Introduction
Colorectal cancer (CRC) is the most common cancer
and the second leading cause of cancer-related mortality in Japan.
Tumor markers, including carcinoembryonic antigen (CEA) and
carbohydrate antigen (CA)19-9, have been used for the screening of
CRC in clinical practice. These markers are useful for monitoring
cancer recurrence and the efficacy of chemotherapy in advanced CRC
patients with positive expression of CEA or CA19-9. However, the
sensitivity of these markers is very low in early-stage CRC.
Therefore, the development of novel molecular markers is required,
using blood testing as a non-invasive method. The serum anti-p53
antibody (Ap53Ab) has been applied as a novel tumor marker for the
detection of several cancers, including esophageal cancer (1,2), breast
cancer (1,3) and CRC (1,4,5), from 2007 onwards in Japan. Mutations of
the TP53 gene, which were detected in half of CRC cases
(6), were strongly associated with
carcinogenesis. The accumulation of mutated-TP53 protein induces
the synthesis of Ap53Ab, depending on the condition of the host
immune system (7).
Previous reports (1,4–6,8,9) have demonstrated that positive Ap53Ab
expression was detected in 24.0–33.1% of CRC patients. These
reports suggested that the measurement of Ap53Ab was useful for the
screening of CRC patients with early-stage (stage 0 and I) disease,
as the positive rate of Ap53Ab expression was higher compared with
that of CEA and CA19-9. However, a consensus on the correlation
between Ap53Ab expression and poor survival rate was not obtained
(4,8,10). The
aim of the present study was to investigate the positive rate and
clinical significance of Ap53Ab expression in 674 CRC patients, and
elucidate the association between Ap53Ab expression and
survival.
Patients and methods
Patients and clinical procedures
The subjects included 674 CRC patients (237 women
and 437 men), who were primarily diagnosed with CRC at the Saitama
Medical Center (Kawagoe, Japan) between January 2010 and December
2014. A total of 115 healthy volunteers were also selected among
patients who had been diagnosed with hemorrhoids or inguinal hernia
at the Saitama Medical Center between October 2008 and July 2010.
The median age of CRC patients and healthy volunteers was 69 years
(range, 25–92 years) and 58 years (range, 16–84 years),
respectively. Of the 674 patients investigated, the cancer
localization was as follows: Cecum, n=45; ascending colon, n=99;
transverse colon, n=56; descending colon, n=24; sigmoid colon,
n=173; and rectum, n=277. The histological diagnosis was
well-differentiated adenocarcinoma in 153 patients, moderately
differentiated adenocarcinoma in 463, poorly differentiated
adenocarcinoma in 32, mucinous adenocarcinoma in 17, and other
types in 8 patients. The carcinomas at the time of primary tumor
resection were staged according to the Union for International
Cancer Control classification (11)
as follows: Stage 0, n=38; stage I, n=130; stage II, n=206; stage
III, n=194; and stage IV, n=106. Of the 194 patients with stage III
CRC, 107 (55.2%) received mFOLFOX6 or XELOX chemotherapy and 32
patients (16.5%) received capecitabine or tegafur-uracil and
leucovorin (UFT/LV) for 6 months as postoperative adjuvant
chemotherapy. The mFOLFOX6 regimen comprises intravenous infusion
of oxaliplatin (85 mg/m2) and LV (200 mg/m2)
for 2 h, followed by rapid intravenous bolus infusion of
5-fluorouracil (5-FU; 400 mg/m2) for 5 min, and
continuous intravenous infusion of 5-FU (2,400 mg/m2)
for 46 h. This regimen was repeated every 2 weeks. The XELOX
regimen was administered as follows: Oxaliplatin (130
mg/m2) was injected intravenously. From day 1 to day 14,
capecitabine (2,000 mg/m2/day) was orally administered.
Each cycle was repeated every 3 weeks. The capecitabine or UFT/LV
group received 8 cycles of oral capecitabine (2,400
mg/m2 for 14 days followed by a 7-day rest per cycle),
or 5 cycles of adjuvant UFT/LV (UFT 300 mg/m2 and LV 75
mg/day for 28 days followed by a 7-day rest per cycle),
respectively.
The present study was performed in accordance with
the ethical guidelines for clinical research with the approval of
our Institutional Ethics Committee. Informed consent was obtained
from all individuals included in the study.
Measurement of Ap53Ab, CEA and
CA19-9
Total blood samples were routinely collected from
CRC patients and healthy volunteers at the time of diagnosis. The
serum samples were processed using the MESACUP anti-p53Ab Test
ELISA kit (MBL, Nagoya, Japan). The cut-off level of the serum
Ap53Ab was set to 1.3 U/ml according to the manufacturer's
instructions. The minimum value of Ap53Ab was reported at <0.69
U/ml; thus, values <0.69 were set to 0.69 for the analysis. The
measurement of serum CEA and CA19-9 was also performed using ELISA.
The normal level of CEA and CA19-9 was <6.7 ng/ml and <37.0
U/ml, respectively.
Statistical analysis of Ap53Ab and CEA
half-life
The half-life of Ap53Ab and CEA was calculated based
on the following formula: T=loge2xt/(loge N0-loge
N)=0.693xt/2.303(log N0-log N), where T, half-life time (days); t,
days from the operation to next blood testing; N0, preoperative
value of Ap53Ab or CEA; and N, postoperative value of Ap53Ab or
CEA.
Of the Ap53Ab-positive patients with values >10
U/ml, 39 patients were selected. In addition, 19 patients with a
CEA level elevated to >20 ng/ml were selected. These CRC
patients underwent curative surgery, with no evidence of recurrence
during the 5-year follow-up. The measurement of Ap53Ab and CEA was
performed within 60 days after surgery.
Statistical analysis
The Mann-Whitney U-test, Fisher's exact probability
test and Chi-squared test were used where applicable. Survival
analysis was conducted using the Kaplan-Meier method. The log-rank
test was used to determine the significance of the survival curves.
The period of disease-free survival (DFS) was calculated from the
time of surgery to the time to recurrence and overall survival (OS)
was calculated from the time of surgery to death from any cause.
DFS and OS were censored at the time of the last visit to our
hospital, or December 2015, whichever came first. Differences were
considered statistically significant when P<0.05. All
statistical analyses were performed using a statistical software
package (StatFlex ver.6.0; Artec, Osaka, Japan).
Results
Positive rates of tumor markers,
including Ap53Ab, CEA and CA19-9
Of the 674 CRC patients, 195 (28.9%) were positive
for Ap53Ab, while 12 positive cases (10.4%) were identified in the
control group (Table I). The mean
level ± standard deviation (SD) of Ap53Ab in CRC patients and the
control group was 26.0±132.0 and 1.24±2.42 U/ml, respectively
(Table I). There difference in the
Ap53Ab level between CRC patients and the control group was
significant (P<0.0001; Table
I).
 | Table I.Ap53Ab level and positive rate in
colorectal cancer patients and healthy control group. |
Table I.
Ap53Ab level and positive rate in
colorectal cancer patients and healthy control group.
| Groups | n | Positive expression,
n (%) | Mean ± SD (U/ml) | P-value |
|---|
| Control | 115 | 12
(10.4) | 1.24±2.42 | <0.0001 |
| Colorectal
cancer | 674 | 195 (28.9) | 26.0±132.0 |
|
The positive rates of Ap53Ab, CEA, and CA19-9 in
each CRC stage were as follows: Stage 0: 5.3, 10.5 and 7.9%,
respectively; stage I: 20.0, 13.1 and 12.3%, respectively; stage
II: 31.6, 38.3 and 12.1%, respectively; stage III: 34.0, 43.8 and
12.4%, respectively; and stage IV: 34.0, 79.2 and 43.4%,
respectively (Table II). Positivity
for Ap53Ab alone was observed in 94 patients (13.9%; Table II). The positive rate of any
examined markers was 58.7% (Table
II).
 | Table II.Positive rate of tumor markers in 674
colorectal cancer patients. |
Table II.
Positive rate of tumor markers in 674
colorectal cancer patients.
|
| Stage, n (%) |
|---|
|
|
|
|---|
| Markers | 0 (n=38) | I (n=130) | II (n=206) | III (n=194) | IV (n=106) | Total (n=674) |
|---|
| Ap53Ab | 2 (5.3) | 26
(20.0) | 65
(31.6) | 66
(34.0) | 36
(34.0) | 195 (28.9) |
| CEA | 4
(10.5) | 17
(13.1) | 79
(38.3) | 85
(43.8) | 84
(79.2) | 269 (39.9) |
| CA19-9 | 3 (7.9) | 16
(12.3) | 25
(12.1) | 24
(12.4) | 46
(43.4) | 114 (16.9) |
| Ap53Ab alone | 1 (2.6) | 20
(15.4) | 33
(16.0) | 32
(16.5) | 8
(7.5) | 94
(13.9) |
| Any marker | 7
(18.4) | 49
(37.7) | 120 (58.3) | 123 (63.4) | 94 (88.7) | 393 (58.7) |
Half-life time of Ap53Ab and CEA
The mean ± SD half-life of Ap53Ab was 30.7±27.2 days
(range, 8.5–118.1 days) in 39 CRC patients, while that of CEA was
11.3±4.4 days (range, 4.4–21.8 days) in 19 CRC patients. Of the 39
patients with elevated Ap53Ab level, the level returned to normal
within 1 year in only 4 patients (10.3%).
Association between Ap53Ab expression
and clinicopathological characteristics and prognosis
Positive expression of Ap53Ab was significantly
associated with the depth of tumor invasion (P<0.001), lymph
node metastasis (P=0.024), stage (P<0.001) and CEA level
(P=0.005) (Table III). There was
no association of Ap53Ab expression with gender, age, tumor
location, histology, lymphatic invasion, venous invasion, liver
metastasis or recurrence (Table
III).
 | Table III.Clinicopathological correlation of
Ap53Ab expression in CRC. |
Table III.
Clinicopathological correlation of
Ap53Ab expression in CRC.
|
| Ap53Ab expression,
n (%) |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive
(n=195) | Negative
(n=479) | P-value |
|---|
| Gender |
|
| 0.33 |
|
Male | 121 (62.1) | 316 (66.0) |
|
|
Female | 74
(37.9) | 163 (34.0) |
|
| Age (years) |
|
| 0.42 |
|
<70 | 104 (53.3) | 239 (49.7) |
|
|
≥70 | 91
(46.7) | 240 (50.3) |
|
| Tumor location |
|
| 0.53 |
|
Right | 55 (28.2) | 145 (30.3) |
|
|
Left | 63 (32.3) | 134 (28.0) |
|
|
Rectum | 77 (39.5) | 200 (41.8) |
|
|
Differentiation |
|
| 0.16 |
|
High | 34
(17.4) | 119 (24.8) |
|
|
Moderate | 144 (73.9) | 319 (66.6) |
|
|
Poor | 11
(5.6) | 21
(4.4) |
|
|
Mucinous | 6
(3.1) | 11
(2.3) |
|
|
Others | 0
(0.0) | 8
(1.9) |
|
| Depth |
|
|
<0.001 |
|
Tis | 2
(1.0) | 36
(7.5) |
|
| T1 | 13
(6.7) | 64
(13.4) |
|
| T2 | 24
(12.3) | 59
(12.3) |
|
| T3 | 103 (52.8) | 207 (43.2) |
|
| T4 | 8
(4.1) | 103 (21.5) |
|
|
Unknown | 4
(2.1) | 010 (2.1) |
|
| Lymphatic
invasion |
|
| 0.1 |
|
Absent | 79
(40.5) | 227 (47.4) |
|
|
Present | 111 (56.9) | 240 (50.1) |
|
|
Unknown | 5
(2.6) | 12
(2.5) |
|
| Venous
invasion |
|
| 0.15 |
|
Absent | 59
(30.2) | 173 (36.1) |
|
|
Present | 131 (67.2) | 295 (61.6) |
|
|
Unknown | 5
(2.6) | 11
(2.3) |
|
| Lymph node
metastasis |
|
| 0.024 |
|
Negative | 99 (50.8) | 289 (59.8) |
|
|
Positive | 93 (47.7) | 184 (38.1) |
|
|
Unknown | 3
(1.5) | 10
(2.1) |
|
| Liver
metastasis |
|
| 0.28 |
|
Absent | 169 (86.7) | 429 (89.6) |
|
|
Present | 26
(13.3) | 50
(10.4) |
|
| UICC stage |
|
|
<0.001 |
| 0 | 2
(1.0) | 36
(7.5) |
|
| I | 26 (13.3) | 104 (21.8) |
|
| II | 65 (33.3) | 141 (29.4) |
|
|
III | 66 (33.9) | 128 (26.7) |
|
| IV | 36 (18.5) | 70
(14.6) |
|
| CEA |
|
| 0.005 |
|
≤6.7 | 101 (51.8) | 303 (63.3) |
|
|
>6.7 | 94 (48.2) | 175 (36.5) |
|
|
Unknown | 0
(0.0) | 1 (0.2) |
|
| Recurrence |
|
| 0.45 |
|
Absent | 139 (87.4) | 368 (90.0) |
|
|
Present | 18 (11.3) | 38 (9.3) |
|
|
Unknown | 2 (1.3) | 3 (0.7) |
|
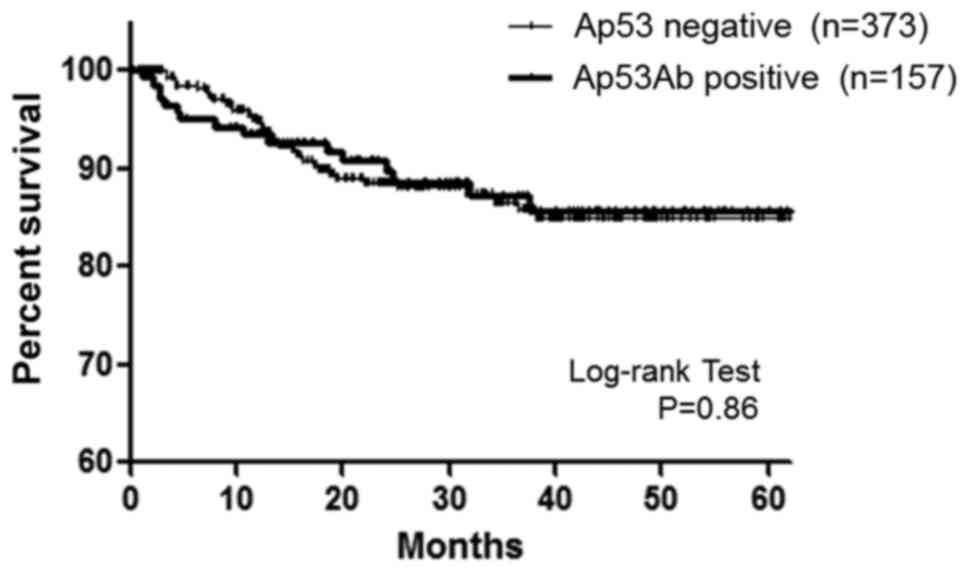
Regarding DFS in CRC patients with stage I–III
disease, no significant difference was observed between patients
with positive Ap53Ab expression (n=157) and those with negative
Ap53Ab expression (n=373) (P=0.86, Fig.
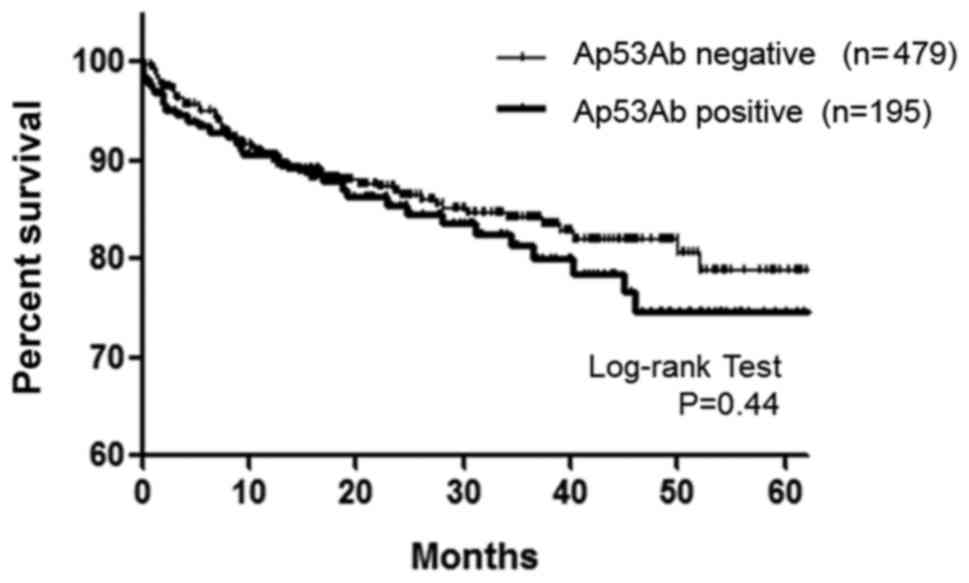
1). Moreover, no association between Ap53Ab expression and OS
was observed (P=0.44, Fig. 2).
Discussion
In the present study, positive Ap53Ab expression was
detected in 28.9% of 674 CRC patients. Previous studies (1,4–6,8,9) reported a positive Ap53Ab expression
rate of 24.0–33.1% in CRC patients. Our data were consistent with
those reports in terms of the positive Ap53Ab rate. Previous
reports (4,5) suggested that the measurement of Ap53Ab
was useful for the screening of CRC patients with early-stage
(stage 0 and I) disease, since the positive rate of Ap53Ab
expression was higher compared with that of CEA and CA19-9. There
was an advantage of Ap53Ab for the detection of CRC patients with
stage I disease, while the positive rate was low in CRC patients
with stage 0 disease. When stage 0 and I patients were collectively
analyzed, the positive rates of Ap53Ab, CEA and CA19-9 expression
were 16.7, 12.5 and 11.3%, respectively. The number of studies
investigating Ap53Ab expression in a large population of stage 0
and I CRC patients is limited. A recent report (4) demonstrated that positive Ap53Ab
expression was detected in 72 (15.1%) of 478 CRC patients with
stage 0 and I disease. Another study (5) reported that 9 of 38 CRC patients
(23.7%) with stage I disease exhibited positive Ap53Ab expression.
In our series, the positive rate of Ap53Ab expression in stage 0
and I and only stage I cases was 16.7 and 20.0%, respectively,
while that of CEA in stage 0 and I and only stage I cases was 12.5
and 13.1%, respectively. Therefore, the positive rate of Ap53Ab
expression was higher compared with that of CEA in CRC patients
with early-stage disease. Of note, one in three CRC patients with
stage 0 and I disease may be detected using the combination of
Ap53Ab, CEA and CA19-9. Of 168 patients with stage 0 and I disease,
any one of these markers was positive in 56 patients (33.3%). Since
measurement of CEA alone was able to detect 12.5% of CRC cases, the
detection rate with measurement of all markers is approximately
three times higher compared with that of CEA alone.
The positive rate of Ap53Ab expression in stage II,
III, and IV CRC is similar (31.6, 34.0 and 34.0%, respectively). A
similar result was also reported by Yamaguchi et al
(4). Thus, it was hypothesized that
repeated exposure to the p53 antigen may have reduced the
production of Ap53Ab in the serum via induction of immunological
tolerance (12). Positive expression
of Ap53Ab alone was observed in 94 patients (13.9%). This rate was
similar with previously reported rates of 13.6 and 15% (4,5).
Consequently, the combined measurement of Ap53Ab, CEA and CA19-9
improved the diagnosis of CRC up to ~60%.
Based on a previous study (1), the cut-off value was defined as <1.3
U/ml. The upper values of Ap53Ab were 4.39 and 16.9 U/ml in 205
healthy control donors and 189 patients with benign disease,
respectively (1). In the present
study, positive Ap53Ab expression was observed in 12 (10.4%) of 115
healthy volunteers using the same detection method. The range of
the Ap53Ab level in those volunteers was 1.5–19.5 U/ml. The 12
volunteers with positive Ap53Ab expression were followed up for ~5
years, and there was no cancer occurrence during that time.
Previous reports indicated that false-positives may occur as a
result of a severely dysplastic gastric mucosa (13), or lung tissues with squamous
metaplasia and dysplasia (14). When
the Ap53Ab value is as high as 20 U/ml, a thorough physical
examination should be performed, bearing in mind the possibility of
false-positive readings. Of note, when positive Ap53Ab expression
is detected in clinical practice, physical examination should be
performed to rule out the possibility of various cancers, including
head and neck, esophageal, uterine, breast, prostatic, biliary
tract, lung, bladder, gastric and pancreatic cancer (1).
In the present study, the Ap53Ab level decreased
slowly following curative resection. Ap53Ab is an IgG antibody and
the half-life of IgG is considered to be ~21 days (15). The mean half-life of CEA was reported
to be 4–12 days, depending on the status of recurrence (16,17). In
our results, the mean ± SD half-life of Ap53Ab was 30.7±27.2 days
in 39 CRC patients, while that of CEA was 11.3±4.4 days in 19 CRC
patients. A previous study (18)
reported that the Ap53Ab level decreased to normal following
curative resection within at least 6 months in almost all
Ap53Ab-positive CRC patients. Recently, Kawahara et al
(19) reported data supporting our
results, as the positive Ap53Ab rate was 75% at 6 months, 70.8% at
12 months, and 54.2% at 24 months after curative operation in 24
CRC patients with no recurrence. In our results, the Ap53Ab level
had returned to normal within 12 months in only 4 (10.3%) of the 39
CRC patients with elevated Ap53Ab level, whereas in some cases
without recurrence it took ~5 years to return to the normal range.
Therefore, the measurement of Ap53Ab should be limited to prior to
surgery. When the elevated Ap53Ab level decreases slowly, even if
it requires a long time, it does not necessarily indicate that the
Ap53Ab level reflects the presence of residual tumor and/or
recurrence.
It has been reported that positive expression of
Ap53Ab was significantly associated with lymph node metastasis and
lymphatic invasion (4,5,20).
Furthermore, Yamaguchi et al (4) reported that other factors, including
tumor location, histology, depth of tumor invasion, vessel
invasion, distant metastasis, CEA and recurrence, were also
significantly associated with positive Ap53Ab expression. Other
studies (9,10) reported that no significant
correlation was observed between positive Ap53Ab expression and
clinicopathological factors. In the present study, several factors,
including depth of tumor invasion, lymph node metastasis, stage and
CEA, were found to be significantly associated with positive Ap53Ab
expression. The association between elevated Ap53Ab and lymph node
metastasis was consistent with previous data from an Asian
population (4,5,7). In
gastric cancer, an elevated Ap53Ab level tended to be associated
with lymph node metastasis (21),
but positive Ap53Ab expression was not found to be correlated with
lymph node metastasis in esophageal squamous cell carcinoma
(22). Therefore, the elevated
Ap53Ab level may be consistently associated with deeper depth of
invasion and lymph node metastasis in CRC, that is to say that
deeper depth of invasion and lymph node metastasis may be involved
in the production of Ap53Ab.
In the present study, no correlation was observed
between Ap53Ab expression and poor survival rate, including DFS and
OS. Although previous reports (8,23)
demonstrated that positive Ap53Ab expression was associated with
poor prognosis in CRC patients, there appears to be no consensus
(4,6,10).
Recently, Yamaguchi et al (4)
reported that no association between Ap53Ab expression and OS was
found in 1384 CRC patients, although Ap53Ab expression was
associated with relapse-free survival in 1212 CRC patients who
underwent curative surgery. Of those 1212 CRC patients, 339 (28%)
received adjuvant chemotherapy. In our series, >70% of CRC
patients with stage III disease were treated with adjuvant
chemotherapy. Of those patients, 77% underwent oxaliplatin-based
adjuvant chemotherapy, including mFOLFOX6 and XELOX. It may be
hypothesized that oxaliplatin-based chemotherapy may improve the
prognosis of CRC patients regardless of Ap53Ab expression. Several
researchers have analyzed the prognosis of cancer patients with
TP53 expression using immunohistochemical examination and genetic
testing (6,24–26).
Since the association between TP53 expression and prognosis using
has not yet reached a firm conclusion, even with these methods, it
is difficult to predict prognosis using Ap53Ab expression. Chang
et al (6) investigated
genetic alteration of TP53, overexpression of intratumoral
p53 protein and Ap53Ab expression in CRC, and found that the
positive rates of each examination were 56.3, 44.9 and 28.1%,
respectively; they concluded that only genetic alterations of
TP53 were significantly associated with poor prognosis,
while intratumoral TP53 and Ap53Ab expression were not. Moreover,
they mentioned that TP53 mutations at exons 6 and 7 were
associated with the presence of Ap53Ab. The frequency of CRC
patients with positive Ap53Ab expression was estimated at ~50%
among CRC patients with genetic alterations of TP53. Further
studies are required to validate the association between Ap53Ab
expression and survival.
Taken together, our results indicate that the
measurement of Ap53Ab may contribute to the detection of
early-stage CRC in clinical practice. The use of Ap53Ab with CEA
and CA19-9 may increase the diagnostic yield for CRC up to ~60%.
Furthermore, the time to normalization of the Ap53Ab level was
longer than expected in CRC patients with elevated Ap53Ab level
preoperatively. Ap53Ab expression was associated with lymph node
metastasis, although the association between Ap53Ab expression and
poor prognosis could not be fully elucidated.
References
|
1
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group: Titration of serum p53 antibodies in
1,085 patients with various types of malignant tumors: A
multiinstitutional analysis by the Japan p53 Antibody Research
Group. Cancer. 97:682–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto S, Chishima T, Adachi S, Harada
F, Toda Y, Arioka H, Hasegawa N and Kakuta Y: Serum p53 antibody in
breast cancer. Cancer Biomark. 14:203–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi T, Takii Y and Maruyama S:
Usefulness of serum p53 antibody measurement in colorectal cancer:
An examination of 1384 primary colorectal cancer patients. Surg
Today. 44:1529–1535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ochiai H, Ohishi T, Osumi K, Tokuyama J,
Urakami H, Seki S, Shimada A, Matsui A, Isobe Y, Murata Y, et al:
Reevaluation of serum p53 antibody as a tumor marker in colorectal
cancer patients. Surg Today. 42:164–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang SC, Lin JK, Lin TC and Liang WY:
Genetic alteration of p53, but not overexpression of intratumoral
p53 protein, or serum p53 antibody is a prognostic factor in
sporadic colorectal adenocarcinoma. Int J Oncol. 26:65–75.
2005.PubMed/NCBI
|
|
7
|
Tang R, Ko MC, Wang JY, Changchien CR,
Chen HH, Chen JS, Hsu KC, Chiang JM and Hsieh LL: Humoral response
to p53 in human colorectal tumors: A prospective study of 1,209
patients. Int J Cancer. 94:859–863. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiota G, Ishida M, Noguchi N, Oyama K,
Takano Y, Okubo M, Katayama S, Tomie Y, Harada K, Hori K, et al:
Circulating p53 antibody in patients with colorectal cancer:
Relation to clinicopathologic features and survival. Dig Dis Sci.
45:122–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noaki R, Kawahara H, Watanabe K, Ushigome
T, Kobayashi S and Yanaga K: Serum p53 antibody is a useful tumor
marker of early colorectal cancer. Int Surg. 95:287–292.
2010.PubMed/NCBI
|
|
10
|
Suppiah A, Alabi A, Madden L, Hartley JE,
Monson JR and Greenman J: Anti-p53 autoantibody in colorectal
cancer: Prognostic significance in long-term follow-up. Int J
Colorectal Dis. 23:595–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. (7th edition).
2010.
|
|
12
|
Takeda A, Shimada H, Nakajima K, Yoshimura
S, Suzuki T, Asano T, Ochiai T and Isono K: Serum p53 antibody as a
useful marker for monitoring of treatment of superficial colorectal
adenocarcinoma after endoscopic resection. Int J Clin Oncol.
6:45–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joypaul BV, Newman EL, Hopwood D, Grant A,
Qureshi S, Lane DP and Cuschieri A: Expression of p53 protein in
normal, dysplastic and malignant gastric mucosa: An
immunohistochemical study. J Pathol. 170:279–283. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bennett WP, Colby TV, Travis WD, Borkowski
A, Jones RT, Lane DP, Metcalf RA, Samet JM, Takeshima Y, Gu JR, et
al: p53 protein accumulates frequently in early bronchial
neoplasia. Cancer Res. 53:4817–4822. 1993.PubMed/NCBI
|
|
15
|
Morell A, Terry WD and Waldmann TA:
Metabolic properties of IgG subclasses in man. J Clin Invest.
49:673–680. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito K, Hibi K, Ando H, Hidemura K,
Yamazaki T, Akiyama S and Nakao A: Usefulness of analytical CEA
doubling time and half-life time for overlooked synchronous
metastases in colorectal carcinoma. Jpn J Clin Oncol. 32:54–58.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JS and Min JS: Significance of
postoperative serum level of carcinoembryonic antigen (CEA) and
actual half life of CEA in colorectal cancer patients. Yonsei Med
J. 38:1–7. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeda A, Shimada H, Nakajima K, Imaseki
H, Suzuki T, Asano T, Ochiai T and Isono K: Monitoring of p53
autoantibodies after resection of colorectal cancer: Relationship
to operative curability. Eur J Surg. 167:50–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawahara H, Watanabe K, Enomoto H, Toyama
Y, Akiba T and Yanaga K: Normalization of serum p53 antibody levels
in patients after curative resection for colorectal cancer.
Anticancer Res. 33:2221–2225. 2013.PubMed/NCBI
|
|
20
|
Nozoe T, Yasuda M, Honda M, Inutsuka S and
Korenaga D: Clinicopathologic significance in serum presence of
anti-p53 antibody in patients with colorectal carcinoma.
Hepatogastroenterology. 54:1422–1425. 2007.PubMed/NCBI
|
|
21
|
Nakajima K, Suzuki T, Shimada H, Hayashi
H, Takeda A and Ochiai T: Detection of preoperative serum anti-p53
antibodies in gastric cancer. Tumour Biol. 20:147–152. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimada H, Nakajima K, Ochiai T, Koide Y,
Okazumi SI, Matsubara H, Takeda A, Miyazawa Y, Arima M and Isono K:
Detection of serum p53 antibodies in patients with esophageal
squamous cell carcinoma: Correlation with clinicopathologic
features and tumor markers. Oncol Rep. 5:871–874. 1998.PubMed/NCBI
|
|
23
|
Kressner U, Glimelius B, Bergström R,
Påhlman L, Larsson A and Lindmark G: Increased serum p53 antibody
levels indicate poor prognosis in patients with colorectal cancer.
Br J Cancer. 77:1848–1851. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morikawa T, Kuchiba A, Liao X, Imamura Y,
Yamauchi M, Qian ZR, Nishihara R, Sato K, Meyerhardt JA, Fuchs CS
and Ogino S: Tumor TP53 expression status, body mass index and
prognosis in colorectal cancer. Int J Cancer. 131:1169–1178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Russo A, Bazan V, Iacopetta B, Kerr D,
Soussi T and Gebbia N: TP53-CRC Collaborative Study Group: The TP53
colorectal cancer international collaborative study on the
prognostic and predictive significance of p53 mutation: Influence
of tumor site, type of mutation and adjuvant treatment. J Clin
Oncol. 23:7518–7528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Munro AJ, Lain S and Lane DP: P53
abnormalities and outcomes in colorectal cancer: A systematic
review. Br J Cancer. 92:434–444. 2005.PubMed/NCBI
|