Introduction
Extranodal natural killer/T-cell lymphoma (ENKL) is
a type of lymphoma associated with Epstein-Barr virus (EBV)
infection, which predominantly affects the nasal cavity. However,
ENKL with an orbital lesion as the initial presentation is
extremely rare, with only a small number of cases reported in the
literature to date, as such cases are easily missed or delayed.
Currently, the treatment of ENKL is difficult due to its aggressive
behavior. Although there is no gold standard for a specific
protocol in ENKL, several aspects are widely accepted in the
treatment of this disease. Radiotherapy (RT) must be administered
alone or in combination with chemotherapy as initial therapy for
stage I/II disease; in advanced disease (stage III and IV),
asparaginase-based therapy, such as the combination of
dexamethasone, methotrexate, ifosfamide, L-asparaginase and
etoposide (SMILE regimen), has achieved the best results (1). However, ENKL patients progressing from
asparaginase-based chemotherapy represent a challenge. Bortezomib
or fludarabine monotherapy has demonstrated considerable efficacy
in the treatment of various types of non-Hodgkin lymphoma (NHL). It
was demonstrated that the cytotoxicity of bortezomib and
fludarabine was synergistic in chronic lymphocytic leukemia cell
line models (2), but their efficacy
remains unclear in ENKL. To the best of our knowledge, this is the
first case report of a relapsed ENKL patient with orbital
involvement as the initial presentation treated with RT and the
SMILE regimen being successfully salvaged with the combination of
bortezomib and fludarabine.
Case report
A 40-year-old woman presented with a 3-week history
of fever and unilateral periorbital edema on the left side. Orbital
computed tomography (CT) scans identified a mass in the left orbit,
and fine-needle aspiration cytology (FNAC) examination of the mass
in another hospital revealed atypical lymphocytic infiltration. The
patient was admitted to our hospital in November 2014. On
admission, thorough physical examination identified no other
positive signs, apart from fever and the abnormality of the left
eye. Hematological parameters, thyroid, liver, kidney function and
coagulation system tests were all normal. Rheumatoid factor,
antinuclear antibody, myeloperoxidase antineutrophil cytoplasmic
antibodies and proteinase 3 antineutrophil cytoplasmic antibodies
were negative. Serological tests for hepatitis B, Coxsackie virus,
cytomegalovirus, influenza virus, antihuman parvovirus B19 and
herpes simplex virus were all negative, but the results of the EBV
serology were IgM− and IgG++. Blood culture
revealed no evidence of bacteremia, and chest radiography revealed
no evidence of cardiopulmonary disease or a mediastinal mass. On
abdominal ultrasound examination, the liver and spleen were normal,
without enlarged lymph nodes.
There was no evidence of sepsis, infectious
mononucleosis, Graves' disease or vasculitides. Orbital pseudotumor
was suspected based on the CT scans and FNAC examination. The
patient was treated with methylprednisolone 500 mg/day for 3 days,
and became afebrile, whereas the edema of the orbit significantly
regressed. Methylprednisolone was gradually tapered down to 50
mg/day. Orbital edema and fever recurred, without other positive
signs on physical examination. Bone marrow cytomorphological
examination and biopsy were normal. CT scans identified a mass in
the left orbit and increased thickness of the nasal mucosa; the
neck, chest, abdomen and pelvis were normal. Bioptic specimens were
collected from the orbital mass and the thickened nasal mucosa.
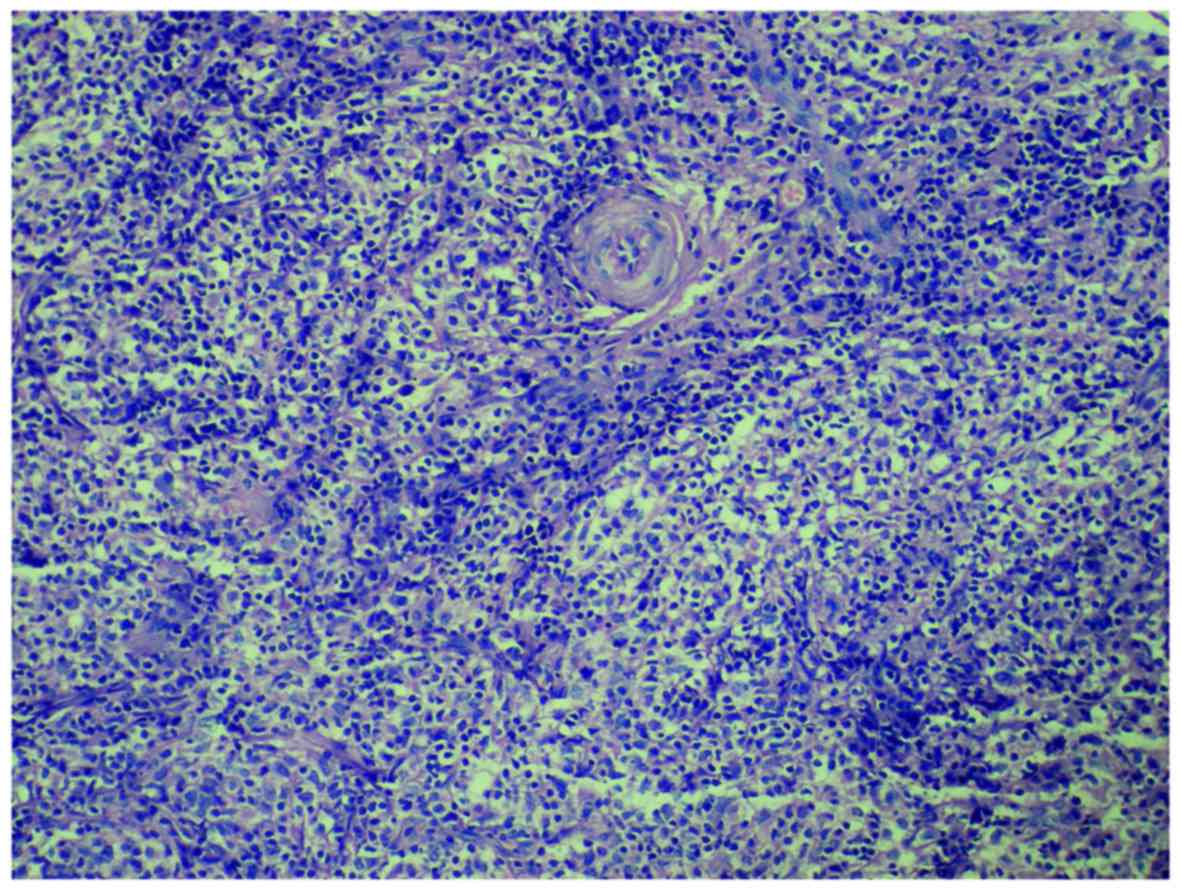
Histopathological examination revealed infiltration by atypical
lymphoid cells with an angiocentric growth pattern (Fig. 1). Immunohistochemistry of the
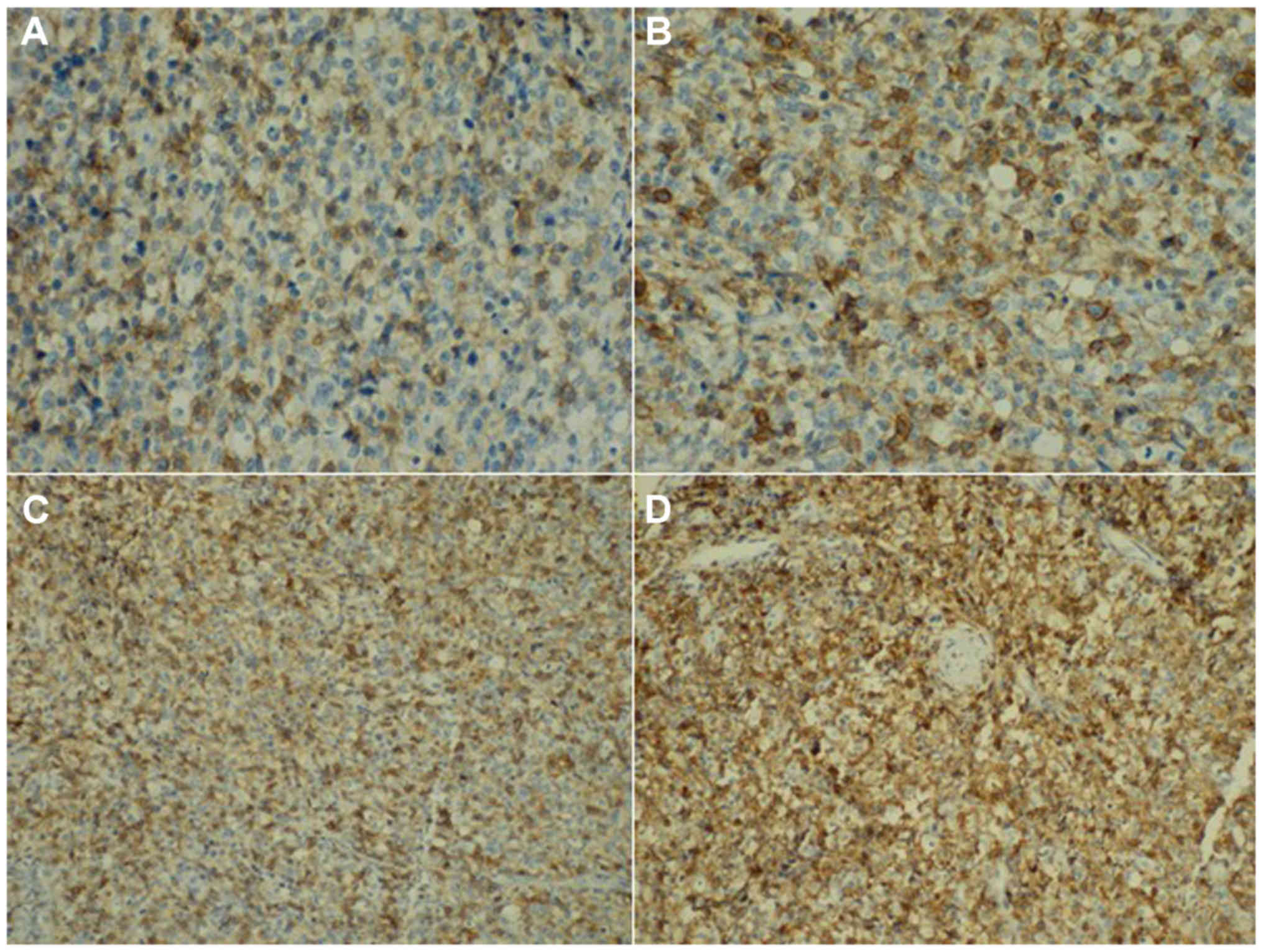
neoplastic cells was positive for CD2, CD56, CD45RO, cytoplasmic
CD3 (Fig. 2), TIA1, granzyme B,
perforin and latent membrane protein 1, and negative for CD5, CD20,
CD21 and surface CD3. Moreover, in situ hybridization of the
neoplastic cells was positive for EBV-encoded small RNA. The
findings supported the diagnosis of ENKL (Ann Arbor stage IE).
The patient was treated with involved-field RT (50
Gy) followed by 2 cycles of SMILE chemotherapy with growth factor
support, repeated every 28 days. A CT scan revealed disappearance
of the orbital tumor. Subsequently, the SMILE regimen was applied
as maintenance therapy but was complicated by toxicities including
grade 4 neutropenia and grade 3 infection. Despite the initial good
response, the patient developed disease relapse and progression
(stage IIIE) after a total of 4 cycles of SMILE, with hyperpyrexia,
painful swelling of the throat and edema of the orbit; the
bilateral tonsils protruded from the tonsillar fossa and exhibited
an irregular surface. The bilateral cervical, axillary and inguinal
lymph nodes were enlarged, with accompanying splenomegaly. A
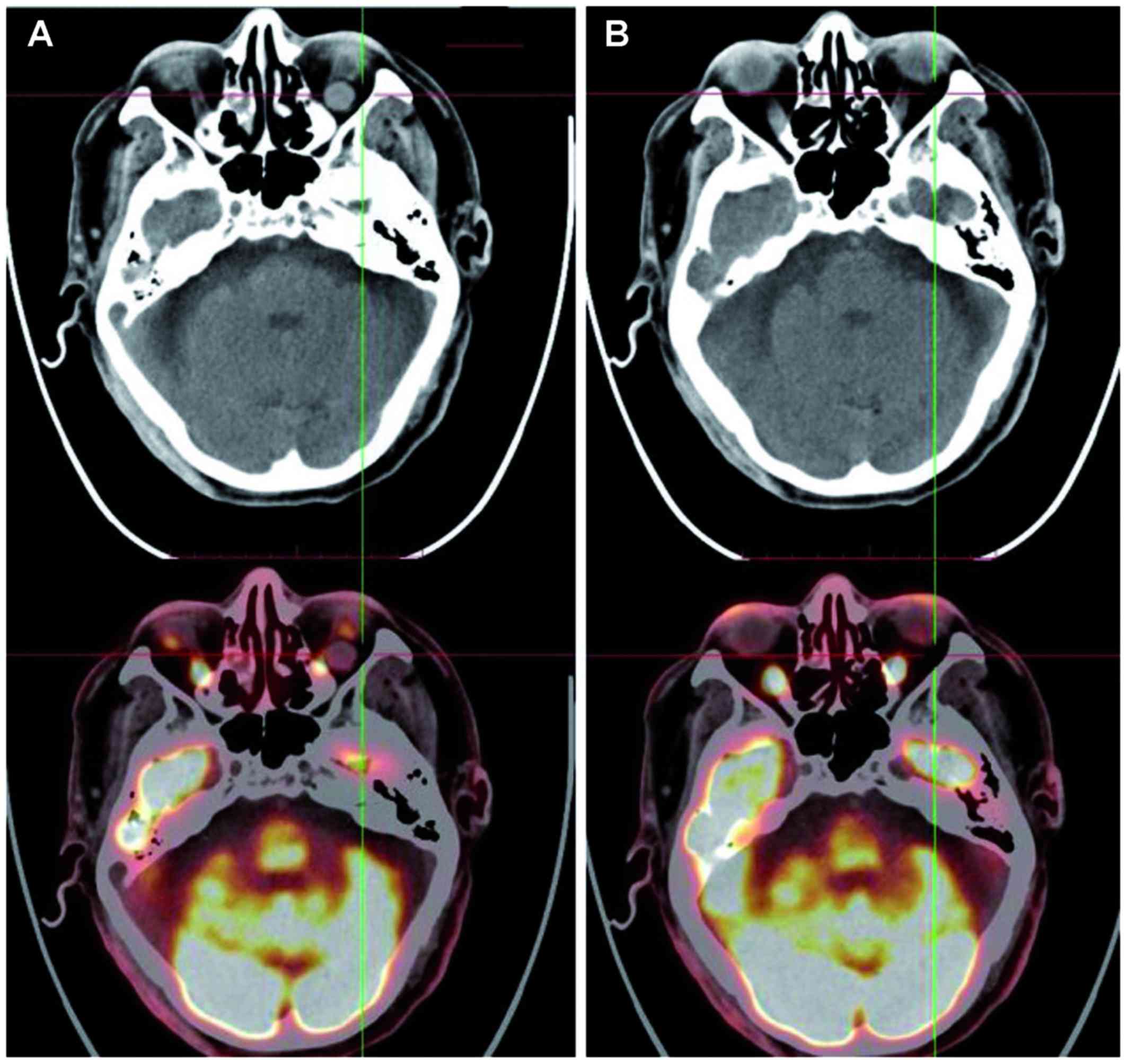
positron emission tomography (PET) scan revealed a mass in the left
orbit (Fig. 3A). After obtaining
informed consent from the patient, treatment was switched to a
novel regimen comprising bortezomib (1.3 mg/m2 on days
1, 4, 8 and 11) and fludarabine (25 mg/m2 on days 1–5)
as salvage therapy, repeated every 3 weeks. The hematological
toxicities of bortezomib plus fludarabine were well-tolerated, with
only grade 3 neutropenia (according to World Health Organization
criteria); the non-hematological toxicities were mild. After two
courses of chemotherapy, PET scan revealed disappearance of the
orbital tumor (Fig. 3B).
Subsequently, the patient received autologous hematopoietic stem
cell transplantation (HSCT).
Discussion
ENKL is a lymphoma of cytotoxic lymphocytes
characterized by vascular destruction and necrosis. ENKL occurs
more commonly in Asia and South America compared with Europe, and
predominantly affects the nasal cavity. Other extranasal sites have
been occasionally reported, such as the upper aerodigestive tract,
gastrointestinal tract, liver, spleen, central nervous system, bone
marrow, skin, soft tissue and testis (3). However, ENKL with an orbital lesion as
the initial presentation is very unusual, with only a small number
of cases reported in the literature. The orbit is a common
extranodal site of lymphoid tumors. However, the majority of
orbital lymphomas are B-cell lymphomas of mucosa-associated
lymphoid tissue (MALT) lymphomas (4), composed mainly of non-active and mature
small lymphocytes, often confined to the orbit, and exhibiting
low-grade malignant characteristics. Among 68 patients with adnexal
lymphomas, 61 were of the MALT type, 2 were of the diffuse large
B-cell type, 2 were of the mantle-cell type, 1 was an anaplastic
large-cell lymphoma, and only 2 cases were NK/T-cell lymphomas
(5). Due to rarity and variable
clinical manifestations, ENKL with orbital involvement may be
easily misdiagnosed. In the early stages of the disease, relatively
few neoplastic cells are present, and the neoplastic cells are
usually located angiocentrically, with areas of surrounding
necrosis. Thus, biopsy may not include the tumor cells. Due to
these factors, multiple or extensive biopsies are often required to
obtain sufficient viable tissue. Atypical manifestations, including
swollen periorbital tissues, high orbital pressure and edematous
skin of the eyelids and the inner canthus, the rapid progression of
the disease and massive tissue necrosis may lead to the
misdiagnosis as an acute orbital pseudotumor, such as our patient.
Orbital ENKL is most often misdiagnosed as orbital cellulitis and
thyroid ophthalmopathy (6,7). In addition, conditions that may cause
or mimic ENKL with orbital involvement also include lymphangioma,
metastatic carcinoma and rheumatological disorders, such as
Wegner's granulomatosis.
Although optimal treatment strategies for ENKL have
not been clearly determined, several aspects are widely accepted in
the treatment of this disease. For localized disease, RT must be
administered alone or in combination with chemotherapy as initial
therapy. In disseminated NK/T-cell lymphomas, systemic chemotherapy
is the mainstay of treatment. Allogeneic HSCT has also been
evaluated in the management of ENKL in several retrospective
patient series, but the results are difficult to interpret, as the
majority of the studies included a small number of cases, with
heterogeneous indications and inclusion criteria (8–10). ENKL
is radiosensitive, and RT alone (~50 Gy) has achieved overall
response rates of 77–100% for localized disease. However, RT alone
is inadequate, with reported systemic relapse rates of 25–40%, and
chemotherapy is required to decrease the risk of systemic failure
(11). A multicenter retrospective
study reported that, in 36 patients with ENKL (nasal type), the use
of RT with chemotherapy (either concurrent or sequential) compared
with chemotherapy alone was associated with significantly increased
complete response (CR) rate (90 vs. 33%, respectively) and a higher
5-year overall survival (OS) rate (75 vs. 35%, respectively)
(12). Anthracycline-based regimens
(such as CHOP) followed by involved-field RT yielded unsatisfactory
response rates. Kim et al used 4 cycles of CHOP chemotherapy
followed by involved-field RT (45 Gy) for stage I/II nasal
lymphoma, with a CR rate of 58% and a 3-year OS rate of 59%
(13). The poor response to CHOP may
be due to the intrinsic properties of the ENKL. Generally, NK cells
overexpress P-glycoprotein (14),
which results in drug efflux and intracellular decrease of
cytotoxic agents. SMILE is one of the most promising protocols,
comprising L-asparaginase, 3 non-P-glycoprotein-dependent drugs
(dexamethasone, methotrexate and ifosfamide) and etoposide, which
has been tested in a multicenter phase 2 study conducted by the NK
Tumor Study Group, where 38 patients with stage IV,
relapsed/refractory ENKL were treated, and the results showed a CR
rate of 45% and a PR rate of 34% (15). Kwong et al treated 43 newly
diagnosed and 44 relapsed/refractory ENKL patients with the SMILE
regimen, with an overall response rate of 81% (CR rate of 66% and
partial response rate of 15%), and the response rates were similar
between newly diagnosed and relapsed/refractory patients. At a
median follow-up of 31 months, the 5-year OS was 50% and the 4-year
disease-free-survival rate was 64% (16). However, the management of patients
with ENKL progressing while on asparaginase-based chemotherapy
represents a challenge.
ENKL cells constitutively express nuclear factor
(NF)-κB, and NF-κB is associated with oncogenesis, cell cycle
progression, apoptosis and multiple drug resistance. Bortezomib is
a dipeptidyl boronic acid that is a specific and selective
inhibitor of the 26S proteasome, which leads to stabilization of
IκBα, an NF-κB inhibitory protein, thereby reducing NF-κB activity.
Moreover, bortezomib may act by inhibiting DNA repair kinases
(17), supporting the use of
bortezomib in combination with cytotoxic agents. Currently, the
clinical efficacy of bortezomib for hematological neoplasms is an
interesting issue. A phase I study of bortezomib plus CHOP in
patients with advanced, aggressive T-cell or NK/T-cell lymphoma
demonstrated that 1 in 3 patients achieved CR (18). Fludarabine, a purine nucleoside
analogue, is a potent cytotoxic agent that acts by inhibiting DNA
polymerase and ribonucleotide reductase, thus terminating DNA
strand replication. Clinical studies have demonstrated that
fludarabine (alone or, particularly, as a component of combination
therapy) may result in high overall response rate and CR rate in
adults with various types of NHL (19). However, the clinical efficacy of
fludarabine has not been well estimated in ENKL.
It was demonstrated that the cytotoxicity of
fludarabine and bortezomib was synergistic in chronic lymphocytic
leukemia cell line models (2). The
potential synergistic mechanism may involve inhibiting the repair
of the fludarabine-induced DNA lesions by bortezomib, thereby
enhancing its antineoplastic effect. Moreover, another study also
suggested that bortezomib may reverse resistance to fludarabine by
inhibiting the NF-κB pathway (20).
Hence, it was hypothesized that chemotherapy with bortezomib plus
fludarabine may be an effective salvage strategy for relapsed ENKL.
After obtaining informed consent from the patient, bortezomib in
combination with fludarabine was administered as salvage treatment,
which achieved a promising result.
The present case suggests that bortezomib plus
fludarabine appears to be a safe and effective alternative for
relapsed ENKL. However, the efficacy of this combination should be
evaluated in further larger-sized clinical trials.
References
|
1
|
Tse E and Kwong YL: How I treat NK/T-cell
lymphomas. Blood. 121:4997–5005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duechler M, Linke A, Cebula B, Shehata M,
Schwarzmeier JD, Robak T and Smolewski P: In vitro cytotoxic effect
of proteasome inhibitor bortezomib in combination with purine
nucleoside analogues on chronic lymphocytic leukaemia cells. Eur J
Haematol. 74:407–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vega F, Lin P and Medeiros LJ: Extranodal
lymphomas of the head and neck. Ann Diagn Pathol. 9:340–350. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferry JA, Fung CY, Zukerberg L, Lucarelli
MJ, Hasserjian RP, Preffer FI and Harris NL: Lymphoma of the ocular
adnexa: A study of 353 cases. Am J Surg Pathol. 31:170–184. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho EY, Han JJ, Ree HJ, Ko YH, Kang YK,
Ahn HS, Ahn SD, Park CJ and Huh J: Clinicopathologic analysis of
ocular adnexal lymphomas: Extranodal marginal zone b-cell lymphoma
constitutes the vast majority of ocular lymphomas among Koreans and
affects younger patients. Am J Hematol. 73:87–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charton J, Witherspoon SR, Itani K, Jones
FR, Marple B and Morse B: Natural killer/T-cell lymphoma
masquerading as orbital cellulitis. Ophthal Plast Reconstr Surg.
24:143–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai W, Zhong M, Shen W, Zou K and Bai CG:
Natural killer T-cell lymphoma originating from the orbit. Chin Med
J (Engl). 125:1677–1680. 2012.PubMed/NCBI
|
|
8
|
Ennishi D, Maeda Y, Fujii N, Kondo E,
Shinagawa K, Ikeda K, Ichimura K, Yoshino T and Tanimoto M:
Allogeneic hematopoietic stem cell transplantation for advanced
extranodal natural killer/T-cell lymphoma, nasal type. Leuk
Lymphoma. 52:1255–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwong YL: Hematopoietic stem cell
transplantation in natural killer cell lymphoma and leukemia. Int J
Hematol. 92:702–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Gao C, Li H, Wang Z, Cao Y, Huang W,
Li X, Wang S, Yu L and Da W: Allogeneic haematopoietic stem cell
transplantation as a salvage strategy for relapsed or refractory
nasal NK/T-cell lymphoma. Med Oncol. 28:840–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SJ and Kim WS: Treatment of localized
extranodal NK/T cell lymphoma, nasal type. Int J Hematol.
92:690–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chauchet A, Michallet AS, Berger F,
Bedgedjian I, Deconinck E, Sebban C, Antal D, Orfeuvre H, Corront
B, Petrella T, et al: Complete remission after first-line
radio-chemotherapy as predictor of survival in extranodal NK/T cell
lymphoma. J Hematol Oncol. 5:272012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim WS, Song SY, Ahn YC, Ko YH, Baek CH,
Kim DY, Yoon SS, Lee HG, Kang WK, Lee HJ, et al: CHOP followed by
involved field radiation: Is it optimal for localized nasal natural
killer/T-cell lymphoma? Ann Oncol. 12:349–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Li XQ, Ma X, Hong X, Lu H and Guo
Y: Immunohistochemical expression and clinical significance of
P-glycoprotein in previously untreated extranodal NK/T-cell
lymphoma, nasal type. Am J Hematol. 83:795–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M, Kwong YL, Kim WS, Maeda Y,
Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, et al:
Phase II study of SMILE chemotherapy for newly diagnosed stage IV,
relapsed, or refractory extranodalnatural killer (NK)/T-cell
lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin
Oncol. 29:4410–4416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T,
Tse E, Leung AY and Chim CS: SMILE for natural killer/T-cell
lymphoma: Analysis of safety and efficacy from the Asia Lymphoma
Study Group. Blood. 120:2973–2980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hideshima T, Mitsiades C, Akiyama M,
Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi
NC, Mitsiades N and Anderson KC: Molecular mechanisms mediating
antimyeloma activity of proteasome inhibitor PS-341. Blood.
101:1530–1534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee J, Suh C, Kang HJ, Ryoo BY, Huh J, Ko
YH, Eom HS, Kim K, Park K and Kim WS: Phase I study of proteasome
inhibitor bortezomib plus CHOP in patients with advanced,
aggressive T-cell or NK/T-cell lymphoma. Ann Oncol. 19:2079–2083.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson VR and Perry CM: Fludarabine: A
review of its use in non-Hodgkin's lymphoma. Drugs. 67:1633–1655.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hewamana S, Alghazal S, Lin TT, Clement M,
Jenkins C, Guzman ML, Jordan CT, Neelakantan S, Crooks PA, Burnett
AK, et al: The NF-kappaB subunit Rel A is associated with in vitro
survival and clinical disease progression in chronic lymphocytic
leukemia and represents a promising therapeutic target. Blood.
111:4681–4689. 2008. View Article : Google Scholar : PubMed/NCBI
|