Introduction
An estimated 14.1 million new cancer cases and 8.2
million cancer deaths occurred in 2012 worldwide (1). Surgical resection is one of the major
methods of treating cancer. However, the morbidity after surgery
has been reported to range from 20 to 65% (2–4).
Delirium is a particularly common morbidity after surgery (5). Postoperative delirium makes patient
management much more difficult, increases costs, and causes severe
discomfort to the patient (6,7).
Delirium is also associated with increased postoperative mortality
and morbidity and with delayed functional recovery (8,9).
Although a range of interventions have been developed to prevent
and treat postoperative delirium, a more rational approach is
necessary.
Yokukansan (TJ-54) is a traditional Japanese herbal
medicine (Kampo; granules) extracted from seven medicinal
herbs (Atractylodes lancea rhizome, Poria sclerotium,
Cnidium rhizome, Uncaria hook, Japanese angelica
root, Bupleurum root and Glycyrrhiza). Originally used to treat
neurosis, insomnia and irritability and/or agitation in infants,
TJ-54 has been approved in Japan as a prescription drug (10). The mechanisms of action of TJ-54 are
as follows: i) Partial agonistic effects for 5-HT 1A receptors, ii)
antagonistic effects for 5-HT 2A receptors and iii) protective
effects against glutamate-induced excitatory neurotoxicity by
amelioration of astrocyte dysfunction (11–15).
Recently, Arai et al found that TJ-54 alleviated
preoperative anxiety without undesirable sedation when compared
with diazepam (16).
Given these previous clinical and biochemical study
findings, in the present study, the efficacy of TJ-54 in the
prevention and/or treatment of delirium was investigated in a
randomized phase II clinical trial in the patients receiving
surgery for gastrointestinal or lung malignancy.
Materials and methods
Study design
A prospective, multi-institutional, randomized,
phase II trial was performed in patients receiving surgery for
gastrointestinal or lung malignancy in Japan. The eligible patients
were centrally randomized to receive either TJ-54 or control during
their perioperative care (between day 7 before surgery to day 4
after surgery, excluding the operation day). The patients were
stratified according to gender, performance status before surgery,
type of malignancies and institution before randomization at a 1:1
ratio.
The primary aim of the present study was to
determine the efficacy and safety of TJ-54 compared with the
control group. The primary endpoint was the incidence of delirium
after surgery and safety. The secondary endpoints were the length
of the hospital stay.
Ethics
The study data and informed consent were obtained in
accordance with the Declaration of Helsinki, and the study protocol
was approved by the Ethics Review Board of each institution (nine
institutions participated the present study. The details were as
follows: Miura City Hospital, Japanese Red Cross Hadano Hospital,
International University of Health and Welfare Atami Hospital,
Kanagawa Prefectural Ashigarakami Hospital, Yokohama Minami Kyosai
Hospital, Hiratsuka Kyosai Hospital, Fujisawa Shonandai Hospital,
Yokohama Kamishirane Hospital and Yokohama City University). All
the patients were given a written explanation of the study protocol
and they all provided their written informed consent before
participating. This trial was registered in the University Hospital
Medical Information Network (UMIN) center (ID UMIN000005423).
Inclusion and exclusion criteria
Patients ≥70 years of age who underwent surgery for
gastrointestinal or lung malignancies were considered eligible for
this study. All the participants were required to have an Eastern
Cooperative Oncology Group performance status ≤2; to receive a
mini-mental state examination (MMSE) before enrollment and to have
an adequate hepatic, renal, and bone marrow function [white blood
cell (WBC) count ≥3,000/mm3 and ≤12,000/mm3,
platelet count ≥75,000/mm3, GOT and GPT ≤100 U/l, total
bilirubin <1.5 mg/dl, and creatinine <1.5 mg/dl]. Patients
with any of the following characteristics were not eligible for the
study: A history of severe hypersensitivity (allergy) to any
medicine containing antiphlogistic, analgesics, opioids or
steroids. Patients with serious constipation or who were pregnant
or lactating were excluded from the study. Other medical conditions
that rendered a patient unsuitable for inclusion in the study
according to the opinion of the investigator were also considered
to be exclusion criteria for this study.
Study drug
The study medication Yokukansan [Tsumura Yokukansan
Extract Granules for Ethical Use; TJ-54 (Tsumura, Japan)] was
administered 3 times a day (2.5 g each time, 7.5 g/day). The amount
of TJ-54 could be decreased depending on the participant's
condition or adverse reactions.
Study assessment
The signs and symptoms of delirium were assessed by
the investigator during the perioperative period. The Diagnostic
and Statistical Manual of Mental Disorders (DSM)-IV was used to
assess delirium (17). Delirium was
independently evaluated by two physicians who were previously
trained on the algorithm. The time to healing of delirium was
defined as the period from the date of onset of delirium to the
date when the delirium symptoms disappeared. If the delirium
symptoms failed to disappear within the study treatment period,
observation was continued until symptom disappearance. Safety was
assessed throughout the study using physical examinations,
hematology and serum chemistry laboratory tests and adverse event
reporting. Any adverse event, whether related or unrelated to the
study drug, was reported with the date and time of onset, severity,
pattern, action taken and outcome. If the adverse event was not
resolved at the time the case report forms were collected, a
follow-up report was provided at a later date. If no follow-up
report was provided, the investigator had to provide justification.
The adverse events were followed until they were either resolved or
the investigator determined that the event was no longer clinically
significant.
Statistical analysis
Eligible patients were randomly assigned at a 1:1
ratio to receive TJ-54 or control. After checking patient
eligibility, randomization was carried out centrally at the data
center using dynamic randomization with gender, performance status
before surgery, type of malignancies and institution.
Assuming an incidence of delirium of 5% in the TJ-54
group and 20% in the control group, a sample size of 88 for each
group was estimated to have ≥80% power under a two-sided
significance level of 10%. Thus, to account for possible dropouts,
a target sample size of 200 patients was required.
The risk ratios of the incidence of delirium between
the groups and its 95% confidence interval (CI) were calculated. A
risk ratio <1 indicated that TJ-54 was better than the control.
Comparisons were made using the Chi-squared test. The baseline
characteristics were compared using the Chi-squared test for
categorical variables and the Wilcoxon test for continuous
variables. The frequencies of adverse events were compared using
the Fisher's exact test. All the P-values were two-sided. The
statistical analyses were performed using the SAS software package
for Windows, release 9.3 (SAS Institute, Cary, NC, USA).
Results
Patients
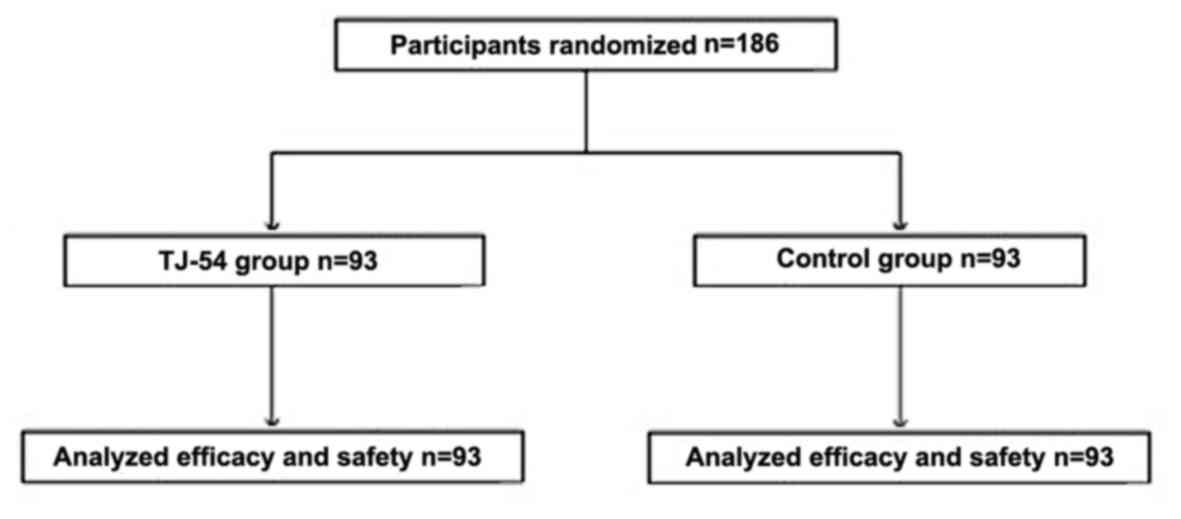
Of the patients receiving surgery for
gastrointestinal or lung malignancy patients, 186 provided informed
consent were randomized to either the TJ-54 (n=93) or control
(n=93) group. A flow diagram of the participants' progress through
the study protocol is shown in Fig.
1. The baseline demographics and disease characteristics of the
per protocol set (PPS) population are shown in Table I. Male subjects comprised 64.5%, and
35.5% of the subjects were female, and the median age was 77 years
(range: 70–89 years). The majority of patients (90%) had
histologically confirmed gastrointestinal malignancy, and 10% had
histologically confirmed lung malignancy. There were no marked
differences between the two PPS randomized groups.
 | Table I.Patient characteristics of the TJ-54
and control groups. |
Table I.
Patient characteristics of the TJ-54
and control groups.
| Factors | Control (N=93) | TJ-54 (N=93) | P-value |
|---|
| Sex (%) |
|
| 1.000 |
| Male | 60 (64.5) | 60 (64.5) |
|
|
Female | 33 (35.5) | 33 (35.5) |
|
| Age |
|
| 0.406 |
| Median
(range) | 76 (70–89) | 77
(70–88) |
|
| PS (%) |
|
| 0.620 |
| 0–1 | 83 (89.3) | 85 (91.4) |
|
| 2 | 10 (10.8) | 8 (8.6) |
|
| Type of malignancy
(%) |
|
| 0.261 |
| Gastric
cancer | 48 (51.6) | 39 (41.9) |
|
|
Colorectal cancer | 36 (38.7) | 35 (37.6) |
|
| Lung
cancer | 9 (9.7) | 10 (10.8) |
|
| MMSE score |
|
| 0.736 |
| Median
(range) | 29 (9–30) | 29
(16–30) |
|
| Comorbidity (%) |
|
|
|
|
Hypertension | 51 (54.8) | 49 (52.7) |
|
| COPD | 6 (6.5) | 10 (10.8) |
|
| Diabetes
mellitus | 17 (18.3) | 22 (23.7) |
|
Surgical details and postoperative
course
The median amount of bleeding was significantly less
in the TJ-54 group than in the control group (P=0.035) (Table II). The median duration of surgery
was marginally significantly longer in the control group than in
the TG-54 group (P=0.067). No marked differences were observed
between the groups in terms of the postoperative course.
 | Table II.Surgical details and postoperative
course of the TJ-54 and control groups. |
Table II.
Surgical details and postoperative
course of the TJ-54 and control groups.
| Factors | Control (N=93) | TJ-54 (N=93) | P-value |
|---|
| Operation time |
|
| 0.067 |
| Median
(range) | 247 min | 222 min |
|
|
| (50–59.49) | (83–482) |
|
| Blood loss |
|
| 0.035 |
| Median
(range) | 136 ml | 67 ml |
|
|
| (5–31.00) | (5–15.34) |
|
| Type of surgery
(%) |
|
| 0.809 |
|
Gastrointestinal | 84 (90.3) | 83 (89.2) |
|
|
Thoracic | 9 (9.7) | 10 (10.8) |
|
| Type of approach
(%) |
|
| 1.000 |
|
Conventional | 41 (44.1) | 41 (44.1) |
|
|
Laparoscopic or
thoracoscopic | 52 (55.9) | 52 (55.9) |
|
| First oral
intake |
|
| 0.576 |
| Median
(range) | 4 day | 4 day |
|
|
| (2–69) | (2–81) |
|
| Surgical
complications (%) |
|
| 0.306 |
|
Yes | 23 (24.7) | 26 (28.0) |
|
| No | 70 (75.3) | 67 (72.0) |
|
| Length of hospital
stay |
|
| 0.867 |
| Median (range) | 16 day | 15 day |
|
|
| (7–101) | (7–267) |
|
Incidence of delirium and
postoperative course
The incidence of delirium was 6.5% (6 patients) in
the TJ-54 group and 9.7% (9 patients) in the control group, and
there was no significant difference between the two groups
(P=0.471). Of note, the primary endpoint was not met in this
study.
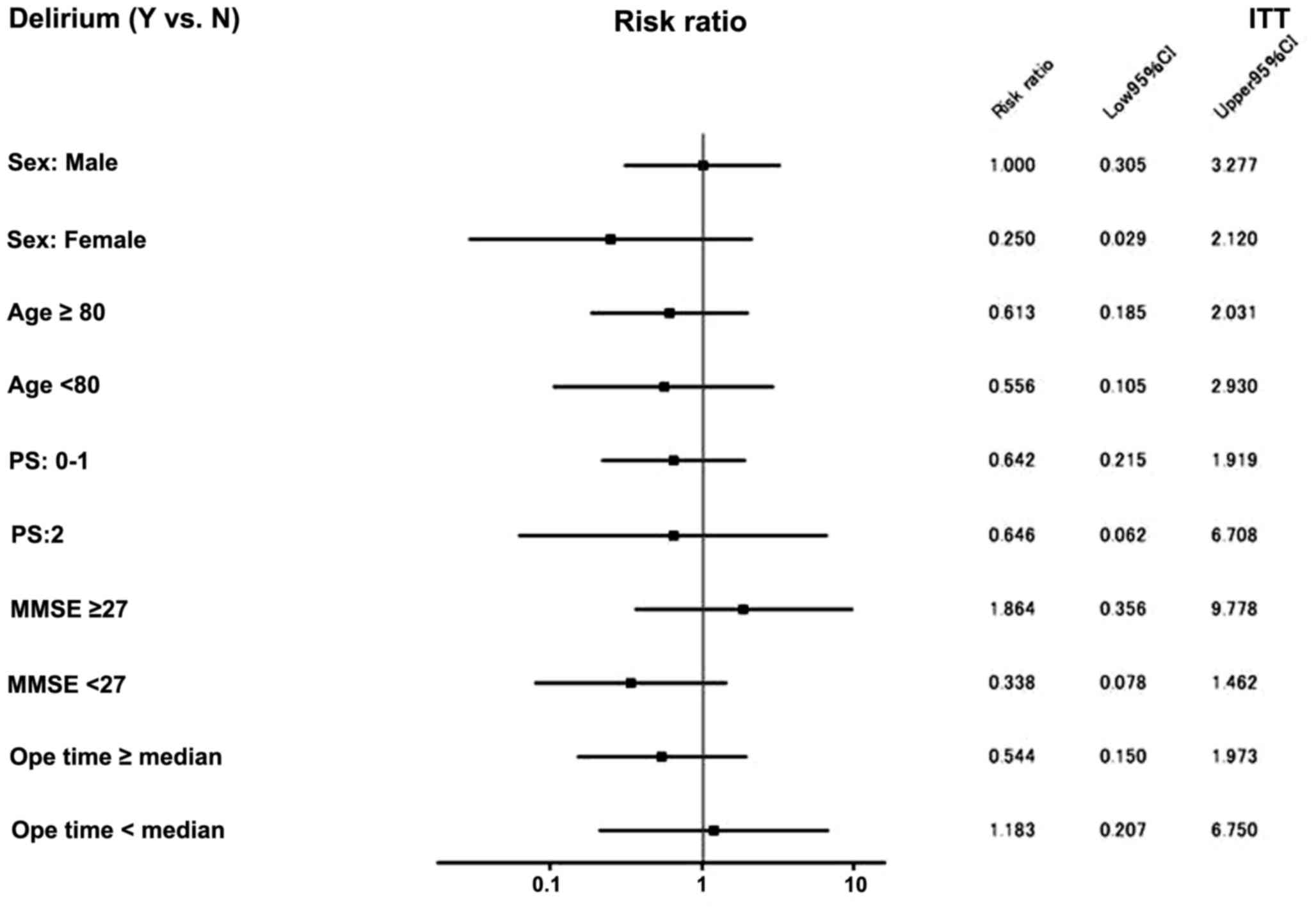
The subgroup analysis of the present study is shown
in Fig. 2. Among the patients who
were MMSE ≤26, the incidence of postoperative delirium was 9.1% in
the TJ-54 group and 26.9% in the control group [risk ratio: 0.338;
95% CI (0.078–1.462), P=0.115], while among the patients who were
MMSE ≥27, the incidence of postoperative delirium was 6.8% in the
TJ-54 group and 3.6% in the control group [risk ratio: 1.864; 95%
CI (0.356–9.778), P=0.453]. Thus, treatment with TJ-54 may reduce
the incidence of postoperative delirium compared with the control
group.
Safety and postoperative course
Hematological, blood biochemistry and
non-hematological toxicities were analyzed (Table III). The majority of these events
were mild to moderate in severity and considered to be unrelated to
the study drug. The length of the hospital stay was also similar
between the TJ-54 group and the control group (16 vs. 15 days,
P=0.867).
 | Table III.Hematological and biochemical
toxicities greater than Grade 2 or more observed during
treatment. |
Table III.
Hematological and biochemical
toxicities greater than Grade 2 or more observed during
treatment.
| Toxicity type | Contoral
(N=93) | TJ-54 (N=93) | P-value |
|---|
| Hematological
toxicity (%) |
|
|
|
|
Leucopenia | 0 (0) | 0 (0) | – |
|
Neutropenia | 0 (0) | 0 (0) | – |
|
Hemoglobin | 0 (0) | 0 (0) | – |
|
Platelet | 0 (0) | 0 (0) | – |
|
T-Bil | 3
(3.2) | 1
(1.1) | 0.312 |
|
AST | 15 (16.2) | 8
(8.6) | 0.119 |
|
ALT | 10 (10.8) | 6
(6.5) | 0.296 |
| Non-hematological
toxicity (%) |
|
|
|
|
Anorexia | 0 (0) | 0 (0) | – |
|
Nausea | 0 (0) | 0 (0) | – |
|
Vomiting | 0 (0) | 0 (0) | – |
|
Diarrhea | 0 (0) | 0 (0) | – |
|
Constipation | 0 (0) | 0 (0) | – |
|
Peripheral neuropathy | 0 (0) | 0 (0) | – |
|
Lassitude | 0 (0) | 0 (0) | – |
| Skin
reaction | 0 (0) | 0 (0) | – |
|
Dyspepsia | 0 (0) | 0 (0) | – |
|
Edema | 0 (0) | 0 (0) | – |
| Change
in PS | 0 (0) | 0 (0) | – |
Discussion
This randomized trial is the first evaluation of the
utility of TJ-54 for preventing and/or treating postoperative
delirium in patients undergoing gastrointestinal or lung malignancy
surgery in a randomized phase II trial. The primary aim of this
study was to prove the effects of TJ-54 in reducing the incidence
of postoperative delirium. The incidence of postoperative delirium
was 6.5% in the TJ-14 group and 9.7% in the control group in the
overall study population. Therefore, treatment with TJ-54 did not
show any obvious efficacy against postoperative delirium in
patients receiving surgery for gastrointestinal and lung
malignancy.
Several reasons may explain why this trial did not
meet its primary objective. First, TJ-54 may not actually be able
to prevent and/or treat postoperative delirium. Second, the
incidence of expected postoperative delirium was not observed in
the control arm in the present study. Previous findings have shown
that the incidence of delirium after major non-cardiovascular
surgery ranged from 13 to 50% (4,18,19).
Given these data, we predicted an incidence of delirium of 20% in
the control group. However, the present results showed that the
incidence of delirium was only 9.7% in the control group. This
discrepancy may be due to the following: First, the rate of
postoperative delirium may have been underestimated. In the present
study, the physicians checked the mental condition of all the
patients at their bedside during the perioperative period and
recorded the psychiatric symptoms. However, the physicians were
able to detect only hyperactive delirium and could not detect
hypoactive delirium, which is often misdiagnosed as depression or
fatigue (7). Second, the surgical
stress, such as blood loss and operative time, was lower in our
study than in previous reports. Risk factors for postoperative
delirium that have been reported include increased blood loss and
increased operative time (20,21).
However, the median blood loss in our study was 67 and 136 ml, and
the median operative time was 222 and 247 min, which was lower than
in previous reports. Furthermore, 50% of the patients received
laparoscopic or thoracspoic surgery. Generally, the surgical
stresses are lower for laparoscopic or thoracspoic surgery than
conventional procedures (22,23).
One important limitation of the present study is the
lack of information for the preoperative nutrition status. Some of
the patients with gastrointestinal cancer may lose their weight
before operation and nutritional status is potentially poor.
Nutrition status, such as BMI or serum albumin, could affect the
results. Thus, further studies taking nutrition status into
consideration are necessary.
However, a borderline significant difference was
observed between the two groups among patients who were MMSE ≤26:
the incidence of postoperative delirium was 9.1% in the TJ-54 group
and 26.9% in the control group (risk ratio: 0.338; 95% CI
[0.078–1.462], P=0.453). By contrast, among the patients who were
MMSE ≥27, the incidence of postoperative delirium was 6.8% in the
TJ-54 group and 3.6% in the control group [risk ratio: 1.864; 95%
CI (0.356–9.778), P=0.453]. Treatment with TJ-54 reduced the
incidence of postoperative delirium compared with the control
group. Among the patients who were MMSE ≤26, an obvious reduction
in the risk of delirium (hazard ratio 0.338) was demonstrated. As
mentioned above, it was previously reported that TJ-54 exerts
slight agonistic effects on 5-HT 1A receptors, antagonistic effects
on 5-HT 2A receptors, and protective effects against
glutamate-induced excitatory neurotoxicity by amelioration of
astrocyte dysfunction. Further studies are needed to clarify the
exact mechanisms underlying these observations.
In conclusion, this study showed no beneficial
effects of TJ-54 in reducing the incidence of postoperative
delirium as the primary endpoint. However, among patients who were
MMSE ≤26, an obvious reduction in the risk of postoperative
delirium (risk ratio: 0.338) was demonstrated. Further analyses may
lead to a better interpretation of the study results by examining
subgroups that will particularly benefit from TJ-54 treatment. A
more definitive design in a future trial of TJ-54 for postoperative
delirium is needed.
Acknowledgements
The authors would like to thank Professor Satoshi
Morita and Ms. Maho Sato for excellent data management in this
study. The authors would like to express their deepest appreciation
to Yukihiro Ozawa, Miura City Hospital, Kimiatsu Hasuo, Japanese
Red Cross Hadano Hospital, Hiroyasu Tanabe, International
University of Health and Welfare Atami Hospital, Katsuya Yoneyama,
Kanagawa Prefectural Ashigarakami Hospital for cooperation in this
study.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasako M, Sano T, Yamamoto S, Kurokawa Y,
Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T and
Arai K: D2 lymphadenectomy alone or with para-aortic nodal
dissection for gastric cancer. N Engl J Med. 359:453–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA,
Campbell KA, Sauter PK, Coleman J, Abrams RA and Hruban RH:
Pancreaticoduodenectomy with or without distal gastrectomy and
extended retroperitoneal lymphadenectomy for periampullary
adenocarcinoma, part 2: Randomized controlled trial evaluating
survival, morbidity, and mortality. Ann Surg. 236:355–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto S, Inomata M, Katayama H,
Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y and
Kitano S: Japan Clinical Oncology Group Colorectal Cancer Study
Group: Short-term surgical outcomes from a randomized controlled
trial to evaluate laparoscopic and open D3 dissection for stage
II/III colon cancer: Japan clinical oncology group study JCOG 0404.
Ann Surg. 260:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inouye SK, Westendorp RG and Saczynski JS:
Delirium in elderly people. Lancet. 383:911–922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marcantonio ER, Goldman L, Mangione CM,
Ludwig LE, Muraca B, Haslauer CM, Donaldson MC, Whittemore AD,
Sugarbaker DJ and Poss R: A clinical prediction rule for delirium
after elective noncardiac surgery. JAMA. 271:134–139. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeuchi M, Takeuchi H, Fujisawa D,
Miyajima K, Yoshimura K, Hashiguchi S, Ozawa S, Ando N, Shirahase
J, Kitagawa Y and Mimura M: Incidence and risk factors of
postoperative delirium in patients with esophageal cancer. Ann Surg
Oncol. 19:3963–3970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Francis J, Martin D and Kapoor WN: A
prospective study of delirium in hospitalized elderly. JAMA.
263:1097–1101. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inouye SK, van Dyck CH, Alessi CA, Balkin
S, Siegal AP and Horwitz RI: Clarifying confusion: The confusion
assessment method. A new method for detection of delirium. Ann
Intern Med. 113:941–948. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furukawa K, Tomita N, Uematsu D, Okahara
K, Shimada H, Ikeda M, Matsui T, Kozaki K, Fujii M and Ogawa T:
Randomized double-blind placebo-controlled multicenter trial of
Yokukansan for neuropsychiatric symptoms in Alzheimer's disease.
Geriatr Gerontol Int. 17:211–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueki T, Mizoguchi K, Yamaguchi T, Nishi A,
Ikarashi Y, Hattori T and Kase Y: Yokukansan increases 5-HT1A
receptors in the prefrontal cortex and enhances 5-HT1A receptor
agonist-induced behavioral responses in socially isolated mice.
Evid Based Complement Alternat Med. 2015:7264712015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto H, Iyo M, Ueda K, Han C, Hirasaki
Y and Namiki T: Yokukan-san: A review of the evidence for use of
this Kampo herbal formula in dementia and psychiatric conditions.
Neuropsychiatr Dis Treat. 10:1727–1742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeda A, Itoh H, Tamano H, Yuzurihara M
and Oku N: Suppressive effect of Yokukansan on excessive release of
glutamate and aspartate in the hippocampus of zinc-deficient rats.
Nutr Neurosci. 11:41–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda A, Tamano H, Itoh H and Oku N:
Attenuation of abnormal glutamate release in zinc deficiency by
zinc and yokukansan. Neurochem Int. 53:230–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakami Z, Kanno H, Ueki T, Terawaki K,
Tabuchi M, Ikarashi Y and Kase Y: Neuroprotective effects of
yokukansan, a traditional Japanese medicine, on glutamate-mediated
excitotoxicity in cultured cells. Neuroscience. 159:1397–1407.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arai YC, Kawanishi J, Sakakima Y, Sueoka
S, Ito A, Tawada Y, Maruyama Y, Banno S, Takayama H and Nishihara
M: The effect of the kampo medicine yokukansan on preoperative
anxiety and sedation levels. Evid Based Complement Alternat Med.
2014:9650452014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
American Psychiatric Association, .
Diagnostic and statistical manual of mental disorders (4th).
American Psychiatric Association. Washington, DC: 1994.
|
|
18
|
Edlund A, Lundström M, Lundström G,
Hedqvist B and Gustafson Y: Clinical profile of delirium in
patients treated for femoral neck fractures. Dement Geriatr Cogn
Disord. 10:325–329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Ha YC, Lee YK, Kang H and Koo KH:
Frequency, risk factors, and prognosis of prolonged delirium in
elderly patients after hip fracture surgery. Clin Orthop Relat Res.
469:2612–2620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raats JW, Steunenberg SL, de Lange DC and
van der Laan L: Risk factors of post-operative delirium after
elective vascular surgery in the elderly: A systematic review. Int
J Surg. 35:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamagata K, Onizawa K, Yusa H, Wakatsuki
T, Yanagawa T and Yoshida H: Risk factors for postoperative
delirium in patients undergoing head and neck cancer surgery. Int J
Oral Maxillofac Surg. 34:33–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng YK, Yang ZL, Peng JS, Lin HS and Cai
L: Laparoscopy-assisted versus open distal gastrectomy for early
gastric cancer: Evidence from randomized and nonrandomized clinical
trials. Ann Surg. 256:39–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adachi Y, Shiraishi N, Shiromizu A, Bandoh
T, Aramaki M and Kitano S: Laparoscopy-assisted Billroth I
gastrectomy compared with conventional open gastrectomy. Arch Surg.
135:806–810. 2000. View Article : Google Scholar : PubMed/NCBI
|