Introduction
Hepatocellular carcinoma (HCC) is a leading cause of
cancer-related mortality worldwide and its incidence is increasing
(1). The majority of patients with
HCC are unsuitable for curative surgical resection. However,
multimodal approaches, such as transarterial chemoembolization
(TACE) and radiofrequency ablation (RFA), may be used in clinical
practice. Although there are no widely accepted diagnostic
criteria, acute-on-chronic liver failure (ACLF) is a newly
recognized clinical entity characterized by sudden deterioration of
a chronic underlying liver disease (2). Sorafenib is a multikinase inhibitor
that targets both Raf and vascular endothelial growth factor and
platelet-derived growth factor receptor tyrosine kinase signaling.
More recently, sorafenib has come to be considered as the standard
treatment for patients with advanced HCC. Liver dysfunction was
reported in <1% of sorafenib-treated patients in the SHARP and
Asia-Pacific trials (3,4) and only few cases of severe
sorafenib-induced hepatitis have been described. We herein present
the case of a patient who developed ACLF with a fatal outcome
during treatment with sorafenib following TACE and RFA.
Case report
A 63-year-old woman was admitted to Lingnan Hospital
(Guangzhou, China) in September, 2013 due to upper abdominal
discomfort, fatigue, anorexia and a weight loss of 2 kg within 1
month. The patient has suffered from hepatitis B for 10 years
without any medical treatment. Other past medical history and
family history were unremarkable. Upon admission the patient was
asymptomatic and there was no evidence of hepatic encephalopathy,
ascites or peripheral edema. Physical examination revealed mild
abdominal distention without tenderness, while the findings of the
gynecological examination were normal.
The laboratory results revealed microcytic anemia
(haemoglobin 9.3 g/dl, haematocrit 28%, mean corpuscular volume 63
fl). Liver tests revealed mild hypoalbuminemia (3.3 g/dl; normal,
3.5–5.5 g/dl), aspartate aminotransferase (AST) 51 U/l (normal
range, 10–40 U/l) and alanine aminotransferase (ALT) 68 U/l (normal
range, 7–56 U/l), whereas all other liver function tests were
normal. The serum tumor markers α-fetoprotein (AFP), carbohydrate
antigen (CA)19-9, CA-125, CA-153 and carcinoembryonic antigen (CEA)
were all within the normal range. The following hepatitis B virus
(HBV) markers were also detected: HBsAg+;
HBeAg−; HBeAb+; HBcAb+; and
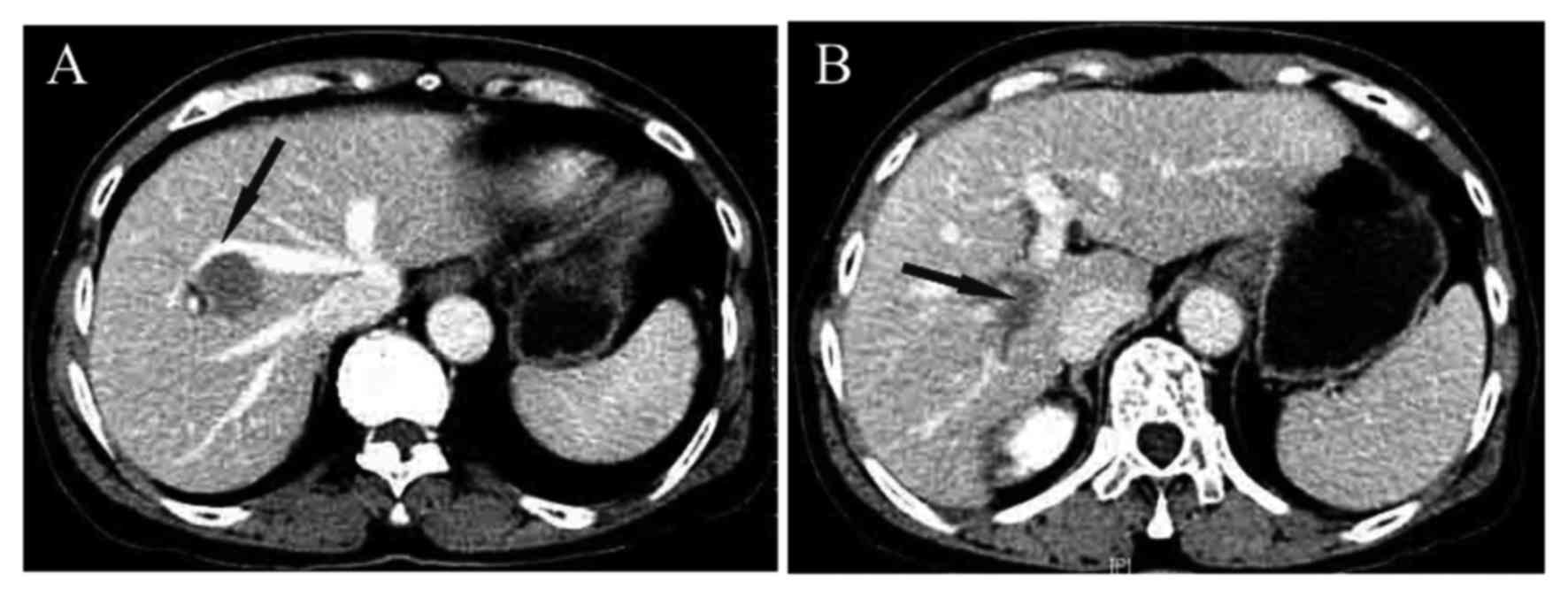
HBV-DNA 2.84×107 copies/ml. Abdominal contrast-enhanced computed
tomography (CT) revealed a 3.0×2.6-cm unifocal tumor in the V/VIII
hepatic segment (Fig. 1).
As the liver lesion invaded the middle hepatic and
right portal veins, the patient was unsuitable for potentially
curative surgical resection. TACE was used to treat the primary
cancer and, at the same time, comprehensive treatments, such as
effective inhibition of HBV replication, were applied. The patient
successfully recovered and was discharged 10 days after TACE.
Following diagnosis with advanced incurable HCC, sorafenib was
initiated the first week of October at a dosage of 400 mg twice
daily. The side effects of sorafenib were generally mild, including
anorexia, tinnitus, nausea and vomiting, which were well-controlled
medically. One month later, a repeated CT scan of the liver
indicated that the initial tumor had not completely regressed
following treatment. In November, ultrasound-guided RFA was
performed. The patient recovered soon and sorafenib treatment,
which interrupted immediately before and after RFA, was
resumed.
Seven weeks later, the patient was readmitted with
abdominal pain, progressive jaundice and abdominal distension. The
liver function parameters were as follows: ALT 970 U/l, AST 1166
U/l, total bilirubin/conjugated bilirubin 335/204 µmol/l, albumin
26 g/l, prothrombin time 39 sec and activated partial
thromboplastin time 54 sec. HBV DNA was repeatedly undetectable and
AFP remained within the normal range. These results were consistent
with the diagnosed characteristics of ACLF. Liver biopsy revealed
intrahepatic cholestasis, parenchymal necrosis, inflammatory
infiltrates, as well as significant fibrosis, indicating both acute
and chronic liver injury (Fig. 2).
Although several therapies were applied, the patient succumbed to
severe liver decompensation after 10 days. Written informed consent
was obtained from the patient's family for the publication of the
case details and associated images.
Discussion
There are multiple treatment options for HCC,
including liver resection, local ablation, TACE or selective
internal radiotherapy, targeted therapy with sorafenib and liver
transplantation (5). The majority of
the cases with HCC are diagnosed at an advanced stage and,
therefore, patients cannot benefit from curative therapy. For our
patient, as the primary liver tumor invading the major veins, a
multidisciplinary treatment approach was applied.
TACE has become the first choice of treatment for
patients with non-surgical HCC. However, the outcome in the present
case was not satisfactory due to the aggressive nature of the
tumor. The SHARP and Asia-Pacific trials demonstrated a significant
survival benefit and good tolerance in patients with advanced HCC,
making sorafenib the new reference standard for systemic therapy of
patients with advanced HCC (3,4).
Ineffective TACE is associated with a negative harm-benefit
balance, without prolongation of the overall survival, and should
not be repeated. TACE failure is an indication for sorafenib
therapy in patients with advanced HCC (6). Based on these analyses, sorafenib was
administered at a dose of 400 mg/bid. TACE and the specific
anti-angiogenesis effect of sorafenib is one of the major
strategies for a combination treatment approach. The CT scan after
TACE indicated that the initial tumor did not fully regress. RFA is
considered a safe and feasible procedure, with a low complication
rate; thus, ultrasound-guided RFA was also applied to control tumor
progression.
Hyperbilirubinemia is almost invariably present and
jaundice is considered an essential criterion for the diagnosis of
ACLF. In addition to jaundice, another hallmark of liver
dysfunction is coagulopathy. Prolongation of the prothrombin time
is common. Coagulation tests are usually abnormal in cirrhotic
patients due to impaired synthesis and increased consumption of
coagulation factors. In the present case, the liver function
parameters revealed that the total bilirubin was 335 µmol/l and the
prothrombin time was 39 sec. The clinical symptoms after
hospitalization also include the development of hepatic
encephalopathy, ascites and hepatorenal syndrome.
To date, the most commonly reported
sorafenib-related adverse events have been diarrhea, fatigue, rash,
alopecia, anorexia and nausea. The side effects in our patient were
mild and effectively controlled. Liver failure was uncommon among
sorafenib-treated patients in the SHARP and Asia-Pacific trials
(3,4). However, Ozenne et al reported
that 7 (21%) French patients with Child-Pugh A class liver disease
experienced liver failure (7). In a
retrospective study from Japan, Ogasawara et al also
reported a higher frequency of liver failure (8): Of the 54 patients treated, liver
failure occurred in 10 (19%).
ACLF is a rare syndrome with diverse etiology, which
is associated with a high mortality rate (9). Although the exact pathogenesis remains
to be elucidated, a number of causes may contribute to the
development of ACLF. In Asia, the continuous replication and
expression of HBV is considered as one of the most common acute
inducers leading to ACLF (10).
Another acute precipitant for ACLF is infection. Infection plays an
important role in the progression and management decisions of ACLF.
However, there was no evidence of HBV reactivation or specific
infection after hospitalization.
The therapeutic challenge in this patient was that
treatment had to be performed in a patient with an already damaged
cirrhotic liver, which by itself causes significant morbidity and
mortality and increases drug toxicity. Recent studies also
indicated that the effectiveness of the combination of sorafenib
and TACE remains questionable (11)
and two recent meta-analyses reported discrepant results (12,13).
However, the combination of TACE and sorafenib induced two-way
targeting of vascularization through embolization and inhibition of
angiogenesis. Thus, we considered that, as Ogasawara et al
had reported, patients treated with sorafenib may require a dose
reduction.
In conclusion, although rare, the possible
development of ACLF in patients undergoing sorafenib treatment must
be kept in mind. Our experience highlights that combination therapy
with sorafenib should be applied with caution and patients treated
with sorafenib may require a dose reduction following
interventional treatment.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Program of Guangdong Province (no.
2014A020212715).
References
|
1
|
Gomaa AI and Waked I: Recent advances in
multidisciplinary management of hepatocellular carcinoma. World J
Hepatol. 7:673–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jalan R, Gines P, Olson JC, Mookerjee RP,
Moreau R, Garcia-Tsao G, Arroyo V and Kamath PS: Acute-on chronic
liver failure. J Hepatol. 57:1336–1348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arizumi T, Ueshima K, Chishina H, Kono M,
Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, et al:
Validation of the criteria of transcatheter arterial
chemoembolization failure or refractoriness in patients with
advanced hepatocellular carcinoma proposed by the LCSGJ. Oncology.
(87 Suppl 1). 32–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozenne V, Paradis V, Pernot S, Castelnau
C, Vullierme MP, Bouattour M, Valla D, Farges O and Degos F:
Tolerance and outcome of patients with unresectable hepatocellular
carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol.
22:1106–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogasawara S, Kanai F, Obi S, Sato S,
Yamaguchi T, Azemoto R, Mizumoto H, Koushima Y, Morimoto N, Hirata
N, et al: Safety and tolerance of sorafenib in Japanese patients
with advanced hepatocellular carcinoma. Hepatol Int. 5:850–856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jalan R and Williams R: Acute-on-chronic
liver failure: Pathophysiological basis of therapeutic options.
Blood Purif. 20:252–261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Liu Z, Wang T, Wang Q, Shi X and
Dao W: Characteristics of acute and sub-acute liver failure in
China: Nomination, classification and interval. J Gastroenterol
Hepatol. 22:2101–2106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu QH, Zhang Q, Bai XL, Hu QD, Su W, Chen
YW, Su RG and Liang TB: Sorafenib enhances effects of transarterial
chemoembolization for hepatocellular carcinoma: A systematic review
and meta-analysis. J Cancer Res Clin Oncol. 140:1429–1440. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X
and Han G: Combination therapy of sorafenib and TACE for
unresectable HCC: A systematic review and meta-analysis. PLoS One.
9:e911242014. View Article : Google Scholar : PubMed/NCBI
|