Introduction
Bortezomib is a proteasome inhibitor that has
demonstrated therapeutic efficacy for patients with progressive
multiple myeloma, with the benefit of a milder toxicity profile
compared with traditional chemotherapies. However, the side effects
of bortezomib may affect a number of systems, including the
gastrointestinal, hematological, nervous and musculoskeletal
systems and, infrequently, the endocrine system (1–8).
The syndrome of inappropriate antidiuretic hormone
secretion (SIADH) was first described in 1957 (9), and is an important cause of
hyponatremia. SIADH may be idiopathic or secondary to numerous
causes, including various drugs, malignancies, central nervous
system disorders and pulmonary abnormalities (Table I). The diagnosis of SIADH is made
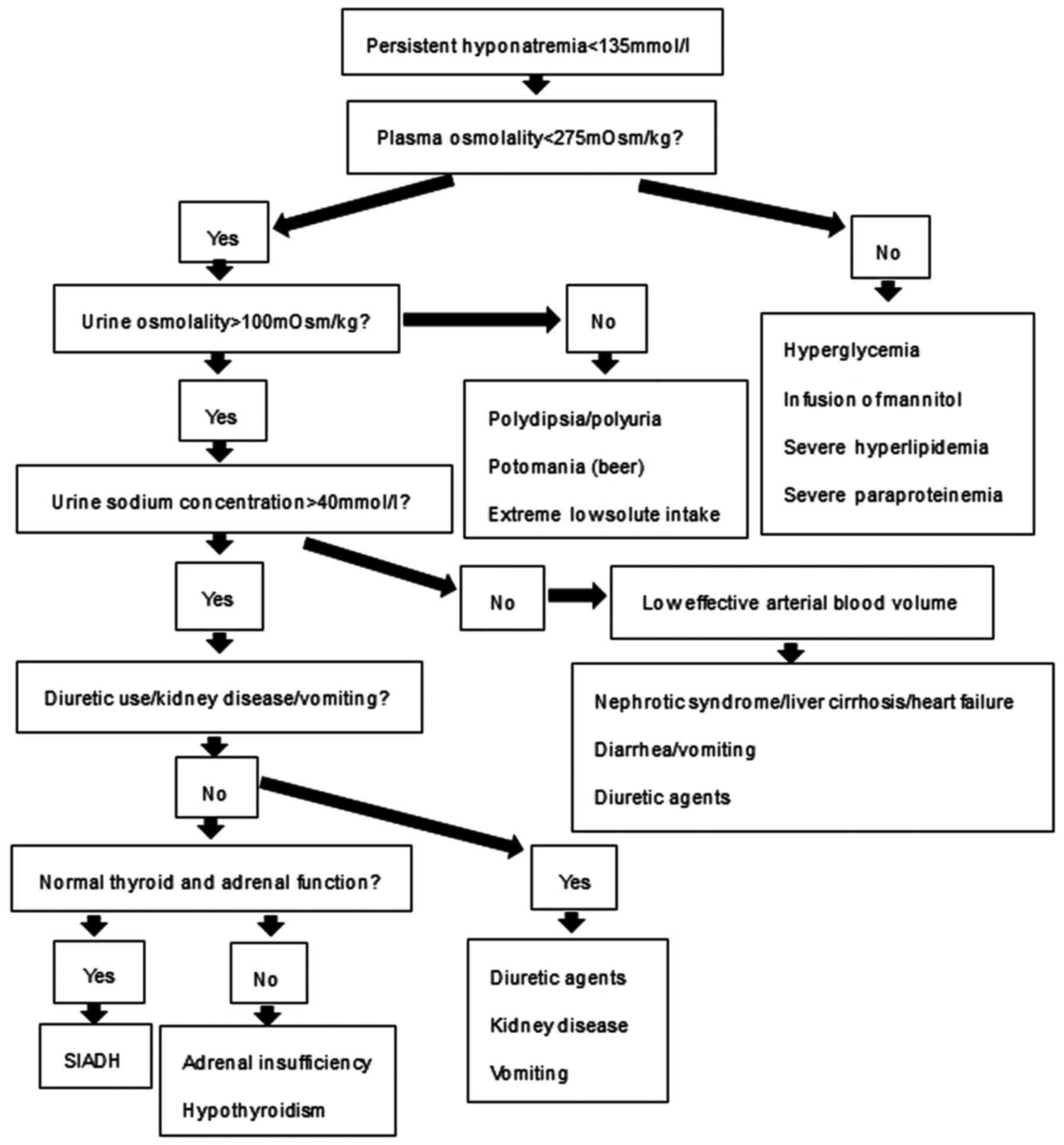
based on the assessment of clinical criteria (Fig. 1) (10).
 | Table I.Causes of SIADH. |
Table I.
Causes of SIADH.
| Malignancies | CNS disorders | Drugs | Pulmonary
disorders | Other causes |
|---|
| Bronchogenic | Tumors | Tricyclic | Tuberculosis | NSIAD
(hereditary) |
| carcinoma | Subdural | antidepressants | Pneumonia | AIDS and ARC |
| Oat cell
carcinoma | Hematoma | Carbamazepine | Lung abscess | Prolonged
strenuous |
| Thymoma | Brain abscesses | Valproate | Bronchiectasis | Exercise |
| Nasopharyngeal | Encephalitis | Oxycarbamazepine | Acute | Prolonged nausea
and |
| carcinoma | Meningitis | Clofibrate | respiratory | vomiting |
| Pancreatic
carcinoma | Systemic lupus | Ciprofloxacin | failure | Senile atrophy |
| Stomach
carcinoma | erythematous | Ethionamide | COPD | Idiopathic
(elderly) |
| Duodenum
carcinoma | Acute
intermittent | Amiodarone | Asthma | Urinary
retention |
| Ewing's sarcoma | porphyria | SSRI | Cystic fibrosis | Hypothyroidism |
| Mesothelioma | Multiple
sclerosis | Omeprazole | Positive | Hypocorticism |
| Bladder
carcinoma | Guillain-Barré | AVP analogues | pressure | Strongyloidiasis |
|
Ureteral/prostate | syndrome | Oxytocin | ventilation |
|
| carcinoma | Spinal cord | Narcotics |
|
|
| Uterine/cervical | lesions | Chlorpropamide |
|
|
| carcinoma | Cerebrovascular | Vincristine |
|
|
| Olfactory | accident | Vinblastine |
|
|
| neuroblastoma | Subarachnoid | Vinorelbine |
|
|
| Leukemia | hemorrhage | Ifosfamide |
|
|
| Lymphoma | Head trauma | Cyclophosphamide |
|
|
|
Macroglobulinemia | Acute psychosis | Nicotine |
|
|
|
| Delirium tremens | Antipsychotic |
|
|
|
| Pituitary stalk
section | drugs |
|
|
|
|
|
| Trans-sphenoidal | ACE inhibitors |
|
|
|
| adenomectomy | Mirtazapine |
|
|
|
| Hydrocephalus | Amiodarone |
|
|
|
| Antiphospholipid | Ciprofloxacin |
|
|
|
| syndrome | Venlafaxine |
|
|
|
| Shy-Drager | Lamotrigine |
|
|
|
| syndrome | Fluoxetine |
|
|
Case report
A 44-year-old male patient with a 3-month history of
multiple myeloma and no other previous medical history was
prescribed a chemotherapy regimen of bortezomib and dexamethasone:
Bortezomib (1.3 mg/m2) was administered on days 1, 4, 8
and 11 of a 28-day cycle, with dexamethasone (40 mg) administered
IV on days 1–4. After 3 cycles of chemotherapy, the patient
presented to the Affiliated Drum Tower Hospital of Nanjing
University Medical School (Nanjing, China) in July 2015,
complaining of fatigue and numbness of the distal extremities 6
days after his last chemotherapy administration. There had been no
recent changes to his diet or water intake, and he had not used
diuretic agents during the month prior to presentation.
Upon physical examination, the body temperature,
blood pressure, heart rate and respiratory rate were normal.
Auscultation of the heart and lungs was normal. There were no signs
of edema, ascites, or dry mucous membranes. The patient had mild
hypesthesia and muscle weakness (<grade 5) of the
extremities.
Serum electrolyte assessment revealed a mild
decrease in serum sodium concentration (133.6 mmol/l). Despite
treatment with oral hypertonic saline, the patient's symptoms
progressed. Two days later, the patient's serum sodium level was
121.6 mmol/l. Blood analysis revealed that the patient's low serum
sodium was accompanied by low plasma osmolality (248 mOsm/kg), low
creatinine (74 µmol/l), low blood urea nitrogen (2.1 mmol/l), and
low uric acid (134 µmol/l). Additionally, the urinary osmolality
was high at 443 mOsm/kg, and urinary sodium was high at 147.7
mmol/l. The patient's potassium, calcium, phosphate, glucose, white
blood cell count, hemoglobin, platelet, lactate dehydrogenase,
total protein and triglycerides were all within the normal range
throughout the duration of the clinical assessment. In addition,
the patient's thyroid and adrenal function were evaluated and found
to be normal (Table II). The
patient had neither clinical signs of contraction nor expansion of
the extracellular fluid and no diuretic agents had been used prior
to the development of hyponatremia. Taking into consideration the
patient's laboratory values, the diagnostic criteria were met to
make a diagnosis of hyponatremia due to SIADH (Fig. 1) (10).
 | Table II.Laboratory values of the patient. |
Table II.
Laboratory values of the patient.
| Laboratory
mesurements | Results | Normal range |
|---|
| Blood pressure
(mmHg) | 105/60 | 100–130/60–80 |
| Blood glucose
(mmol/l) | 5.15 | 3.9–6.1 |
| Serum [Na+]
(mmol/l) | 121.6 | 135–145 |
| Serum chloride
(mmol/l) | 82 | 98–108 |
| Serum calcium
(mmol/l) | 2.32 | 2.25–2.75 |
| Serum phosphate
(mmol/l) | 0.91 | 0.96–1.62 |
| White blood cell
(x109/l) | 9.5 | 4–10 |
| Blood platelet count
(x109/l) | 105 | 100–300 |
| Hemoglobin (g/l) | 130 | 130–172 |
| Hematocrit
(proportion of 1.0) | 0.4 | 0.38–0.508 |
| Serum LDH (U/l) | 380 | 109–245 |
| Serum urea nitrogen
(mmol/l) | 2.1 | 2.9–7.5 |
| Serum creatinine
(µmol/l) | 74 | 44–106 |
| Serum uric acid
(µmol/l) | 134 | 90–420 |
| Serum [K+]
(mmol/l) | 4.22 | 3.5–5.5 |
| Serum total protein
(g/l) | 65.5 | 62–85 |
| Serum Albumin
(g/l) | 41.2 | 35–51 |
| Serum triglycerides
(mmol/l) | 2.5 | 0.56–1.7 |
| Serum cholesterol
(mmol/l) | 4.62 | 2.9–6 |
| Serum cortisol
(nmol/l) | 574 (08:00)-414
(16:00) | 138-690
(08:00)/69-345 (16:00) |
| Serum ACTH
(pmol/l) | 2.51 (08:00)-1.59
(16:00) | 0-10.13 |
| Serum urine
specific gravity | 1.005 | 1.003–1.030 |
| TSH (mIU/l) | 5.98 | 0.27–4.2 |
| Free T4
(pmol/l) | 20.58 | 12–22 |
| Anti-thyroglobulin
antibody (U/ml) | 17.8 | 0–115 |
| Antimicrosomal
antibody(U/ml) | 18.18 | 0–34 |
| Urinary
K+ (mmol/24 h) | 32.15 | 25–125 |
| Urinary
Na+ (mmol/l) | 147.7 | <20–30 |
| Plasma osmolality
(mOsm/kg) | 248 | 275–305 |
| Urine osmolality
(mOsm/kg) | 443 | 300–1000 |
When investigating the etiology of the patient's
SIADH, reasons secondary to central nervous system disorders and
pulmonary abnormalities were excluded through analyzing the
patient's medical history, physical examination, and recent
laboratory and radiological tests. Idiopathic reasons, listed in
Table I, did not fit the clinical
profile of the patient. Certain malignancies, such as bronchogenic
carcinoma, thymoma, lymphoma and macroglobulinemia, may also cause
SIADH. The patient had high serum immunoglobulin A levels during
the early stages of multiple myeloma, but the high level of serum
immunoglobulin A normalized after two cycles of chemotherapy. At
the time of presentation for SIADH, the patient had already
achieved complete remission (CR) from his multiple myeloma, as
determined through evaluation of the patient's blood and bone
marrow. This decreased the likelihood that SIADH was directly
associated with his diagnosis of multiple myeloma and disease
progression. By process of elimination, the most likely cause was
SIADH secondary to medications. Dexamethasone is unlikely to cause
SIADH, based on its mechanism of action and pharmacokinetics, and
has never been reported. Bortezomib was suspected as the offending
agent, given its recent introduction into the patient's regimen and
its documented risk (0.1–1%) of causing endocrine disorders, such
as SIADH (2).
The patient was treated with an infusion of
hypertonic saline and fluid restriction. Despite appropriate
treatment, his serum sodium level decreased further, reaching a
nadir of 114.9 mmol/l. However, the serum sodium level eventually
started to normalize and, when it had reached 128.9 mmol/l, the
patient experienced an improvement of his fatigue and numbness in
the distal extremities, and he asked to be discharged from the
hospital against advice from the treating physicians, who
recommended continued hospitalization for longer treatment. The
patient was followed up with outpatient tests and fluid
restriction, but discontinued the infusion of hypertonic saline. At
78 days after initially presenting with hyponatremia, the patient's
serum sodium level had normalized (135 mmol/l) and his symptoms had
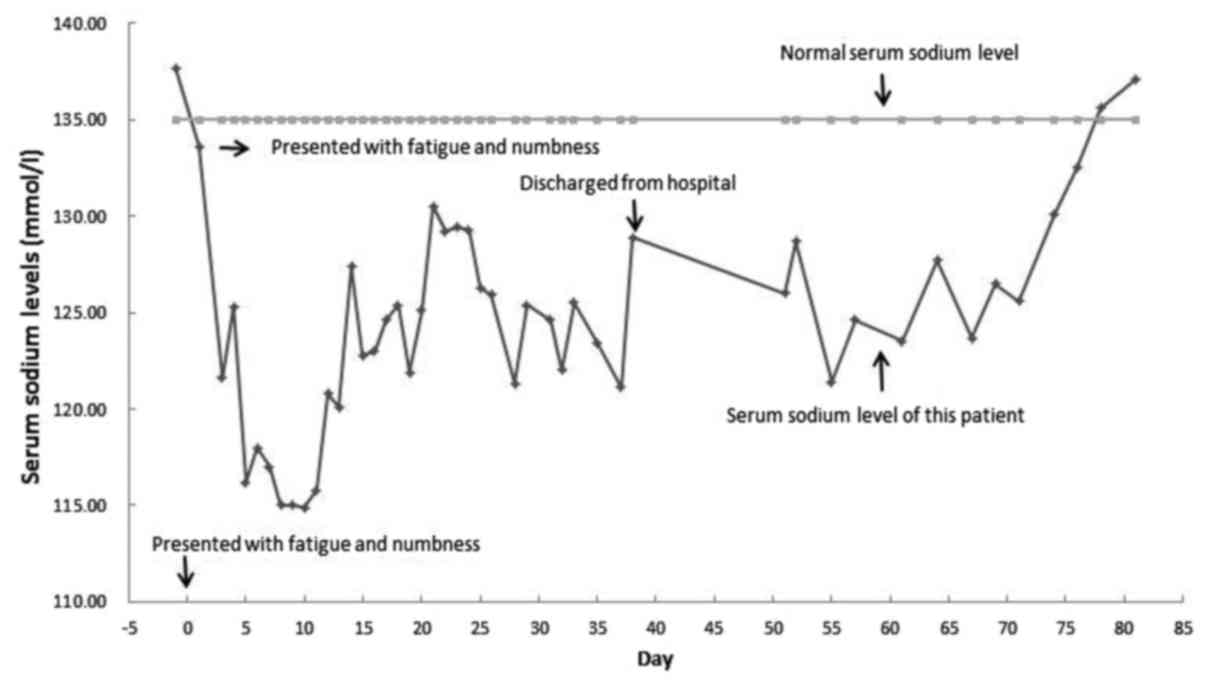
disappeared (Fig. 2). Fluid
restriction was discontinued, and the serum sodium levels remained
normal. Bortezomib was not added to the next chemotherapy cycle.
The patient subsequently received melphalan, prednisone and
thalidomide for one course, after which time treatment for multiple
myeloma was discontinued. The patient maintained CR on the last
follow-up in February 2017, and the sodium levels remained
normal.
Discussion
We herein report a case of SIADH suspected to be
secondary to bortezomib treatment. A diagnosis of SIADH was made
according to the diagnostic clinical criteria (Fig. 1) (10). SIADH is a common cause of
hyponatremia, and it is associated with significant morbidity and
mortality (11), longer
hospitalization (12), osteoporosis
(13), falls and fractures (13,14).
SIADH may be idiopathic or secondary to numerous drugs,
malignancies, central nervous system disorders, or pulmonary
abnormalities (Table I). Due to this
patient's recent history of malignancy, tumor-related SIADH was
considered as a possibility. However, to the best of our knowledge,
multiple myeloma has never been associated with SIADH to date.
Additionally, the patient in this case study had successfully
achieved CR after chemotherapy; hence, a direct malignancy-related
cause was unlikely. After ruling out other causes, including
central nervous system disorders, pulmonary abnormalities and
idiopathic forms through collectively analyzing the patient's
symptoms, physical examination and tests, a medication side effect
was deemed the most probable cause. The only medication that was
co-administered was dexamethasone. However, glucocorticoids have
never been previously associated with SIADH, so it was highly
unlikely that dexamethasone was the cause of the electrolyte
disorder. Therefore, bortezomib was suspected to be the cause of
SIADH in this patient.
Bortezomib was approved for the treatment of
patients with multiple myeloma in 2003 by the U.S. Food and Drug
Administration. Bortezomib inhibits the proteolytic activity of the
proteasome complex in mammalian cells. This inhibition of the
intracellular protein degradation pathway alters the levels of
several intracellular regulatory and signaling proteins,
effectively altering downstream cellular processes in a manner that
leads to apoptosis or growth arrest (15,16).
Numerous studies have demonstrated the promising effect of
bortezomib in treating patients with progressive multiple myeloma.
Bortezomib also has a significantly more tolerable side effect
profile compared with other traditional chemotherapeutic agents for
multiple myeloma.
Bortezomib has been reported to have a wide-ranging
side effect profile affecting a number of systems (1). However, the endocrine system is seldom
affected in patients receiving bortezomib (2–8),
although severe hyponatremia has been reported in 2.6–25.9% of such
patients (Table III) (3–8,17,18).
 | Table III.Frequency of severe hyponatremia in
patients treated with bortezomib as reported in previous
studies. |
Table III.
Frequency of severe hyponatremia in
patients treated with bortezomib as reported in previous
studies.
| Authors | Frequency, %
(adverse event/total number) | (Refs.) |
|---|
| Richardson et
al | Undetailed | (3) |
| Belch et
al | 3.3 (1/30) | (4) |
| O'Connor et
al | 12.5 (3/24) | (5) |
| Orlowski et
al | 25.9 (7/27) | (6) |
| Van Waes et
al | 11 (1/9) | (7) |
| Jagannath et
al | 9.2 (5/54) | (8) |
| Richardson et
al | 2.6 (1/38) | (17) |
| Davies et
al | 3.8 (1/26) | (18) |
When patients experience hyponatremia, the symptoms
are associated with the severity of the hyponatremia and the
duration of time over which the serum sodium level decreased
(19). Sudden-onset (<48 h) and
severe hyponatremia may cause emergent, life-threatening symptoms
and manifest as seizures, coma, or signs of brain herniation. By
contrast, patients with chronic hyponatremia (onset >48 h) may
display minimal neurological manifestations. Some patients with
long-standing hyponatremia may even be asymptomatic, even in the
presence of dangerously low serum sodium levels. The patient in
this case study presented with progressive fatigue and numbness of
the distal of arms and legs, accompanied by mild hypesthesia and
muscle weakness <grade 5. The patient exhibited a mildly
decreased serum sodium level, but the serum sodium level was normal
2 days prior. After treating the patient with oral hypertonic
saline for 2 days, the serum sodium level decreased further (114.9
mmol/l). Despite the patient's very low sodium levels, his symptoms
were not severe symptoms. This is likely due to the 4-day interval
(>48 h) over which his serum sodium decreased from normal (137.7
mmol/l) to severely low (121.6 mmol/l).
Fluid restriction is considered to be the first-line
therapy in SIADH patients, with an initial fluid intake reduction
to 800–1,200 ml/24 h (20). This
volume of fluid intake is 500 ml/24 h below the average daily urine
volume (21). To prevent a further
reduction of serum sodium levels, hypertonic saline (3% NaCl) may
be infused. The osmolality of hypertonic saline exceeds the
osmolality of the urine. Isotonic saline should be avoided, as it
may worsen hyponatremia (22).
Vaptans, such as tolvaptan, lixivaptan, satavaptan and conivaptan,
are V2-receptor vasopressin antagonists and are a
promising alternative treatment for hyponatremia. Vaptans
counteract the binding of antidiuretic hormone to V2
receptors and promote solute-free water excretion by the kidneys,
decrease sodium excretion, further increasing the serum sodium
levels (23). The patient in this
case report was first treated with hypertonic saline infusion and
fluid restriction. While the serum sodium level increased, it never
exceeded 131 mmol/l throughout the duration of his hospital stay
(Fig. 2). Vaptans were considered
for further treatment to accelerate serum sodium normalization, but
were unavailable at the treating hospital and, per hospital policy,
could not be obtained from elsewhere. Approximately 80 days after
the initial presentation, the patient's serum sodium had normalized
(135 mmol/l) and remained normal throughout the duration of the
follow-up period. Bortezomib was permanently discontinued.
In conclusion, treatment with bortezomib is an
effective approach for multiple myeloma, but may have side effects
on a number of systems, although the endocrine system is
infrequently affected. The symptoms of SIADH are non-specific,
including fatigue, numbness, coma and seizures, and may be
misinterpreted as symptoms of a neurological disorder.
Consequently, the diagnosis of SIADH may often be missed, delayed,
or misdiagnosed. We herein provide evidence that supports
bortezomib as a possible cause of SIADH. Physicians treating
patients taking bortezomib who start to feel fatigue, numbness and
other neurological symptoms should consider SIADH in the
differential diagnosis and consider fluid restriction and infusion
of hypertonic saline as treatment options. The patient consented to
the publication of this case and associated details.
Acknowledgements
The authors wish to sincerely thank all the
colleagues from the Hematology Department of the Affiliated Drum
Tower Hospital of Nanjing University Medical School.
References
|
1
|
Kane RC, Bross PF, Farrell AT and Pazdur
R: Velcade: U.S. FDA approval for the treatment of multiple myeloma
progressing on prior therapy. Oncologist. 8:508–513. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodmann S, GyrKlaas E, Cathomas R,
Girardi V and von Moos R: Severe hyponatremia in a patient with
mantle cell lymphoma treated with bortezomib. A case report and
review of the literature. Onkologie. 30:651–654. 2007.PubMed/NCBI
|
|
3
|
Richardson PG, Sonneveld P, Schuster MW,
Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D,
Lonial S, Goldschmidt H, et al: Bortezomib or high-dose
dexamethasone for relapsed multiple myeloma. N Engl J Med.
352:2487–2498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Belch A, Kouroukis CT, Crump M, Sehn L,
Gascoyne RD, Klasa R, Powers J, Wright J and Eisenhauer EA: A phase
II study of bortezomib in mantle cell lymphoma: The National cancer
institute of Canada clinical trials group trial IND.150. Ann Oncol.
18:116–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Connor OA, Wright J, Moskowitz C, Muzzy
J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C,
Hamlin P, Choi E, et al: Phase II clinical experience with the
novel proteasome inhibitor bortezomib in patients with indolent
non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol.
23:676–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orlowski RZ, Stinchcombe TE, Mitchell BS,
Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ,
Pien CS, et al: Phase I trial of the proteasome inhibitor PS-341 in
patients with refractory hematologic malignancies. J ClinOncol.
20:4420–4427. 2002. View Article : Google Scholar
|
|
7
|
Van Waes C, Chang AA, Lebowitz PF, Druzgal
CH, Chen Z, Elsayed YA, Sunwoo JB, Rudy SF, Morris JC, Mitchell JB,
et al: Inhibition of nuclear factor-kappaB and target genes during
combined therapy with proteasome inhibitor bortezomib and
reirradiation in patients with recurrent head-and-neck squamous
cell carcinoma. Int J Radiat Oncol Biol Phys. 63:1400–1412. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jagannath S, Barlogie B, Berenson J,
Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R,
Limentani SA, Alsina M, et al: A phase 2 study of two doses of
bortezomib in relapsed or refractory myeloma. Br J Haematol.
127:165–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz WB, Bennett W, Curelop S and
Bartter FC: Syndrome of renal sodium loss and hyponatremia probably
resulting from inappropriate secretion of antidiuretic hormone. Am
J Med. 23:529–542. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellison DH and Berl T: The syndrome of
inappropriate antidiuresis. N Engl J Med. 356:2064–2072. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waikar SS, Mount DB and Curhan GC:
Mortality after hospitalization with mild, moderate, and severe
hyponatremia. Am J Med. 122:857–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wald R, Jaber BL, Price LL, Upadhyay A and
Madias NE: Impact of hospital-associated hyponatremia on selected
outcomes. Arch Intern Med. 170:294–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verbalis JG, Barsony J, Sugimura Y, Tian
Y, Adams DJ, Carter EA and Resnick HE: Hyponatremia-induced
osteoporosis. J Bone Miner Res. 25:554–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinsella S, Moran S, Sullivan MO, Molloy
MG and Eustace JA: Hyponatremia independent of osteoporosis is
associated with fracture occurrence. Clin J Am Soc Nephrol.
5:275–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gillessen S, Groettup M and Cerny T: The
proteasome, a new target for cancer therapy. Onkologie. 25:534–539.
2002.PubMed/NCBI
|
|
16
|
Ludwig H, Khayat D, Giaccone G and Facon
T: Proteasome inhibition and its clinical prospects in the
treatment of hematologic and solid malignancies. Cancer.
104:1794–1807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richardson PG, Weller E, Jagannath S,
Avigon DE, Alsina M, Schlossman RL, Mazumder A, Munshi NC, Ghobrial
IM, Doss D, et al: Multicenter, phase I, dose-escalation trial of
lenalidomide plus bortezomib for relapsed and relapsed/refractory
multiple myeloma. J Clin Oncol. 27:5713–5719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies AM, Ruel C, Lara PN, Lau DH,
Gumerlock PH, Bold R, Shibata S, Lenz HJ, Schenkein DP and Gandara
DR: The proteasome inhibitor bortezomib in combination with
gemcitabine and carboplatin in advanced non-small cell lung cancer:
A California cancer consortium phase I study. J Thorac Oncol.
3:68–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reynolds RM and Seckl JR: Hyponatraemia
for the clinical endocrinologists. Clin Endocrinol (Oxf).
63:366–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherlock M and Thompson CJ: The syndrome
of inappropriate antidiuretic hormone: Current and future
management options. Eur J Endocrinol. 162 Suppl 1:S13–S18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robertson GL: Posterior pituitary. Felig
P, Baxter JD, Broadus AE and Frohman LA: Endocrinology and
Metabolism. (1st edition). (New York). McGraw-Hill. 338–385.
1986.
|
|
22
|
Palmer BF: Hyponatremia in patients with
central nervous system disease: SIADH versus CSW. Trends Endocrinol
Metab. 14:182–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin PY, Abraham WT, Lieming X, Olson
BR, Oren RM, Ohara M and Schrier RW: Selective V2-receptor
vasopressin antagonism decreases urinary aquaporin-2 excretion in
patients with chronic heart failure. J Am Soc Nephrol.
10:2165–2170. 1999.PubMed/NCBI
|