Introduction
Prostate cancer is the second most commonly
diagnosed malignancy in males globally, with an estimated 1,111,700
novel cases and 307,500 mortalities per year (1). Metastatic prostate cancer is
characterized by a period during which the suppression of serum
testosterone using androgen deprivation therapy is sufficient to
control the disease (2). However,
this period is followed by a transition to castration resistance,
during which progression occurs despite the continued suppression
of testosterone. This is referred to as metastatic
castration-resistant prostate cancer (mCRPC) (1). Formerly, this disease state was known
as hormone-refractory prostate cancer. As it is now understood that
androgen receptor (AR) signaling remains critical to disease
progression in castration-resistant disease, this term is no longer
used (3). The fact that
prostate-specific antigen (PSA) rises during mCRPC progression
exemplifies this point, as PSA is an androgen-regulated gene
(1). The clinical relevance of
targeting androgen signaling in mCRPC is demonstrated by the
survival advantage conferred by abiraterone acetate (AA) and
enzalutamide (EZL) in this disease state (1).
Docetaxel (DOC) was the first agent identified to
prolong patient survival in mCRPC, and it gained regulatory
approval from the FDA for this indication in 2004 (4). In recent years there has been a rapid
increase in the number of drugs available to treat this disease,
following the approval of cabazitaxel (2010) (5), sipuleucel-T (2010) (6), AA (post-DOC, 2011; chemotherapy-naïve,
2012) (7), EZL (post-DOC, 2012;
chemotherapy-naïve, 2014) (8,9) and
radium-223 (2013) (10). In the case
of AA and EZL, approvals following DOC use were initially granted
based on the COU-AA-301 and AFFIRM trials (9), respectively. Subsequent trials
involving patients with chemotherapy-naïve mCRPC were conducted for
AA (COU-AA-302) and EZL (PREVAIL), leading to an extension of the
approval to the aforementioned population.
The present review focuses on the use of AA in
patients with chemotherapy-naïve mCRPC. In this retrospective
analysis, a variety of treatment sequences were evaluated in order
to determine the optimal treatment sequence for patients with
mCRPC.
Patients and methods
A total of 65 patients with CRPC treated with EZL
(160 mg/day) were retrospectively analyzed at the aforementioned
institutions between June 2014 and July 2015. A total of 23
patients were pre-treated with DOC, and 42 patients were DOC-naïve.
Following the initiation of EZL treatment, the PSA level was
evaluated in 46/65 cases. This case series was conducted with those
46 sequential patients (median age: 77, range 59–89; median serum
PSA level: 56 ng/ml, range 1.5–3211 ng/ml) with CRPC treated with
EZL (160 mg/day) at the aforementioned institutions from June 2014
to July 2015 (Fig. 1).
Results
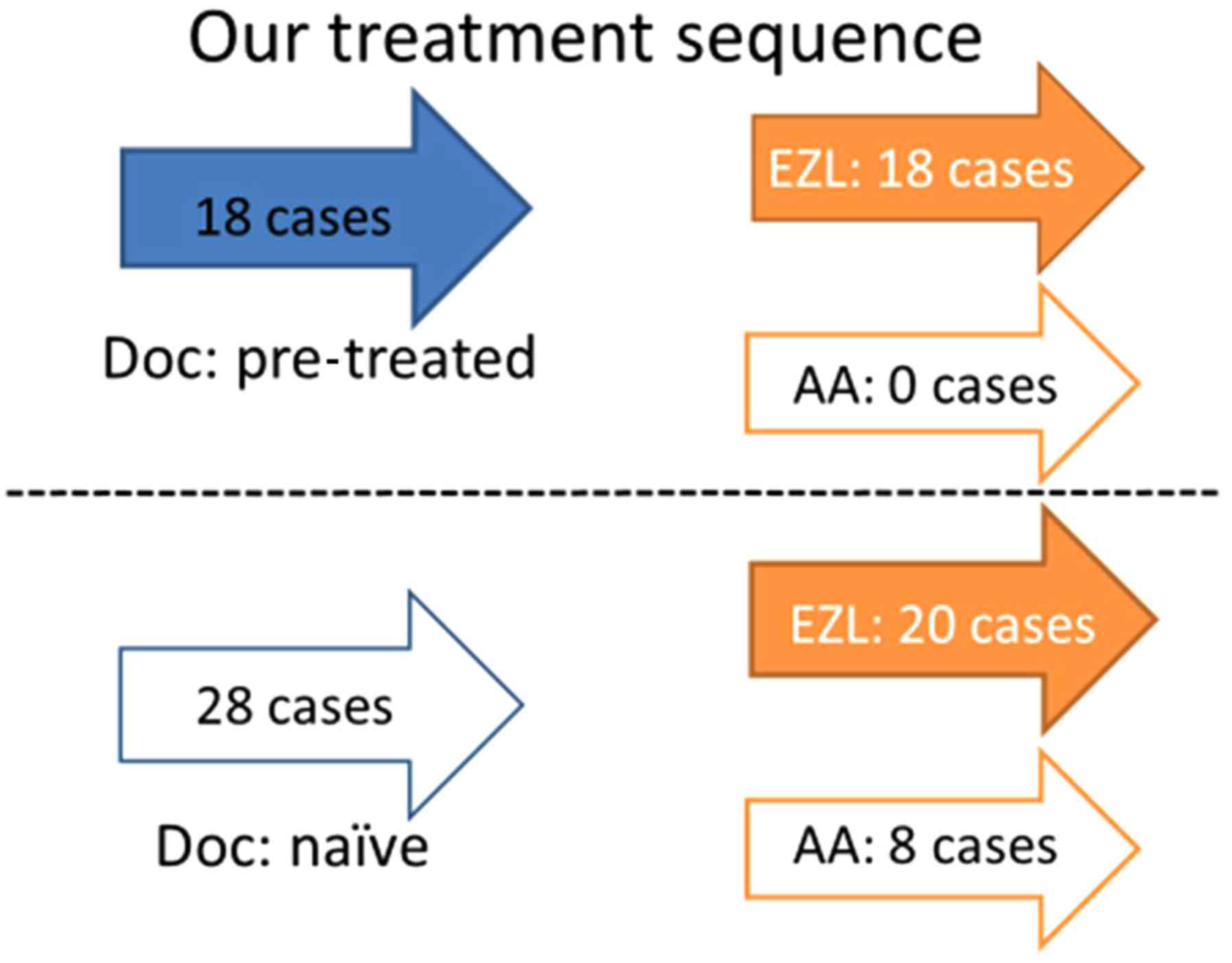
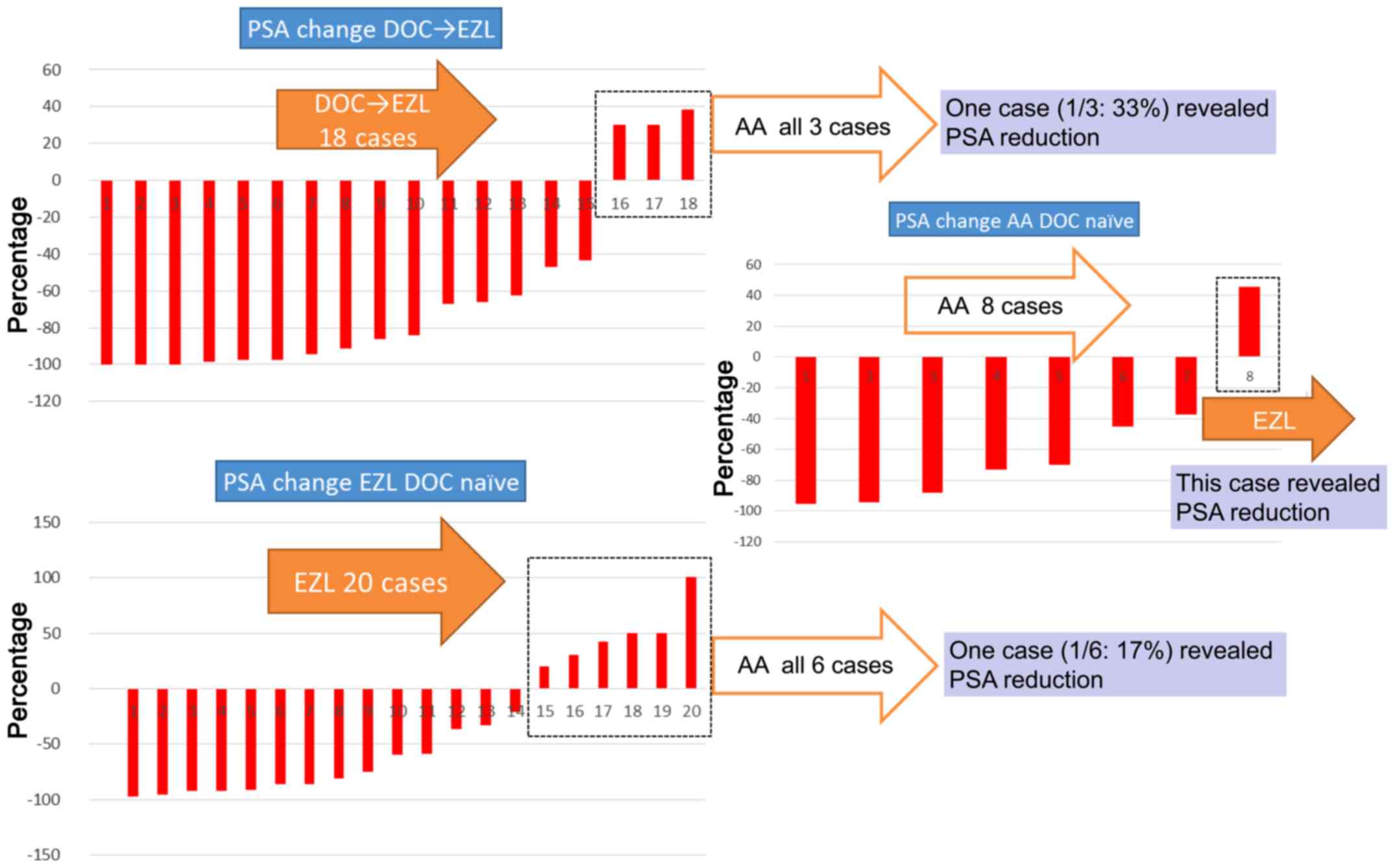
Pretreatment with DOC had been administered in 18/46
cases (Fig. 2), and the remaining 28
cases were DOC-naïve. EZL was administered to the aforementioned 18
patients following the pretreatment with DOC; subsequently, a
reduction in PSA was observed in 15 of these cases. The remaining
three cases, in which PSA reduction had not been recorded, received
AA following EZL administration, and a reduction in PSA was later
revealed in one of the three cases. Of the 28 DOC-naïve cases, EZL
was prescribed for 20 patients, and a reduction in PSA was
identified in 14 of these cases. Of the six cases who had not
exhibited a reduction in PSA levels, and for whom AA was prescribed
after EZL, only one case then revealed a reduction in PSA. Of eight
cases without pretreatment with DOC for which AA was prescribed,
seven cases exhibited a reduction in PSA levels; in the remaining
one case without PSA reduction, EZL was prescribed following AA
administration and a reduction in PSA was subsequently
identified.
Discussion
During the study, the EZL dose was reduced in three
cases due to adverse events (body weight loss, fatigue and nausea).
In the single case of weight loss, the dose was gradually reduced,
leading to the complete discontinuation of EZL; however, the
decrease in the PSA level persisted for three months.
AA and EZL are currently available in Japan
(11). A prior clinical trial has
demonstrated the benefits of these agents in male patients with
CRPC (11). The optimal sequencing
of therapies in the context of drug efficacy and known
cross-resistance remains uncertain. Due to the known mechanisms of
action and the available clinical data, AA and EZL may be indicated
for the treatment of the early stages of prostate cancer (11,12).
Individualized therapy will remain a requirement for each patient
based on the clinical and disease characteristics until further
clinical trials are able to determine the optimal treatment
sequence.
The efficacy of EZL was limited in AA-pre-treated
patients following DOC administration. The possibility of DOC
rechallenge in the treatment of patients with CRPC has been limited
predominantly by the introduction of AA, EZL and cabazitaxel
(5). However, it must be considered
that the reintroduction of DOC may reduce the possibility that one
of the novel treatment options available could then be
administered. Furthermore, the situation is complicated by recent
clinical trials that may lead to the early administration of DOC in
combination with androgen-deprivation therapy, or to novel
indications of AA and EZL in pre-DOC patients (13,14). In
this setting, certain prior reports have indicated the possibility
of the occurrence of cross-resistance when first-line chemotherapy
with DOC was administered after the novel hormonal agent AA; by
contrast, there have been few instances of DOC rechallenge
following failure to respond to AA or other agents (15,16). The
cross-resistance to AA and EZL, as well as EWS, has been attributed
to the expression of an AR splice variant-7 (17,18). In
conclusion, further prospective studies are required in order to
determine the optimal treatment sequence in this new anti-androgen
era.
References
|
1
|
Gartrell BA and Saad F: Abiraterone in the
management of castration-resistant prostate cancer prior to
chemotherapy. Ther Adv Urol. 7:194–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirby M, Hirst C and Crawford ED:
Characterising the castration-resistant prostate cancer population:
A systematic review. Int J Clin Pract. 65:1180–1192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCrea E, Sissung TM, Price DK, Chau CH
and Figg WD: Androgen receptor variation affects prostate cancer
progression and drug resistance. Pharmacol Res. 114:152–162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joensuu T, Joensuu G, Kairemo K, Kiljunen
T, Riener M, Aaltonen A, Ala-Opas M, Kangasmäki A, Alanko T,
Taipale L, et al: Multimodal primary treatment of metastatic
prostate cancer with androgen deprivation and radiation. Anticancer
Res. 36:6439–6447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cicero G, De Luca R, Dorangricchia P,
Galvano A, Lo Re G, Serretta V, Dispensa N and Dieli F: Cabazitaxel
in metastatic castration-resistant prostate cancer patients
progressing after docetaxel: A prospective single-center study.
Oncology. 92:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu R, George DJ and Zhang T: What is the
role of sipuleucel-T in the treatment of patients with advanced
prostate cancer? An update on the evidence. Ther Adv Urol.
8:272–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carton E, Noe G, Huillard O, Golmard L,
Giroux J, Cessot A, Saidu NE, Peyromaure M, Zerbib M, Narjoz C, et
al: Relation between plasma trough concentration of abiraterone and
prostate-specific antigen response in metastatic
castration-resistant prostate cancer patients. Eur J Cancer.
72:54–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saad F: Evidence for the efficacy of
enzalutamide in postchemotherapy metastatic castrate-resistant
prostate cancer. Ther Adv Urol. 5:201–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker C, Zhan L, Cislo P, Reuning-Scherer
J, Vogelzang NJ, Nilsson S, Sartor O, O'Sullivan JM and Coleman RE:
Effect of radium-223 dichloride (Ra-223) on hospitalisation: An
analysis from the phase 3 randomised Alpharadin in symptomatic
prostate cancer patients (ALSYMPCA) trial. Eur J Cancer. 71:1–6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akaza H: Influences of the results from
STRIVE trial on the combination androgen depletion therapy for
advanced prostate cancer. Curr Urol Rep. 17:842016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badrising SK, van der Noort V, van den
Eertwegh AJ, Hamberg P, van Oort IM, van den Berg HP, Los M, Aarts
MJ, Coenen JL, Gelderblom H, et al: Prognostic parameters for
response to enzalutamide after docetaxel and abiraterone treatment
in metastatic castration-resistant prostate cancer patients; a
possible time relation. Prostate. 76:32–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oudard S, Kramer G, Caffo O, Creppy L,
Loriot Y, Hansen S, Holmberg M, Rolland F, Machiels JP and Krainer
M: Docetaxel rechallenge after an initial good response in patients
with metastatic castration-resistant prostate cancer. BJU Int.
115:744–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verzoni E, De Giorgi U, Derosa L, Caffo O,
Boccardo F, Facchini G, Porcu L, De Vincenzo F, Zaniboni A, Chiuri
VE, et al: Predictors of long-term response to abiraterone in
patients with metastastic castration-resistant prostate cancer: A
retrospective cohort study. Oncotarget. 7:40085–40094. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lohiya V, Aragon-Ching JB and Sonpavde G:
Role of chemotherapy and mechanisms of resistance to chemotherapy
in metastatic castration-resistant prostate cancer. Clin Med
Insights Oncol. 10 Suppl 1:S57–S66. 2016.
|
|
17
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonarakis ES, Lu C, Luber B, Wang H,
Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA,
et al: Androgen receptor splice variant 7 and efficacy of Taxane
chemotherapy in patients with metastatic castration-resistant
prostate cancer. JAMA Oncol. 1:582–591. 2015. View Article : Google Scholar : PubMed/NCBI
|