laboratory tests
performed prior to surgery included liver function tests, viral
hepatitis markers and tumor markers, such as serum α-fetoprotein
(AFP), carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic
antigen (CEA). The imaging examinations included ultrasonography
(US), computed tomography (CT), magnetic resonance imaging (MRI)
and magnetic resonance cholangiopancreatography (MRCP). After the
surgery, the final diagnosis was confirmed by two independent
pathologists. All the patients were followed up by telephone
communication until the end of this study or until the patients'
death, and follow-up data were obtained.
All the study participants, or their legal
guardians, provided written informed consent prior to study
enrollment. The study was reviewed and approved by the Eastern
Hepatobiliary Surgery Hospital Institutional Review Board.
Results
Patient characteristics
The patients included 7 men and 2 women, aged 35–70
years (mean age, 48.2±10.5 years). Two patients presented with
clinical symptoms, whereas the remaining 7 patients were
asymptomatic. Only 1 patient had increased AFP levels (103.5±279.1
µg/l; normal range, 0–20 µg/l), 4 patients had increased CA19-9
levels (132.1±266.1 µg/l; normal range, 0–39 µg/l), 2 patients had
increased total bilirubin (TBIL): 33.2±56.7 µmol/l (normal range,
3.42–20.52 µmol/l), 2 patients had increased alanine
aminotransferase (ALT) levels: 40.2±20.7 IU/l (normal range, 9–50
IU/l) and 3 patients had increased aspartate aminotransferase (AST)
levels: 31.7±18.0 IU/l (normal range, 15–40 IU/l). The CEA level
was normal (2.6±0.8 µg/l; normal range, 0–10 µg/l), and the
majority of the patients had normal hepatic function. Five cases
were positive for hepatitis B surface antigen. These results
demonstrated that the BDH patients had no typical clinical symptoms
or laboratory test data (Table
I).
 | Table I.Clinical characteristics of patients
with bile duct hamartoma. |
Table I.
Clinical characteristics of patients
with bile duct hamartoma.
| Patient (n) | Sex | Clinical
symptoms | Age symptoms | AFP (µg/l) | CEA (µg/l) | CA19-9 (µ/ml) | TBIL (µmol/l) | ALT (µ/l) | AST (µ/l) | HBsAgt |
|---|
| 1 | M | Abdominal pain | 45 | 2.3 | 2.7 | 13.3 | 14.4 | 58.1 | 29.0 | Negative |
| 2 | M | No symptoms | 35 | 9.6 | 2.1 | 49.4 | 7.2 | 37.0 | 24.0 | Positive |
| 3 | M | No symptoms | 70 | 893 | 3.3 | 112.3 | 30.8 | 41.0 | 56.0 | Positive |
| 4 | F | No symptoms | 49 | 2.4 | 1.8 | 19.0 | 8.4 | 18.0 | 16.0 | Positive |
| 5 | M | No symptoms | 50 | 3.7 | 3.3 | 36.1 | 13.8 | 36.0 | 21.0 | Positive |
| 6 | F | Jaundice | 58 | 2.4 | 4.1 | 878.8 | 192.3 | 86.0 | 65.0 | Negative |
| 7 | M | No symptoms | 41 | 11.2 | 2.4 | 69.2 | 8.1 | 45.0 | 45.0 | Positive |
| 8 | M | No symptoms | 35 | 3.1 | 1.9 | 0.6 | 16.3 | 13.1 | 12.1 | Negative |
| 9 | M | No symptoms | 51 | 3.4 | 1.9 | 9.9 | 7.9 | 28.0 | 17.0 | Negative |
Imaging examinations
Prior to surgery, at least one of the medical
imaging modalities was applied. A total of 8 patients underwent US
examination and the lesions appeared as hypoechoic, hyperechoic and
with mixed echogenicity. A total of 5 patients underwent CT
examination; in all 5 patients, the lesions were low-density on
plain CT, without enhancement in 4 cases and inhomogeneous
enhancement in 1 case. A total of 5 patients underwent MRI
examination, which revealed hypointensity on T1-weighted imaging
(WI) and hyperintensity on T2WI. A total of 4 patients exhibited
enhancement and 1 exhibited no enhancement in the arterial phase.
Two patients underwent MRCP due to jaundice and intrahepatic bile
duct dilation. The preoperative diagnosis was liver cancer in 4,
hepatic hilar biliary obstruction in 1, chronic calculous
cholecystitis in 1, suspected hepatic abscess in 1, and benign
liver tumor in 2 patients (Table II
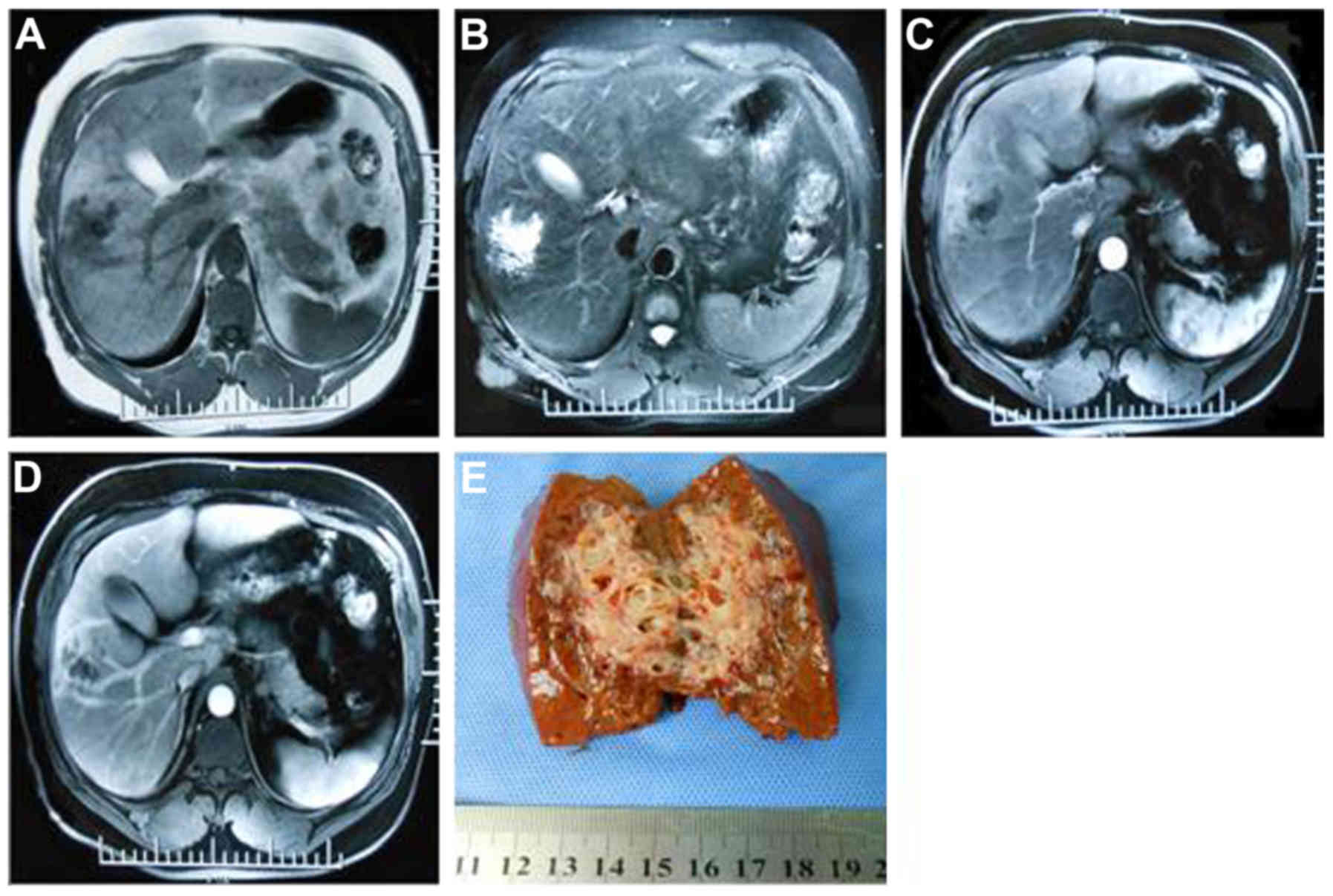
and Fig. 1). Therefore, it was
difficult for the clinicians to reach a definitive diagnosis of BDH
without pathological examination, due to the variable appearance of
the lesions on imaging.
 | Table II.Appearance of bile duct hamartoma on
imaging examinations. |
Table II.
Appearance of bile duct hamartoma on
imaging examinations.
| Patient (n) | US | CT | MRI | MRCP | Preoperative
diagnosis |
|---|
| 1a | ND | ND | ND | ND | Chronic calculous
cholecystitis |
| 2 | Hyperechoic | Low-density on plain
CT; no enhancement in arterial phase | ND | ND | Liver cancer |
| 3 | Mixed echogenicity,
intrahepatic bile duct dilation | Low-density on plain
CT; no enhancement in arterial phase | Hypointense on T1WI,
hyperintense on T2WI, enhancement in arterial phase | ND | Liver cancer |
| 4 | Mixed
echogenicity | Low-density on plain
CT; no enhancement in arterial phase | Hypointense on T1WI,
hyperintense on T2WI, enhancement in arterial phase | ND | Liver cancer |
| 5 | Hyperechoic | ND | Hypointense on T1WI,
hyperintense on T2WI, enhancement in arterial phase | ND | Benign liver
tumor |
| 6 | Intrahepatic bile
duct dilation | ND | ND | Dilated bile
ducts | Hepatic hilar
biliary obstruction |
| 7 | Hypoechoic | Low-density on
plain CT; inhomogeneous enhancement in arterial phase | ND | ND | Liver cancer |
| 8 | Hyperechoic,
obscure boundary | Low-density on
plain CT; no enhancement in arterial phase | Hypointense on
T1WI, hyperintense on T2WI, no enhancement in arterial phase | ND | Hepatic
abscess |
| 9 | Hyperechoic | ND | Hypointense on
T1WI, hyperintense on T2WI, enhancement in arterial phase | Dilated bile
duct | Benign liver
tumor |
Pathological characteristics of the
patients
All the patients underwent surgery. In one of the
patients, during gallstone removal surgery, a small nodule was
incidentally discovered, which was pathologically diagnosed as BDH.
The majority of the tumors were located in the right lobe.
Macroscopically, all the tumors displayed a grayish white surface
and they were mostly non-encapsulated. One patient had accompanying
liver cirrhosis. The mean tumor diameter was 2.4±1.8 cm (range,
0.2–5.8 cm). Microscopically, all cases exhibited bile duct
proliferation. Nest-like formations were observed in 2 patients,
and in 1 patient the lesion had transformed to intrahepatic
cholangiocarcinoma (Table III and
Fig. 1). These results indicated
that BDH has the potential to transform into intrahepatic
cholangiocancinoma.
 | Table III.Pathological characteristics of the
patients with bile duct hamartomas. |
Table III.
Pathological characteristics of the
patients with bile duct hamartomas.
| Patient (n) | Location
(segment) | Pathological
diagnosis | Size (cm) | Gross
appearance | Microscopic
manifestations | Follow-up |
|---|
| 1 | V | BDH | 0.3×0.3 | Grey-white
nodule | Bile duct
proliferation | Alive |
| 2 | VI | BDH, partial
malignant transformation | 3.2×2.6 | Grey-white nodule,
no capsule | Bile duct
proliferation, partly nest-like arrangement | Alive |
| 3 | V | BDH, partial
malignant transformation | 1.2×1.0 | Grey-white nodule,
liver cirrhosis | Bile duct
proliferation, partly nest-like arrangement, liver cirrhosis | Alive |
| 4 | VIII | BDH | 1.4×1.2 | Grey-white nodule,
no capsule | Bile duct
proliferation | Alive |
| 5 | VIII | BDH | 1.4×1.1 | Grey-white
nodule | Bile duct
proliferation | Alive |
| 6 | III | BDH | 0.2×0.2 | Grey-white
nodule | Bile duct
proliferation | Alive |
| 7 | VI | Intrahepatic
cholangiocarcinoma, partial BDH | 5.8×4.1 | Grey-white nodule,
no capsule | Obvious atypical
cells, invasive growth, partly bile duct proliferation,
cholangiocarcinoma |
Deceased |
| 8 | IV | BDH | 4.0×2.8 | Grey-white nodule,
no capsule | Bile duct
proliferation | Alive |
| 9 | V | BDH | 4.0×3.7 | Grey-white nodule,
no capsule | Bile duct
proliferation | Alive |
Follow-up
At the end of the present study, follow-up data were
obtained for all the patients. The patient who was diagnosed with
intrahepatic cholangiocancinoma succumbed to the disease 25 months
after the operation. The other patients remained alive at the last
follow-up and had recovered well.
Discussion
In the present study, the majority of the patients
were asymptomatic and most laboratory test results were considered
normal. As shown in Table II, the
imaging modalities performed included US, CT, MRI and MRCP. On
plain imaging, although the characteristics were variable and
atypical, the majority of the lesions exhibited low density on CT,
hypointensity on T1WI and hyperintensity on T2WI. As shown in
Table III and Fig. 1, 2 cases exhibited partial malignant
transformation and in 1 case the BDH transformed into intrahepatic
cholangiocarcinoma and the patient succumbed to the disease 25
months after the operation. Therefore, BDH is difficult to diagnose
without pathological examination and, once the diagnosis is
confirmed, surgical treatment should be preferred due to the
potential transformation to cholangiocancinoma.
Several studies reported that a proportion of
patients with BDH may present with abdominal distension (6), bacterial infections (7), recurring cholangitis and jaundice
(8), and portal hypertension
(9). Similarly, 2 patients in our
study presented with abdominal pain and jaundice. In the present
case series, the majority of the patients had normal liver function
tests and tumor marker levels, but 2 patients exhibited increased
level of AFP, CA19-9, TBIL and ALT; one was mainly due to liver
cirrhosis and portal hypertension, whereas the other one was
possibly related to the jaundice.
It has been reported that there are various
sonographic appearances of BDH, including hyperechoic, hypoechoic
and mixed echogenicity (2,10–13),
which is similar to our findings. Wohlgemuth et al (14) hypothesized that the variable
sonographic appearance is mainly associated with the different
number and size of dilated biliary ducts and degree of liver
fibrosis. In 1998, Luo et al strongly recommended that the
appearance of multiple comet-tail echoes in the liver may be the
characteristic sonographic feature of BDH (15). However, in the present study, there
were no signs of multiple comet-tail echoes in any of the patients.
We hypothesized that this discrepancy may be attributed to the
meagerness of relevant knowledge and the low incidence rate of
BDH.
On plain CT, all the lesions exhibited low density,
which was consistent with the published literature (11,13). On
enhanced CT, the majority of the studies reported that there was no
enhancement of BDH due to its poor vascularity (16). In the present case series, 1 patient
exhibited inhomogeneous enhancement, which is somewhat similar to
two previously reported cases (17,18). All
the cases exhibited hypointensity on T1WI and hyperintensity on
T2WI, which is similar to previous reports (11,19,20). As
regards the enhancement of the lesion on MRI, several studies
reported enhancement (12,14,19,21),
whereas others reported no enhancement (11,22,23). In
the present series, 4 patients exhibited enhancement and 1 patient
exhibited no enhancement, which suggests that the presence of
enhancement plays no role in the diagnosis of BDH. Compared with CT
and MRI, MRCP exhibits superior sensitivity in identifying bile
duct disorders. In the present case series, only 2 patients
underwent MRCP, in one of whom a clear cluster of dilated bile
ducts without any communication with the main biliary system was
observed, appearing to be a BDH.
The exact pathogenesis of BDH remains unclear and it
is usually considered as a cluster of benign liver malformations
and ductal plate maldevelopment (13). Whether BDH has malignant potential
remains controversial. BDH was previously considered as a benign
liver tumor that should be followed regularly (11,24–26).
However, an increasing number of cases indicated that BDH may
transform to cholangiocarcinoma and hepatocellular carcinoma
(3,4,10,27–29).
In the present case series, 3 patients exhibited malignant
transformation, one of whom succumbed to the disease 25 months
after the operation. The findings of the present study indicate
that BDH has malignant potential and must be resected when the
diagnosis is definitive.
In summary, BDH may transform into
cholangiocarcinoma, which is a life-threatening malignancy; thus,
BDH should be resected if the lesion is operable. More cases should
be investigated to elucidate the mechanism underlying the
transformation of BDH to cholangiocarcinoma.
Acknowledgements
The present study was funded by the Nursery Project
of the Second Military Medical University (grant no. 2014QN19).
References
|
1
|
von Meyenburg H: Uber die Cystenleber.
Beitr Pathol Anat. 64:477–532. 1918.(In German).
|
|
2
|
Singh Y, Cawich SO, Ramjit C and
Naraynsingh V: Rare liver tumor: Symptomatic giant von Meyenburg
complex. J Surg Case Rep. 2016:pii: rjw1952017. View Article : Google Scholar
|
|
3
|
Kim HK and Jin SY: Cholangiocarcinoma
arising in von Meyenburg complexes. Korean J Hepatol. 17:161–164.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parekh V and Peker D: Malignant
transformation in von-meyenburg complexes: Histologic and
immunohistochemical clues with illustrative cases. Appl
Immunohistochem Molecul Morphol: AIMM. 23:607–614. 2015. View Article : Google Scholar
|
|
5
|
Gupta A, Pattnaik B, Das A and Kaman L:
Von Meyenburg complex and complete ductal plate malformation along
with Klatskin tumour: A rare association. BMJ Case Reports.
2016:10.1136/bcr-2016-215220, 2016.
|
|
6
|
Chandramouleeswari K, Anita S and Shivali
B: Mesenchymal hamartoma of the liver: A case report. J Clin Diagn
Res. 6:1552–1554. 2012.PubMed/NCBI
|
|
7
|
Hashimoto M, Ouchi M, Norose J, Suda FS,
Suzuki K, Matsumura N, Lgari Y, Suzuki T, Nakano H, Mizuse M, et
al: Bile duct hamartomas (von Meyenburg complexes) associated with
a bacterial infection: Case report of elderly diabetic patient.
Geriatr Geront Int. 11:534–536. 2011. View Article : Google Scholar
|
|
8
|
Sinakos E, Papalavrentios L, Chourmouzi D,
Dimopoulou D, Drevelegas A and Akriviadis E: The clinical
presentation of Von Meyenburg complexes. Hippokratia. 15:170–173.
2011.PubMed/NCBI
|
|
9
|
Cnossen WR and Drenth JP: Polycystic liver
disease: An overview of pathogenesis, clinical manifestations and
management. Orphanet J Rare Dis. 9:692014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu AM, Xian ZH, Zhang SH and Chen XF:
Intrahepatic cholangiocarcinoma arising in multiple bile duct
hamartomas: Report of two cases and review of the literature. Eur J
Gastroenterol Hepatol. 21:580–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lung PF, Jaffer OS, Akbar N, Sidhu PS and
Ryan SM: Appearances of von meyenburg complex on cross sectional
imaging. J Clin Imaging Sci. 3:222013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin YM: Biliary hamartoma presented as a
single mass. Korean J Hepatol. 17:331–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi QS, Xing LX, Jin LF, Wang H, Lv XH and
Du LF: Imaging findings of bile duct hamartomas: A case report and
literature review. Int J Clin Exp Med. 8:13145–13153.
2015.PubMed/NCBI
|
|
14
|
Wohlgemuth WA, Böttger J and Bohndorf K:
MRI, CT, US and ERCP in the evaluation of bile duct hamartomas (von
Meyenburg complex): A case report. Eur Radiol. 8:1623–1626. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo TY, Itai Y, Eguchi N, Kurosaki Y,
Onaya H, Ahmadi Y, Niitsu M and Tsunoda HS: Von Meyenburg complexes
of the liver: imaging findings. J Comput Assist Tomogr. 22:372–378.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng RQ, Zhang B, Kudo M, Onda H and
Inoue T: Imaging findings of biliary hamartomas. World J
Gastroenterol. 11:6354–6359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki H, Tsurita G, Ishihara S, Akahane
M, Kitayama J and Nagawa H: Resovist-enhanced MRI for preoperative
assessment of colorectal hepatic metastases: A case of multiple
bile duct hamartomas associated with colon cancer. Case Rep
Gastroenterol. 2:509–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patel S, Rajalakshmi BR and Manjunath GV:
Histopathologic findings in autopsies with emphasis on interesting
and incidental findings-a pathologist's perspective. J Clin Diagn
Res. 10:EC08–EC12. 2016.PubMed/NCBI
|
|
19
|
Gong J, Kang W and Xu J: MR imaging and MR
Cholangiopancreatography of multiple biliary hamartomas. Quant
Imaging Med Surg. 2:133–134. 2012.PubMed/NCBI
|
|
20
|
Lin S, Weng Z, Xu J, Wang MF, Zhu YY and
Jiang JJ: A study of multiple biliary hamartomas based on 1697
liver biopsies. Eur J Gastroenterol Hepatol. 25:948–952. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saidi RF, Yoon V, Jabbour N, Shah SA and
Bozorgzadeh A: Liver transplantation from a donor with multiple
biliary hamartomata. Int J Organ Transplant Med. 4:35–37.
2013.PubMed/NCBI
|
|
22
|
Barboi OB, Moisii LG, Albu-Soda A,
Ciortescu I and Drug V: Biliary hamartoma. Clujul Med. 86:383–384.
2013.PubMed/NCBI
|
|
23
|
Yamada N, Sanada Y, Katano T, Tashiro M,
Hirata Y, Okada N, Ihara Y, Miki A, Sasanuma H, Urahashi T, et al:
Pediatric living donor liver transplantation for congenital hepatic
fibrosis using a mother's graft with von Meyenburg complex: A case
report. World J Gastroenterol. 22:9865–9870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Vlerken LG, van Leeuwen MS, Schipper
ME and van Erpecum KJ: The ‘Von Meyenburg complex’: An unusual
cause of cholangitis? Clin Res Hepatol Gastroenterol. 35:762–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ioannidis O, Iordanidis F, Paraskevas G,
Ntoumpara M, Tsigkriki L, Chatzopoulos S, Kotronis A, Papadimitriou
N, Konstantara A, Makrantonakis A, et al: Incidentally discovered
white subcupsular liver nodules during laparoscopic surgery:
Biliary hamartoma and peribiliary gland hamartoma. Klin Onkol.
25:468–470. 2012.PubMed/NCBI
|
|
26
|
Patel SR, Misra V, Verma K, Gupta P and
Dhingra V: Benign hepatic mesenchymal hamartoma (HMH) - A case
report. J Clin Diagn Res. 8:119–120. 2014.PubMed/NCBI
|
|
27
|
Ettel M, Eze O and Xu R: Clinical and
biological significance of precursor lesions of intrahepatic
cholangiocarcinoma. World J Hepatol. 7:2563–2570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Zhao B, Ma J, Li J and Li X:
Lesions of biliary hamartoms can be diagnosed by ultrasonography,
computed tomography and magnetic resonance imaging. Int J Clin Exp
Med. 7:3370–3377. 2014.PubMed/NCBI
|
|
29
|
Lee KB: Histopathology of a benign bile
duct lesion in the liver: Morphologic mimicker or precursor of
intrahepatic cholangiocarcinoma. Clin Mol Hepatol. 22:400–405.
2016. View Article : Google Scholar : PubMed/NCBI
|