Introduction
Radiation therapy is standard treatment for
localized prostate cancer, but late adverse events, such as rectal
bleeding, are a major concern, with a reported risk of 5–20% for
genitourinary (GU) and gastrointestinal (GI) adverse events of
grade ≥2 (1,2). The irradiated dose and volume to an
organ at risk (OAR) are correlated with the frequency of late
adverse events (1,3), but higher local doses also achieve
better local control (4,5). Thus, a high radiation dose to the
target and reduction of OAR doses are critical factors in radiation
therapy. The emergence of image-guided radiotherapy (IGRT),
3-dimentional conformal radiotherapy (3D-CRT), and its successor,
intensity-modulated radiotherapy (IMRT), has significantly lowered
toxicity to the bladder and rectum, although 5–10% of the patients
develop grade 2 or more severe toxicity (5–7).
Charged particle beams, such as those used in proton
beam therapy (PBT), deliver high radiation doses to the target in a
conformal manner, which minimizes the doses to OARs. These
advantages are based on the fundamental physical dose distribution
of charged particle beams (8).
However, the number of clinical trials on PBT for prostate cancer
is limited. A dose escalation study using PBT as a boost yielded
favorable results (9), but with
limited follow-up of patients who received PBT alone. We herein
report a retrospective review of the efficacy and safety of PBT for
prostate cancer.
Patients and methods
Patients
A total of 111 consecutive patients underwent
definitive PBT for prostate cancer at the Department of Radiation
Oncology and Proton Medical Research Center (Tsukuba, Japan)
between 2008 and 2012. A total of 11 patients were excluded due to
incomplete treatment, 6 were lost to follow-up, and 1 had a
different histological type of tumor, namely basal cell carcinoma.
Therefore, a total of 93 patients were analyzed in the present
study. The patient characteristics are summarized in Table I. Staging evaluation was performed by
digital rectal examination, MRI, CT and bone scintigraphy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. (%) |
|---|
| Age, years |
| Median
(range) | 68
(49–81) |
| T stage |
| T1c | 24 (26) |
|
T2a-T2b | 30 (32) |
| T2c | 11 (12) |
| T3a | 20 (22) |
| T3b | 7 (8) |
| T4 | 1 (1) |
| Initial PSA value
(ng/ml) |
|
<10.0 | 47 (51) |
|
10.0–19.9 | 21 (23) |
|
≥20.0 | 25 (27) |
| Gleason score
sum |
| ≤6 | 14 (15) |
| 7 | 33 (35) |
| ≥8 | 46 (49) |
| Tumor risk
group |
|
High | 54 (49) |
|
Intermediate | 32 (35) |
|
Low | 7 (8) |
| Antithrombotic
drugs |
|
Yes | 10 (11) |
| No | 83 (89) |
Risk stratification
In risk classification of prostate cancer (Table II), patients with all low-risk
factors were classified as low-risk; those with any high-risk
factor as high-risk; and those with any other combination as
intermediate-risk. Complete androgen blockade (CAB) was performed
from 6 months prior to PBT for intermediate- or high-risk cases,
and patients at high risk continued CAB for 3 years. No combination
therapy was used for low-risk cases based on our criteria.
 | Table II.Risk classification for treatment in
our institution. |
Table II.
Risk classification for treatment in
our institution.
| Factors | Low-risk | High-risk |
|---|
| T stage | T1c-2b | T3a-b, T4 |
| iPSA (ng/ml) | <10.0 | ≥20.0 |
| Gleason score
sum | ≤6 | ≥8 |
Radiotherapy systems
The PBT system consisted of a 250 MeV synchrotron
equipped with an isocentric rotational gantry, a 15×15-cm passive
scattering port with a 5-mm multileaf collimator, a rotational
treatment couch, and a treatment-planning system (Hitachi 3D
Treatment Planning System ver. 2.0; Hitachi Ltd., Tokyo, Japan)
with a CT scanner and an X-ray simulator without any modifications.
Dose volume histogram (DVH) parameters were calculated using the
same treatment-planning system.
Principles of treatment planning
Target volume and risk organs were defined as
follows: The clinical target volume (CTV) was set as the prostate
plus 1/3 caudal seminal vesicle (whole seminal vesicle for cT3b).
The planning target volume (PTV) was defined as the CTV plus a
10-mm lateral, 12-mm anterior, and 5-mm craniocaudal and posterior
margins. The rectum was contoured from the sigmoid flexure to the
anus or ischial tuberosity, whichever was closer to the PTV.
Low-risk cases received 74 GyE (relative biological
effectiveness=1.1) in 37 fractions, and intermediate- or high-risk
cases received 78 GyE in 39 fractions. To reduce the dose to the
rectum, the posterior edge of the PTV was set in front of the
anterior wall of the rectum by using multileaf collimators after 30
fractions. Two lateral ports (one from either side) were used for
treatment. Dose constraints to the rectum were set to the
following: V50 <30%, V80 <20% and V90 <10%, although
exceptions were permitted when the risk/benefit ratio was
considered clinically acceptable. Dose constraints to the bladder
were not set, since this was not an issue due to the beam angle
set-up.
Patient preparation and fixation
Prior to treatment planning, the patients had
fiducial markers installed in their prostate by a transrectal
method for positioning verification. Image-guided patient position
verification was performed using orthogonal X-ray images for every
fraction. In order to control bladder volume, the patients were
asked to completely void their bladders 30 min prior to treatment
and to drink one cup (~100 ml) of tea. Bladder volume was confirmed
to be ~100 ml, immediately prior to treatment using ultrasonic
bladder volume measuring instruments. Fixation was performed using
foot and leg rests without thermal plastic shells.
Follow-up
Regular follow-up included physical examinations and
prostate-specific antigen (PSA) blood tests at 3- to 4-month
intervals for the first 2 years and at 3- to 6-month intervals
thereafter. Treatment-related morbidities were evaluated by
physical examination and imaging. Events were assessed using the
National Cancer Institute Common Terminology Criteria for Adverse
Effects, version 4 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
Statistical analysis
Statistical analysis was performed using R software
(http://www.r-project.org/). Gray
analysis with death as a competing risk consideration was used for
biochemical relapse-free rate and cumulative toxicity event rate
calculations. Fine-Gray analysis was used for uni- and multivariate
analysis. A P-value of <0.05 was considered to indicate
statistically significant differences.
Results
Follow-up and outcome
The median follow-up time was 55 months (range,
32–97 months). All patients apart from one were PSA failure-free
and the 5-year cumulative biochemical relapse-free rate was 99.0%
(95% CI: 93.2–99.9%). Only one death was reported, which was due to
pancreatic cancer. As regards late GU morbidity, grade 3
non-infectious cystitis occurred in 1 patient (1.5%) and grade 2
urinary frequency and hematuria were observed in 4 (4.3%) and 1
(1.5%) patients, respectively. As regards late GI morbidity, grade
2 rectal bleeding was observed in 4 patients (4.3%). No other grade
≥2 adverse events were observed. The 5-year cumulative incidence of
grade ≥2 GU and GI morbidities was 5.8 and 4.3%, respectively
(Fig. 2). The median maximum dose to
the PTV was 101.5% [±0.21% two standard deviations (2SD)] of the
prescribed dose. The median rectal V30 to V80 in 10% increments
were 32.5% (±16.2% 2SD), 28.0% (±17.9%), 23.8% (±13.4%), 20.0%
(±11.9%), 16.5% (±10.4%) and 12.5% (±8.6%), respectively. The
rectal doses in patients with and without grade 2 GI toxicity are
shown in Table III. All doses were
higher in patients with grade 2 GI toxicity, but the difference was
not statistically significant. On multivariate analysis, the use of
anticoagulants was a significant positive risk factor [hazard ratio
(HR)=5.72, 95% confidence interval (CI): 1.31–24.92] and the PTV
volume was a significant negative risk factor (HR=0.96, 95% CI:
0.937–0.983) for grade 2 rectal bleeding. Age, Gleason score,
initial PSA, prescription dose, and T3b disease were not found to
be significant.
 | Table III.Dose volume histogram comparison with
and without late toxicity. |
Table III.
Dose volume histogram comparison with
and without late toxicity.
|
| Grade <2 | Grade 2 |
|
|---|
|
|
|
|
|
|---|
| Dosimetry | % | 2SD | % | 2SD | P-value |
|---|
| V30 | 33.6 | ±16.3 | 37.3 | ±13.6 | 0.37 |
| V40 | 28.9 | ±15.0 | 32.2 | ±12.0 | 0.387 |
| V50 | 24.8 | ±13.6 | 27.9 | ±10.0 | 0.365 |
| V60 | 20.8 | ±12.1 | 23.4 | ±8.0 | 0.394 |
| V70 | 16.9 | ±10.5 | 19.3 | ±6.1 | 0.365 |
| V80 | 12.5 | ±7.7 | 14.0 | ±4.6 | 0.497 |
Discussion
Late toxicity following radiotherapy for prostate
cancer has been a major concern from the start of use of this
therapy (10). The emergence of
3D-CRT demonstrated that a high radiation dose to the target leads
to better biochemical disease-free survival (4,5,11), but is also associated with higher
toxicity (4,5). Several studies have investigated risk
factors associated with late toxicities following radiotherapy for
prostate cancer, and the irradiation dose and volume to the rectum
and use of anticoagulants are considered to be key factors
(1,3,12,13).
The use of IGRT is also known to improve the actual
dose to the rectum and, therefore, improve clinical results
(14). A significant decrease in
rectal toxicity was observed with the use of IGRT for high-dose
irradiation (15).
Charged particle beams, such as those used in PBT,
deliver high radiation doses to the target in a conformal manner,
which minimizes the doses to OARs. This is realized by the unique
fundamental physical characteristics known as Bragg peak, and by
modulating it with the use of spread-out Bragg peak and collimators
(8,16). Alongside adequate re-planning, as
described earlier, our rectal doses where lower compared with all
parameters in other 3D-CRT studies (1,17). Also,
compared with IMRT studies, the high doses were comparable, and the
intermediate doses were lower (18).
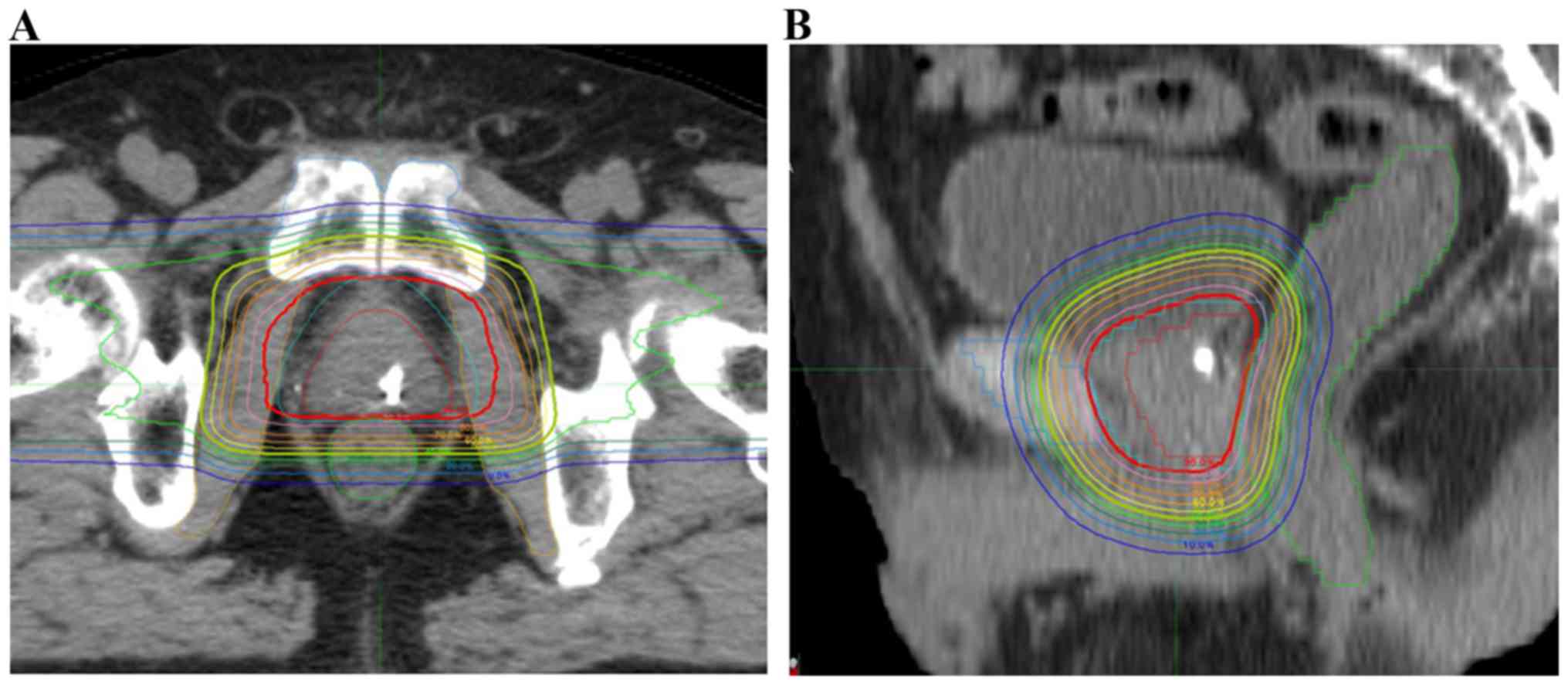
Βladder doses have not been evaluated in detail; however, since we
only utilized lateral irradiation ports (2 ports, Fig. 1), a low to intermediate dose to the
bladder was sufficiently low.
As regards clinical results, with the use of
fiducial markers and daily IGRT, grade 2 rectal bleeding developed
in 4 patients (4.3%), grade ≥2 hematuria was observed in 2 patients
(2.1%) and grade 2 urinary frequency occurred in 4 patients (4.3%).
No other grade ≥2 late adverse events were observed. Rectal
toxicity was comparable with that of IMRT and lower compared with
that of 3D-CRT, and genitourinary toxicity was better compared with
that of both 3D-CRT and IMRT (Table
IV) (7,19–30).
 | Table IV.List of previous reports on the
results of treatment for prostate cancer. |
Table IV.
List of previous reports on the
results of treatment for prostate cancer.
|
|
|
|
| 5-year BFS (%) |
| Grade ≥2
toxicitya |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author | Method | Dose (Gy) | Gy/Fr | Low | Intermediate | High | GI | GU | (Refs.) |
|---|
| D'Amico | 3D-CRT | 66–70 | 2 | 80 | 65–75 | 40 | N/A | N/A | (19) |
| Dearnaley | 3D-CRT | 74 | 2 | 85 | 79 | 57 | 43 | 15 | (20) |
| Vora | 3D-CRT | 66–71 | 1.8–2 | 76 | 50 | 35 | 16 | 22 | (21) |
| Zepatero | 3D-CRT | 76–82 | 2 | N/A | 88 | 88 | 10.1 | 9.9 | (7) |
| Zelefsky | IMRT | 81 | 1.8 | 85 | 76 | 72 | 1.8 | 12.2 | (22) |
| Kupelian | IMRT | 70 | 2.5 | 94 | 83 | 72 | 1.8 | 12.2 | (23) |
| Vora | IMRT | 70.2–77.4 | 1.8 | 88 | 70 | 60 | 24 | 29 | (21) |
| Cahlon | IMRT | 70 | 2.5 | 94 | 83 | 72 | 6 | 7 | (24) |
| Martin | IMRT | 79.8 | 1.77 | 88 | 77 | 78 | 13.7 | 12.1 | (25) |
| Guckeznberger | IMRT | 73.9–76.2 | 2.3 | 88 | 80 | 78 | 4.8 | 22.4 | (26) |
| Schulte | PBT | 74–75 | 1.8–2 |
| 82 |
| 3.5 | 5.4 | (27) |
| Mendenhall | PBT | 78–82 | 2 | 99 | 99 | 76 | 1.0 | 0.9 | (28) |
| Takagi | PBT | 74 | 2 | 99 | 91 | 86 | 3.8 | 1.9 | (29) |
| Bryant | PBT | 72–82 | 2 | 94 | 88 | 88 | N/A | N/A | (30) |
| Present study | PBT | 74–78 | 2 |
| 99 |
| 5.4 | 1.0 |
|
Unfortunately, due to the low toxicity incidence
rate, the availability of statistical analysis data on risk factors
of GI toxicity is limited. Patients with grade 2 GI toxicity had
higher V30 to V80 rectal doses, but the difference was not
significant. On multivariate analysis, the use of anticoagulants
was the only significant positive risk factor, and PTV volume was a
negative risk factor. Age, Gleason score, initial PSA, prescription
dose and T3b disease were not significant factors, and these
findings did not change after excluding PTV volume in the analysis.
DVH analysis revealed higher doses, although without a significant
difference, in patients with grade 2 GI toxicity, which is
consistent with previous studies reporting rectal dose as a risk
factor (1,3). The DVH parameters likely failed to be
significant due to the low event rate, with only 4 patients
suffering grade 2 rectal bleeding, including 2 who received
anticoagulant therapy. A larger PTV volume was not found to be a
risk factor for GI toxicity, in contrast to findings supporting
that a large PTV volume is a positive risk factor in other
modalities (31). In addition to the
low grade 2 incidence rate, this may be explained by our
irradiation method. The PTV volume is enlarged by prostate
hypertrophy and T3b disease, thus affecting the rectal dose. PBT is
known to deliver a lower dose to the rectum and bladder,
particularly in high-risk cases where seminal vesicle irradiation
is required (32,33), which supports the results of the
present study.
GU toxicity was relatively low compared with that in
previous reports. We consider this to be due to the conformal dose
distribution in passive PBT. Heterogeneity of the radiation dose
within the target is reported to predispose patients to urethral
strictures (34). The maximum dose
in the present study was 101.5% (±0.21% 2SD) of the prescribed
dose, which is a difficult value to achieve using IMRT or scanning
PBT (35). This conformal dose may
be an explanation for the low GU toxicity. Also, as discussed
above, only lateral beams were used in PBT for prostate cancer,
thus lowering the low to intermediate doses to the bladder.
Various approaches have been assessed to decrease
late toxicity in prostate cancer treatment. Hypofractionation is
considered to decrease the biological effective dose to the rectum
due to the nature of prostate cancer, although extreme
hypofractionation may result in compromising tumor control due to
the heterogeneity of cancer (36).
The use of carbon-ion radiotherapy is a more straightforward method
for improving dose distribution with promising results, although it
requires specialized equipment and a vast amount of space (3,37).
The biological effectiveness of PBT is considered to
be slightly higher compared with that of high-voltage
X-ray/cobalt-60, raising the relative biological effectiveness to
1.1 (38). This 10% change in
biological effectiveness may contribute to better tumor control,
but it is unclear without a randomized control study to evaluate
such detailed difference. In the present study, all patients but
one were PSA failure-free and the 5-year cumulative biochemical
relapse-free rate was 99.0%. Considering that the follow-up period
was relatively short, with a median follow-up of just under 5
years, it is difficult to suggest better tumor control compared
with other treatments, but the results appear to be promising. In
addition, all the patients were observed for >48 months, which
is sufficient for toxicity analysis, and the observed toxicities
were minimal. We consider these results as promising, and PBT may
be considered as a treatment option for prostate cancer. To
elucidate the advantage of PBT over X-ray therapy, multiple
prospective multi-center single-arm trials are currently
underway.
References
|
1
|
Fiorino C, Sanguineti G, Cozzarini C,
Fellin G, Foppiano F, Menegotti L, Piazzolla A, Vavassori V and
Valdagni R: Rectal dose-volume constraints in high-dose
radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol
Phy. 57:953–962. 2003. View Article : Google Scholar
|
|
2
|
Sheets NC, Goldin GH, Meyer AM, Wu Y,
Chang Y, Stürmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR
and Chen RC: Intensity-modulated radiation therapy, proton therapy,
or conformal radiation therapy and morbidity and disease control in
localized prostate cancer. JAMA. 307:1611–1620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishikawa H, Tsuji H, Kamada T, Hirasawa N,
Yanagi T, Mizoe JE, Akakura K, Suzuki H, Shimazaki J and Tsujii H:
Risk factors of late rectal bleeding after carbon ion therapy for
prostate cancer. Int J Radiat Oncol Biol Phys. 66:1084–1091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michalski J, Winter K, Roach M, Markoe A,
Sandler HM, Ryu J, Parliament M, Purdy JA, Valicenti RK and Cox JD:
Clinical outcome of patients treated with 3D conformal radiation
therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat
Oncol Biol Phys. 83:e363–e370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michalski JM, Bae K, Roach M, Markoe AM,
Sandler HM, Ryu J, Parliament MB, Straube W, Valicenti RK and Cox
JD: Long-term toxicity following 3D conformal radiation therapy for
prostate cancer from the RTOG 9406 phase I/II dose escalation
study. Int J Radiat Oncol Biol Phys. 76:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollack A, Zagars GK, Starkschall G,
Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA and Rosen
I: Prostate cancer radiation dose response: Results of the M. D.
Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys.
53:1097–1105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zapatero A, Guerrero A, Maldonado X,
Alvarez A, Segundo Gonzalez San C, Rodríguez Cabeza MA, Macias V,
Olive Pedro A, Casas F, Boladeras A, et al: High-dose radiotherapy
with short-term or long-term androgen deprivation in localised
prostate cancer (DART01/05 GICOR): A randomised, controlled, phase
3 trial. Lancet Oncol. 16:320–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedroni E, Bacher R, Blattmann H,
Böhringer T, Coray A, Lomax A, Lin S, Munkel G, Scheib S, Schneider
U, et al: The 200-MeV proton therapy project at the Paul Scherrer
Institute: Conceptual design and practical realization. Med Phys.
22:37–53. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zietman AL, DeSilvio ML, Slater JD, Rossi
CJ Jr, Miller DW, Adams JA and Shipley WU: Comparison of
conventional-dose vs high-dose, conformal radiation therapy in
clinically localized adenocarcinoma of the prostate: A randomized
controlled trial. JAMA. 294:1233–1239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz EM and Hanks GE: External beam
radiation therapy for prostate cancer. CA Cancer J Clin.
50:349–375; quiz 376–379. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher
GJ, Fleshner NE, Venkatramen ES, Reuter VE, Fair WR, Ling CC and
Fuks Z: Dose escalation with three-dimensional conformal radiation
therapy affects the outcome in prostate cancer. Int J Radiat Oncol
Biol Phys. 41:491–500. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akimoto T, Katoh H, Kitamoto Y, Tamaki T,
Harada K, Shirai K and Nakano T: Rectal bleeding after
high-dose-rate brachytherapy combined with hypofractionated
external-beam radiotherapy for localized prostate cancer: Impact of
rectal dose in high-dose-rate brachytherapy on occurrence of grade
2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys.
65:364–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fenwick JD, Khoo VS, Nahum AE,
Sanchez-Nieto B and Dearnaley DP: Correlations between dose-surface
histograms and the incidence of long-term rectal bleeding following
conformal or conventional radiotherapy treatment of prostate
cancer. Int J Radiat Oncol Biol Phys. 49:473–480. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaly B, Bauman GS, Song W, Battista JJ
and Van Dyk J: Dosimetric impact of image-guided 3D conformal
radiation therapy of prostate cancer. Phys Med Biol. 50:3083–3101.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zelefsky MJ, Kollmeier M, Cox B, Fidaleo
A, Sperling D, Pei X, Carver B, Coleman J, Lovelock M and Hunt M:
Improved clinical outcomes with high-dose image guided radiotherapy
compared with non-IGRT for the treatment of clinically localized
prostate cancer. Int J Radiat Oncol Biol Phys. 84:125–129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inada T, Hayakawa Y, Tada J, Takada Y and
Maruhashi A: Characteristics of proton beams after field shaping at
PMRC. Med Biol Eng Comput. 31:Suppl. S44–S48. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackson A, Skwarchuk MW, Zelefsky MJ,
Cowen DM, Venkatraman ES, Levegrun S, Burman CM, Kutcher GJ, Fuks
Z, Liebel SA and Ling CC: Late rectal bleeding after conformal
radiotherapy of prostate cancer. II. Volume effects and dose-volume
histograms. Int J Radiat Oncol Biol Phys. 49:685–698. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pederson AW, Fricano J, Correa D,
Pelizzari CA and Liauw SL: Late toxicity after intensity-modulated
radiation therapy for localized prostate cancer: An exploration of
dose-volume histogram parameters to limit genitourinary and
gastrointestinal toxicity. Int J Radiat Oncol Biol Phys.
82:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Amico AV, Whittington R, Malkowicz SB,
Cote K, Loffredo M, Schultz D, Chen MH, Tomaszewski JE, Renshaw AA,
Wein A and Richie JP: Biochemical outcome after radical
prostatectomy or external beam radiation therapy for patients with
clinically localized prostate carcinoma in the prostate specific
antigen era. Cancer. 95:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dearnaley DP, Sydes MR, Graham JD, Aird
EG, Bottomley D, Cowan RA, Huddart RA, Jose CC, Matthews JH, Millar
J, et al: Escalated-dose versus standard-dose conformal
radiotherapy in prostate cancer: First results from the MRC RT01
randomised controlled trial. Lancet Oncol. 8:475–487. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vora SA, Wong WW, Schild SE, Ezzell GA and
Halyard MY: Analysis of biochemical control and prognostic factors
in patients treated with either low-dose three-dimensional
conformal radiation therapy or high-dose intensity-modulated
radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol
Phys. 68:1053–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zelefsky MJ, Chan H, Hunt M, Yamada Y,
Shippy AM and Amols H: Long-term outcome of high dose intensity
modulated radiation therapy for patients with clinically localized
prostate cancer. J Urol. 176:1415–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kupelian PA, Willoughby TR, Reddy CA,
Klein EA and Mahadevan A: Hypofractionated intensity-modulated
radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate
cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys.
68:1424–1430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cahlon O, Zelefsky MJ, Shippy A, Chan H,
Fuks Z, Yamada Y, Hunt M, Greenstein S and Amols H: Ultra-high dose
(86.4 Gy) IMRT for localized prostate cancer: Toxicity and
biochemical outcomes. Int J Radiat Oncol Biol Phys. 71:330–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin JM, Bayley A, Bristow R, Chung P,
Gospodarowicz M, Menard C, Milosevic M, Rosewall T, Warde PR and
Catton CN: Image guided dose escalated prostate radiotherapy: Still
room to improve. Radiat Oncol. 4:502009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guckenberger M, Lawrenz I and Flentje M:
Moderately hypofractionated radiotherapy for localized prostate
cancer: Long-term outcome using IMRT and volumetric IGRT.
Strahlenther Onkol. 190:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulte RW, Slater JD, Rossi CJ Jr and
Slater JM: Value and perspectives of proton radiation therapy for
limited stage prostate cancer. Strahlenther Onkol. 176:3–8. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendenhall NP, Hoppe BS, Nichols RC,
Mendenhall WM, Morris CG, Li Z, Su Z, Williams CR, Costa J and
Henderson RH: Five-year outcomes from 3 prospective trials of
image-guided proton therapy for prostate cancer. Int J Radiat Oncol
Biol Phys. 88:596–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagi M, Mima M, Terashima K, Fujii O,
Demizu Y, Nagano F, Jin D, Okimoto T, Waki T, Murakami M and Fuwa
N: Long-term outcomes in patients treated with proton therapy for
localized prostate cancer. Int J Radiat Oncol Biol Phys.
93:E186–E187. 2015. View Article : Google Scholar
|
|
30
|
Bryant C, Smith TL, Henderson RH, Hoppe
BS, Mendenhall WM, Nichols RC, Morris CG, Williams CR, Su Z, Li Z,
et al: Five-year biochemical results, toxicity and patient-reported
quality of life after delivery of dose-escalated image guided
proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys.
95:422–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vargas C, Yan D, Kestin LL, Krauss D,
Lockman DM, Brabbins DS and Martinez AA: Phase II dose escalation
study of image-guided adaptive radiotherapy for prostate cancer:
Use of dose-volume constraints to achieve rectal isotoxicity. Int J
Radiat Oncol Biol Phys. 63:141–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rana S, Cheng C, Zheng Y, Risalvato D,
Cersonsky N, Ramirej E, Zhao L, Larson G and Vargas C: Proton
therapy vs. VMAT for prostate cancer: A treatment planning study.
International Journal of Particle Therapy. 1:22–33. 2014.
View Article : Google Scholar
|
|
33
|
Rana S, Cheng C, Zhao L, Park S, Larson G,
Vargas C, Dunn M and Zheng Y: Dosimetric and radiobiological impact
of intensity modulated proton therapy and RapidArc planning for
high-risk prostate cancer with seminal vesicles. J Med Radiat Sci.
64:18–24. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
McDonald AM, Baker CB, Popple RA, Cardan
RA and Fiveash JB: Increased radiation dose heterogeneity within
the prostate predisposes to urethral strictures in patients
receiving moderately hypofractionated prostate radiation therapy.
Pract Radiat Oncol. 5:338–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tran A, Zhang J, Woods K, Yu V, Nguyen D,
Gustafson G, Rosen L and Sheng K: Treatment planning comparison of
IMPT, VMAT and 4π radiotherapy for prostate cases. Radiat Oncol.
12:102017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krauss DJ, Ye H, Martinez AA, Mitchell B,
Sebastian E, Limbacher A and Gustafson GS: Favorable preliminary
outcomes for men with low- and intermediate-risk prostate cancer
treated with 19-Gy single-fraction high-dose-rate brachytherapy.
Int J Radiat Oncol Biol Phys. 97:98–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasuya G, Ishikawa H, Tsuji H, Nomiya T,
Makishima H, Kamada T, Akakura K, Suzuki H, Shimazaki J, Haruyama
Y, et al: Significant impact of biochemical recurrence on overall
mortality in patients with high-risk prostate cancer after
carbon-ion radiotherapy combined with androgen deprivation therapy.
Cancer. 122:3225–3231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paganetti H, Niemierko A, Ancukiewicz M,
Gerweck LE, Goitein M, Loeffler JS and Suit HD: Relative biological
effectiveness (RBE) values for proton beam therapy. Int J Radiat
Oncol Biol Phys. 53:407–421. 2002. View Article : Google Scholar : PubMed/NCBI
|