Introduction
There are few established treatments for patients
with non-small-cell lung cancer (NSCLC) with interstitial lung
disease (ILD). The safety and efficacy of albumin-bound paclitaxel
(nab-paclitaxel) in combination with carboplatin is uncertain,
although the combination of carboplatin and paclitaxel is the most
common regimen for treating NSCLC patients with ILD (1–3). Lung
cancer is the leading cause of cancer-related mortality worldwide
(4), although the treatment of
patients with NSCLC is gradually improving (5). For example, targeted therapies have
been developed to inactivate epidermal growth factor receptor
(6) and anaplastic lymphoma kinase
(7). Other therapeutics include
immune checkpoint inhibitors, such as nivolumab (8) and pembrolizumab (9). Clinical trials demonstrated that these
treatments provided survival benefits (5). The median survival was 3.5 years for
patients with an oncogenic driver and genotype-directed therapy,
compared with 2.4 years for those with any oncogenic driver(s) who
did not receive genotype-directed therapy. By contrast, patients
with severe complications, such as ILD, are excluded from a number
of clinical trials, as pre-existing ILD is a risk factor for the
NSCLC therapy related to ILD (10,11).
ILD affects the parenchyma or the alveolar region of
the lung, and it presents as an interstitial shadow on images
acquired using computed tomography, according to the guidelines of
American Thoracic Society and the European Respiratory Society
(12). ILD, particularly idiopathic
pulmonary fibrosis (IPF), is a frequent complication in patients
with NSCLC (13), and the incidence
of lung cancer in patients with ILD is 20–30% (11). Approximately 10% of the patients with
ILD reported acute exacerbations (AE) generally characterized by
suddenly progressive and severe respiratory failure, accompanied by
new lung opacities and diffuse alveolar damage (14). Chemotherapy for lung cancer
occasionally exacerbates AE-ILD and leads to death in 27.9% of the
patients (15). In patients with
advanced or recurrent NSCLC who receive at least one chemotherapy
regimen, the rate of AE-ILD is higher among patients with
pre-existing ILD who are administered chemotherapy, rather than
among those without pre-existing ILD (15,16).
Pre-existing ILD was reported to be a strong risk factor in NSCLC
patients, with an odds ratio of 4.80–25.27 compared with those
without ILD (15).
There is no established standard regimen for NSCLC
patients with ILD. The best characterized regimen for first-line
chemotherapy is carboplatin (CBDCA) plus paclitaxel (PTX). However,
a phase III study found that the administration of albumin-bound
PTX (nab-PTX) in combination with CBDCA achieved a higher response
rate compared with that of CBDCA plus PTX (33 vs. 25%,
respectively) (17). Thus, nab-PTX
is a promising agent for all histological types of NSCLC. Moreover,
nab-PTX may replace the conventional treatment with PTX, as it is
more effective for treating patients with NSCLC. However, to the
best of our knowledge, there is no evidence that CBDCA plus nab-PTX
is safe and effective for treating patients with NSCLC with ILD.
Therefore, the aim of the present retrospective study was to
determine whether nab-PTX plus CBDCA is a feasible treatment option
for NSCLC patients with ILD.
Patients and methods
Patient selection
A total of 9 NSCLC patients with ILD who were
treated at the Kitasato University Hospital (Sagamihara, Japan)
between April 2013 and March 2016 were included in this
retrospective study. ILD was diagnosed according to patients'
medical histories, physical examination and radiological
abnormalities that were consistent with the characteristics of
bilateral lung fibrosis, such as ground-glass opacity and
consolidation, with or without reticular shadow. There were 34
NSCLC patients with ILD in our institution between April 2013 and
March 2016. The present study included the typical interstitial
pneumonia (UIP) pattern and the non-UIP pattern of ILD, according
to the International Consensus Statement of the Japanese
Respiratory Society (18). The
inclusion criteria were as follows: i) Histologically or
cytologically confirmed NSCLC, ii) clinically diagnosed pulmonary
fibrosis, iii) confirmed inoperable disease, iv) age ≤80 years, v)
Eastern Cooperative Oncology Group (ECOG) performance status 0–2,
and vi) sufficient organ function for the administration of
chemotherapy. The Institutional Ethics Review Board of Kitasato
University Hospital approved the study protocol and the research
was conducted in accordance with the principles of the World
Medical Association Declaration of Helsinki; the patients were
informed that they could withdraw consent at any time during
treatment.
Treatment and methods
Patients were administered 100 mg/m2
nab-PTX on days 1, 8 and 15, plus CBDCA on day 1 (area under the
curve=5–6) every 4–6 weeks. The suspension of nab-PTX on days 8 and
15 was permitted due to toxicities, such as hematotoxicity. In our
institution, prior to the start of the treatment of this regimen
and on days 8 and 15, the patients were required to have an
absolute neutrophil count of ≥1,000/mm3 and a platelet
count of ≥50,000/mm3. Furthermore, granulocyte-colony
stimulating factor (G-CSF) was administered for prophylaxis against
hematotoxicity.
Evaluation of response
The Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1 (https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf)
was used to evaluate the response to treatment. Chest and abdominal
CT was performed after every two cycles of CBDCA plus nab-PTX to
evaluate tumor size.
Toxicity and acute exacerbation of
ILD
Adverse events were evaluated according to the
Common Terminology Criteria for Adverse Events, version 4.0
(https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
AE-ILD was confirmed if the following criteria were met: i)
Exacerbation of dyspnea within 1 month, ii) newly developed diffuse
pulmonary opacity, iii) absence of heart failure and infectious
lung disease, as previously described (14,19).
Chemotherapy-related AE-ILD was diagnosed when AE-ILD developed
within 28 days after the last course of chemotherapy.
Statistical analysis
The primary endpoint of the study was the incidence
of AE-ILD, and the secondary endpoints were objective response
rate, toxicity, median progression-free survival (PFS) and overall
survival (OS). PFS and OS were analyzed using the Kaplan-Meier
method. PFS was defined as the period from the first day of therapy
to failure, including death and disease progression. All responses
were defined according to RECIST version 1.1. OS was defined as the
period from the first day of treatment to the day of death. JMP 10
software was used for all statistical analyses.
Results
Patient characteristics
The baseline patient characteristics are listed in
Table I. At the time of the first
administration of CBDCA plus nab-PTX, the median age of the
patients was 67 years (range, 59–76 years). The ECOG performance
status was 0–1 (8/9 patients, 88%). The disease stages were IIIA
(n=1), IIIB (n=1) and IV (n=7). The histological subtypes were
squamous cell carcinoma (n=6) and undifferentiated carcinoma (n=3).
All the patients were current or former smokers. The UIP pattern of
ILD was observed in 6 patients.
 | Table I.Patient characteristics (n=9). |
Table I.
Patient characteristics (n=9).
| Characteristics | No. |
|---|
| Sex |
|
|
Male/female | 9/0 |
| Age, years |
|
| Median
(range) | 67 (59–76) |
| Stage |
|
|
IIIA/IIIB/IV | 1/1/7 |
| Histology |
|
|
AdenoCa/SCC/NOS | 0/6/3 |
| ECOG performance
status |
|
|
0/1/2 | 0/8/1 |
| Smoking status |
|
|
Current/former/never | 2/7/0 |
| ILD pattern |
|
|
UIP/non-UIP | 6/3 |
Treatment delivery and response
Details of the treatment and response are shown in
Table II. The median number of
cycles administered was 4 (range, 1–4), 5 patients experienced a
partial response, 1 patient had stable disease, and the overall
response and disease control rates were 55.6 and 66.7%,
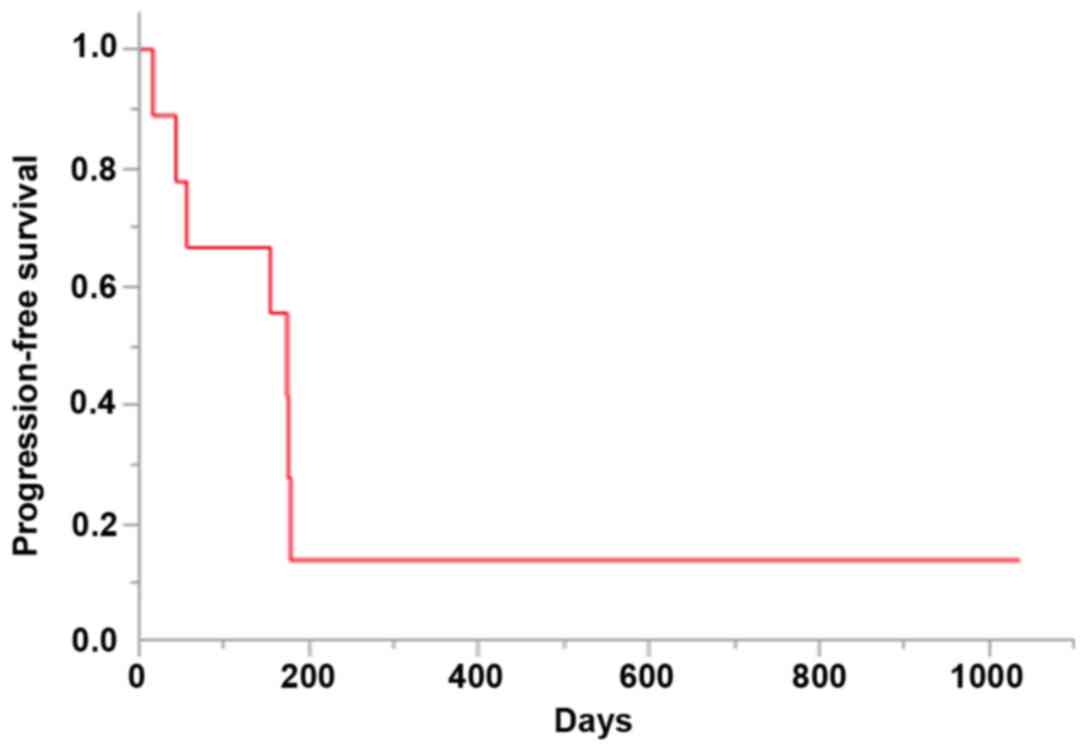
respectively. The median PFS was 174 days [95% confidence interval
(CI): 16–178 days; Fig. 1]. The
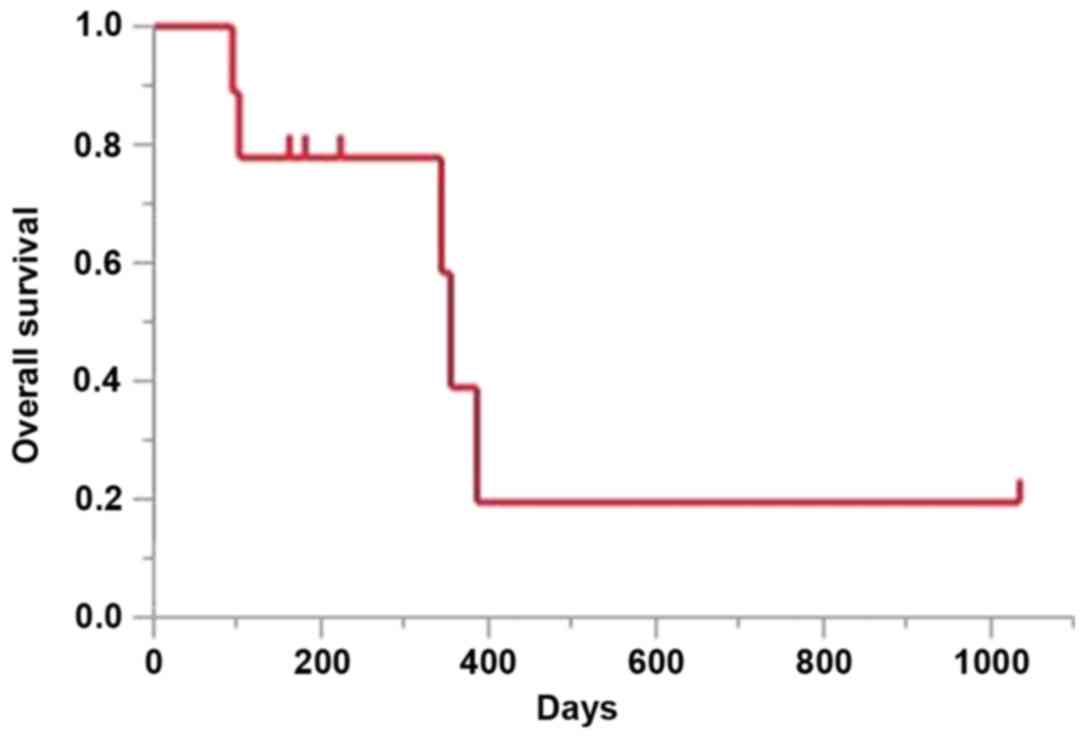
median OS at the time of data cut-off was 355 days (95% CI: 94-not
reached; Fig. 2).
 | Table II.Treatment delivery and response
(n=9). |
Table II.
Treatment delivery and response
(n=9).
| Variables | No. of patients |
|---|
| Treatment delivery
(cycles) |
|
| 1 | 1 |
| 2 | 2 |
| 3 | 0 |
| 4 | 6 |
| Response |
|
| Complete
response | 0 |
| Partial
response | 5 |
| Stable
disease | 1 |
|
Progressive disease | 3 |
| Response rate, % | 55.6 |
| Disease control rate,
% | 66.7 |
Toxicity and safety
The adverse events without pulmonary toxicities are
shown in Table III. The most
frequently reported grade ≥3 hematological adverse events were
leukocytopenia (5/9, 55.6%) and neutropenia (5/9, 55.6%). Febrile
neutropenia (FN) occurred in 3/9 patients (33.3%), but no patient
succumbed to this disorder. The most frequent adverse events were
appetite loss (5/9, 55.6%) and fatigue (5/9, 55.6%).
 | Table III.Treatment-related adverse events
(n=9). |
Table III.
Treatment-related adverse events
(n=9).
|
| Grade, n |
|
|---|
|
|
|
|
|---|
| Events | 1 | 2 | 3 | 4 | All-grade |
|---|
| Leukocytopenia | 0 | 3 | 3 | 2 | 8 |
| Neutropenia | 0 | 3 | 2 | 3 | 8 |
|
Thrombocytopenia | 1 | 2 | 0 | 0 | 3 |
| Anemia | 1 | 0 | 1 | 0 | 2 |
| Febrile
neutropenia | – | – | 3 | 0 | 3 |
| Appetite loss | 4 | 0 | 1 | 0 | 5 |
| Constipation | 4 | 0 | 0 | 0 | 4 |
| Vomiting | 1 | 0 | 0 | 0 | 1 |
| Fatigue | 0 | 1 | 4 | 0 | 5 |
| Rash | 1 | 0 | 0 | 0 | 1 |
| Peripheral
neurotoxicity | 0 | 1 | 0 | 0 | 1 |
| Bleeding | 0 | 0 | 0 | 0 | 0 |
Chemotherapy-induced AE-ILD was not observed. The 2
patients who suffered pulmonary toxicities (pulmonary infections)
during CBDCA plus nab-PTX treatment were successfully treated with
antibiotics.
Post-chemotherapy
After receiving CBDCA plus nab-PTX, 5 patients were
administered other chemotherapies, such as S-1, docetaxel,
pemetrexed and nivolumab. The details of these post-chemotherapies
are presented in Table IV. One
patient succumbed to AE-ILD induced by second-line pemetrexed
monotherapy. A total of 5 patients succumbed to their disease
during follow-up, namely 4 due to lung cancer and 1 due to AE-ILD
during pemetrexed therapy.
 | Table IV.Post-chemotherapy and
chemotherapy-induced AE-ILD. |
Table IV.
Post-chemotherapy and
chemotherapy-induced AE-ILD.
| Patient | Age, years/sex | Histology | ILD pattern | AE-ILD (+/−) | Post-chemotherapy
AE-ILD (+/−) |
|---|
| 1 | 67/M | SCC | Non-UIP | – | BSC |
| 2 | 67/M | SCC | UIP | – | BSC |
| 3 | 73/M | SCC | Non-UIP | – | 2nd-line S-1,
3rd-line nivolumab |
| 4 | 59/M |
Undifferentiated | UIP | – | -Pemetrexed |
| 5 | 64/M | SCC | Non-UIP | – | +S-1 |
| 6 | 61/M |
Undifferentiated | Non-UIP | – | -Docetaxel |
| 7 | 66/M |
Undifferentiated | UIP | – | -BSC |
| 8 | 71/M | SCC | UIP | – | BSC |
| 9 | 76/M | SCC | UIP | – | -S-1 |
Discussion
Drug-induced AE-ILD, a potentially fatal adverse
effect, may occur in NSCLC patients with ILD who undergo
chemotherapy. The optimal chemotherapy for such patients remains
controversial (1,20), and the best-characterized regimen is
CBDCA plus PTX (1–3). The efficacy of CBDCA plus nab-PTX for
treating NSCLC patients without ILD has been established (17). Moreover, patients with ILD were
excluded from the clinical trials (6–8). To the
best of our knowledge, the present study is the first to report the
efficacy and safety of CBDCA and nab-PTX in such patients.
In the present study, the overall response and
disease control rates were 55.6 and 66.7%, respectively, and the
PFS and OS were 174 and 355 days, respectively. Previous studies
analyzed the effects of CBDCA plus PTX (CP regimen) (1–3), as well
as CBDCA plus PTX with bevacizumab (CPB regimen) (21,22) and
CBDCA plus S-1 (23) (Table V). The median PFS and OS of patients
administered the CP regimen were 2.5–5.3 and 7.0–10.6 months,
respectively (1–3) and 5.3–7.2 and 8.5–16.1 months,
respectively, for those administered the CPB regimen (21,22).
Another study reported that the PFS and OS of patients treated with
CBDCA plus S-1 were 4.2 and 9.7 months, respectively (23).
 | Table V.Summary of previous studies. |
Table V.
Summary of previous studies.
| Regimen | N | mPFS (months) | mOS (months) | AE-ILD | FN | (Refs.) |
|---|
| CBDCA+PTX | 15 | 2.5 | 7.0 | 4/15 | 0/15 | (20) |
| CBDCA+PTX | 16 | 5.3 | 10.6 |
1/16 | 1/16 | (19) |
| CBDCA+PTX | 11 | 4.4 | 9.7 |
0/11 | 0/11 | (21) |
| CBDCA+PTX+BEV | 10 | 5.3 | 16.1 |
1/10 | 0/10 | (21) |
| CBDCA+PTX+BEV | 5 | ND | ND | 2/5 | ND | (18) |
| CBDCA+PTX+BEV | 25 | 7.2 | 8.5 | 3/25 | 5/25 | (22) |
| CBDCA+S-1 | 21 |
4.2 | 9.7 | 2/21 | 0/21 | (23) |
| CBDCA+nab-PTX | 9 | 5.8 | 11.8 | 0/9 | 3/9 | Present |
In the present study, chemotherapy-induced AE-ILD
was not observed in patients treated with CBDCA plus nab-PTX.
Previous studies demonstrated that AE-ILD occurs in 0–26.7, 10–40
and 9.7% of patients administered the CP, CPB and CBDCA plus S-1
regimens, respectively. The present study, which was relatively
small, demonstrated that treatment with CBDCA plus nab-PTX may not
be the optimal option for advanced NSCLC patients with ILD.
A notable finding of the present study was that
hematological adverse events were more frequent compared with those
reported by other studies. The majority of the patients in our
study developed leukocytopenia or neutropenia. In particular, FN
occurred in 3/9 patients (Table V).
Thus, the incidence of patients with FN exceeded that of patients
(<1%) without ILD in phase III clinical trials (17). Although the association between ILD
and the rate of FN onset associated with this regimen was not
proven, sufficient prophylaxis should be provided regardless,
including administration of G-CSF, to prevent bone marrow
suppression. CBDCA and nab-PTX are commonly administered triweekly
(nab-PTX is administered weekly). By contrast, CBDCA and nab-PTX
were administered every 4–6 weeks in the present study. The
delivery period of chemotherapy was prolonged due to the frequency
of hematological toxicity.
Patients without lung cancer develop acute
exacerbation of ILD during the typical course of the disease. For
example, a retrospective study reported that the 1-year frequency
of acute exacerbation of ILD is 8.5% after diagnosis (24). Other studies reported that AE-ILD is
associated with infection (25–27). Of
note, we were unable to detect an association between severe
hematological toxicity, including FN, in NSCLC patients with ILD,
and the onset of AE-ILD.
The tyrosine kinase inhibitor nintedanib, which
inhibits the receptors for vascular endothelial growth factor
(VEGF), platelet-derived growth factor, and fibroblast growth
factor, benefits patients with IPF (28). The administration of nintedanib is
associated with a reduction in the decline of lung function, with
fewer AEs and preserved quality of life (28). Furthermore, VEGF is targeted by
bevacizumab and nintedanib (29).
The efficacy of the prognostic extension of the CPB regimen for
patients with non-squamous cell lung cancer is established
(30). Therefore, the regimen of
CBDCA plus nab-PTX with nintedanib may be an alternative useful
choice for treating NSCLC patients with ILD.
There were several limitations to the present study.
First, the study was retrospective and included only a limited
number of patients. As mentioned above, chemotherapy for NSCLC
patients with ILD is associated with a higher risk of AE-ILD. Thus,
several of the previous studies we investigated on NSCLC patients
with ILD were also small-sized. Although the clinical sample of the
present study was small for oncological studies, to the best of our
knowledge, ours is the first retrospective study to evaluate the
efficacy and safety of the CBDCA plus nab-PTX regimen. Thus, the
safety and efficacy of CBDCA plus nab-PTX must be confirmed in a
prospective randomized large-scale study. Second, the follow-up
time was insufficient, and only 3 patients remained alive at the
time of data cut-off.
In conclusion, the present findings indicate that
the CBDCA plus nab-PTX therapy regimen is an effective and safe
option for patients with advanced NSCLC with pre-existing ILD,
although attention must be paid to hematological toxicity.
Moreover, these encouraging results require confirmation by a
large-scale clinical trial.
References
|
1
|
Kenmotsu H, Naito T, Mori K, Ko R, Ono A,
Wakuda K, Imai H, Taira T, Murakami H, Endo M and Takahashi T:
Effect of platinum-based chemotherapy for non-small cell lung
cancer patients with interstitial lung disease. Cancer Chemother
Pharmacol. 75:521–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minegishi Y, Sudoh J, Kuribayasi H,
Mizutani H, Seike M, Azuma A, Yoshimura A, Kudoh S and Gemma A: The
safety and efficacy of weekly paclitaxel in combination with
carboplatin for advanced non-small cell lung cancer with idiopathic
interstitial pneumonias. Lung Cancer. 71:70–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukuya T, Ishiwata T, Hara M, Muraki K,
Shibayama R, Koyama R and Takahashi K: Carboplatin plus weekly
paclitaxel treatment in non-small cell lung cancer patients with
interstitial lung disease. Anticancer Res. 30:4357–4361.
2010.PubMed/NCBI
|
|
4
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2015. CA Cancer J Clin.
65:457–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kris MG, Johnson BE, Berry LD, Kwiatkowski
DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson
SL, Su PF, et al: Using multiplexed assays of oncogenic drivers in
lung cancers to select targeted drugs. JAMA. 311:1998–2006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isobe K, Hata Y, Sakamoto S, Takai Y,
Shibuya K and Homma S: Clinical characteristics of acute
respiratory deterioration in pulmonary fibrosis associated with
lung cancer following anti-cancer therapy. Respirology. 15:88–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minegishi Y, Takenaka K, Mizutani H, Sudoh
J, Noro R, Okano T, Azuma A, Yoshimura A, Ando M, Tsuboi E, et al:
Exacerbation of idiopathic interstitial pneumonias associated with
lung cancer therapy. Intern Med. 48:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
American Thoracic Society; European
Respiratory Society: American Thoracic society/European respiratory
society international multidisciplinary consensus classification of
the idiopathic interstitial pneumonias. This joint statement of the
American thoracic society (ATS) and the European respiratory
society (ERS) was adopted by the ATS board of directors, June 2001
and by the ERS executive committee, June 2001. Am J Respir Crit
Care Med. 165:277–304. 2002.PubMed/NCBI
|
|
13
|
Ozawa Y, Suda T, Naito T, Enomoto N,
Hashimoto D, Fujisawa T, Nakamura Y, Inui N, Nakamura H and Chida
K: Cumulative incidence of and predictive factors for lung cancer
in IPF. Respirology. 14:723–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondoh Y, Taniguchi H, Kawabata Y, Yokoi
T, Suzuki K and Takagi K: Acute exacerbation in idiopathic
pulmonary fibrosis. Analysis of clinical and pathologic findings in
three cases. Chest. 103:1808–1812. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M,
Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, et
al: Interstitial lung disease in Japanese patients with lung
cancer: A cohort and nested case-control study. Am J Respir Crit
Care Med. 177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kenmotsu H, Naito T, Kimura M, Ono A,
Shukuya T, Nakamura Y, Tsuya A, Kaira K, Murakami H, Takahashi T,
et al: The risk of cytotoxic chemotherapy-related exacerbation of
interstitial lung disease with lung cancer. J Thorac Oncol.
6:1242–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small-cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raghu G, Rochwerg B, Zhang Y, Garcia CA,
Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et
al: An official ATS/ERS/JRS/ALAT clinical practice guideline:
Treatment of idiopathic pulmonary fibrosis. An update of the 2011
clinical practice guideline. Am J Respir Crit Care Med. 192:e3–e19.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe
S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al:
Double-blind, placebo-controlled trial of pirfenidone in patients
with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
171:1040–1047. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu R, Fujimoto D, Kato R, Otoshi T,
Kawamura T, Tamai K, Matsumoto T, Nagata K, Otsuka K, Nakagawa A,
et al: The safety and efficacy of paclitaxel and carboplatin with
or without bevacizumab for treating patients with advanced
nonsquamous non-small cell lung cancer with interstitial lung
disease. Cancer Chemother Pharmacol. 74:1159–1166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enomoto Y, Kenmotsu H, Watanabe N, Baba T,
Murakami H, Yoh K, Ogura T, Takahashi T, Goto K and Kato T:
Efficacy and safety of combined carboplatin, paclitaxel, and
bevacizumab for patients with advanced non-squamous non-small cell
lung cancer with pre-existing interstitial lung disease: A
retrospective multi-institutional study. Anticancer Res.
35:4259–4263. 2015.PubMed/NCBI
|
|
23
|
Sekine A, Satoh H, Baba T, Ikeda S, Okuda
R, Shinohara T, Komatsu S, Hagiwara E, Iwasawa T, Ogura T and Kato
T: Safety and efficacy of S-1 in combination with carboplatin in
non-small cell lung cancer patients with interstitial lung disease:
A pilot study. Cancer Chemother Pharmacol. 77:1245–1252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim DS, Park JH, Park BK, Lee JS,
Nicholson AG and Colby T: Acute exacerbation of idiopathic
pulmonary fibrosis: Frequency and clinical features. Eur Respir J.
27:143–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Konishi K, Gibson KF, Lindell KO, Richards
TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, et
al: Gene expression profiles of acute exacerbations of idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 180:167–175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wootton SC, Kim DS, Kondoh Y, Chen E, Lee
JS, Song JW, Huh JW, Taniguchi H, Chiu C, Boushey H, et al: Viral
infection in acute exacerbation of idiopathic pulmonary fibrosis.
Am J Respir Crit Care Med. 183:698–702. 2011. View Article : Google Scholar
|
|
27
|
Ushiki A, Yamazaki Y, Hama M, Yasuo M,
Hanaoka M and Kubo K: Viral infections in patients with an acute
exacerbation of idiopathic interstitial pneumonia. Respir Investig.
52:65–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richeldi L, du Bois RM, Raghu G, Azuma A,
Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y,
et al: Efficacy and safety of nintedanib in idiopathic pulmonary
fibrosis. N Engl J Med. 370:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manzo A, Carillio G, Montanino A, Costanzo
R, Sandomenico C, Rocco G and Morabito A: Focus on nintedanib in
NSCLC and other tumors. Front Med (Lausanne). 3:682016.PubMed/NCBI
|
|
30
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|