Introduction
Lung cancer with preexisting interstitial lung
disease (ILD) is difficult to treat due to the risk of acute
exacerbation of ILD. There are newly developed treatments for
advanced lung cancer, such as molecular targeting agents and immune
checkpoint blockades, which may be successful in prolonging the
progression-free or overall survival of patients with lung cancer.
However, these agents are considered to be associated with a higher
risk of acute exacerbation of ILD in lung cancer with preexisting
ILD, and their use is not recommended in such patients. Therefore,
conventional cytotoxic chemotherapy remains the mainstay of
treatment for lung cancer with preexisting ILD.
Chemotherapy for lung cancer with ILD has been
previously investigated. One of the most effective treatments for
lung cancer with ILD is the combination of platinum agents with
paclitaxel or etoposide (1). The
incidence rate of acute exacerbation of ILD in patients treated
with weekly paclitaxel in combination with carboplatin was reported
to be 5.6–27% (2–4).
Nanoparticle albumin-bound (nab-) paclitaxel is a
130-nm albumin-bound form of paclitaxel. Nab-paclitaxel improves
the overall response rate and reduces neuropathy more efficiently
compared with conventional solvent-based (sb-) paclitaxel in
non-small-cell lung cancer (NSCLC) (5). However, the incidence of acute
exacerbation induced by nab-paclitaxel in lung cancer with ILD has
not been reported.
The aim of the present study was to compare
nab-paclitaxel with sb-paclitaxel regarding the incidence of acute
exacerbation of ILD in patients with advanced lung cancer.
Patients and methods
Patients
The medical records of lung cancer patients with ILD
who were administered nab-paclitaxel or sb-paclitaxel at the Kyoto
University Hospital (Kyoto, Japan) between January 2005 and August
2016 were retrospectively reviewed. Patients who received radiation
therapy to the chest region prior to and during these treatments
were excluded from the study.
Patients with ILD were defined as those who were
found to have reticular shadows, ground-glass opacities,
honeycombing, or traction bronchiectasis on chest computed
tomography (CT). Treatment-related acute exacerbation of ILD was
diagnosed according to the following criteria: i) Worsening of
dyspnea within the previous month; ii) new ground-glass opacities
or consolidation on chest radiography or CT; iii) no evidence of
infection, pneumothorax, pulmonary embolism, or congestive heart
failure as a cause of acute dyspnea; iv) <30 days after the last
administration of nab-paclitaxel or sb-paclitaxel. All the patients
were followed up until November 2016.
High-resolution CT criteria for usual
interstitial pneumonia (UIP) pattern
The classification shown in the ATS/ERS/JRS/ALAT
statement was adopted (6). Cases
inconsistent with the UIP pattern were defined as non-UIP and the
remaining cases were defined as UIP pattern in the present study.
One radiologist and one respiratory physician with expertise in
interstitial pneumonia reviewed the pretreatment CTs; both
reviewers were blinded to the patient data. Disagreements in the CT
findings between the two reviewers was resolved by a consensus.
Treatment method
Patients were administered nab-paclitaxel at a dose
of 100 mg/m2 on days 1, 8 and 15, or sb-paclitaxel at a
dose of 180–200 mg/m2 on day 1 or a dose of 70–80
mg/m2 on days 1, 8 and 15. Certain patients were
co-administered carboplatin with an area under the curve of 4–6 on
day 1. These agents were administered every 3–4 weeks. Dose
reduction and discontinuation of chemotherapy were based on the
physician's discretion. Treatment was continued until disease
progression, intolerable toxicity, or refusal by the patient.
Statistical analysis
The Fisher's exact test, Chi-squared test, Student's
t-test or Wilcoxon's two-sample test were used to compare patient
characteristics and the incidence of acute exacerbation of ILD. All
P-values <0.05 were considered to indicate statistically
significant differences. All statistical analyses were performed
using JMP 12.2 software for Windows (SAS Institute Inc., Cary, NC,
USA). The protocol of the present study was approved by the
Institutional Review Board of the Kyoto University Hospital.
Results
Patient characteristics
A total of 27 patients were included in the study.
The numbers of patients administered nab-paclitaxel and
sb-paclitaxel were 14 and 14, respectively (1 patient received both
agents). There were significantly more patients with small-cell
carcinoma in the sb-paclitaxel group compared with the
nab-paclitaxel group (P=0.0329). The performance status was 0–1 in
25 patients and 2 in 2 patients. None of the patients had been
previously treated for ILD. The mean serum level of Krebs von den
Lungen-6 (KL-6) prior to treatment in the sb-paclitaxel group was
higher compared with that in the nab-paclitaxel group, but the
difference was not statistically significant. Some of the patients
with a higher KL-6 level exhibited tumor progression.
The number of patients with the UIP pattern was
similar to that of patients with the non-UIP pattern.
Inter-observer agreement on the classification of ILD was high
(κ=0.85). There were no significant differences between the groups
regarding other patient characteristics (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Nab-paclitaxel
(n=14) | Sb-paclitaxel
(n=14) | P-value |
|---|
| Age, years | 69.9±9.3 | 70.1±8.4 | 0.950 |
| Sex
(male/female) | 10/4 | 13/1 | 0.326 |
| Smoking history
(yes/no) | 10/4 | 14/0 | 0.699 |
| Histological type
(adenocarcinoma/squamous/SCLC) | 7/6/1 | 4/3/7 |
0.0561 |
| KL-6, U/ml | 800.4±583.2 | 1350.8±1,226.5 | 0.199 |
| SP-D, ng/ml | 186.7±235.6 | 154.0±110.6 | 0.905 |
| Line of chemotherapy
(first-/second- and further-line) | 11/3 | 6/8 |
0.0530 |
| Preexisting COPD
(yes/no) | 2/9 | 1/8 | 1.000 |
| %FVC, % | 90.7±6.0 | 95.6±7.1 | 0.603 |
| Platine combination
(yes/no) | 11/3 | 7/7 | 0.115 |
| Classification of ILD
(UIP/non-UIP) | 12/2 | 11/3 | 1.000 |
Acute exacerbation of ILD
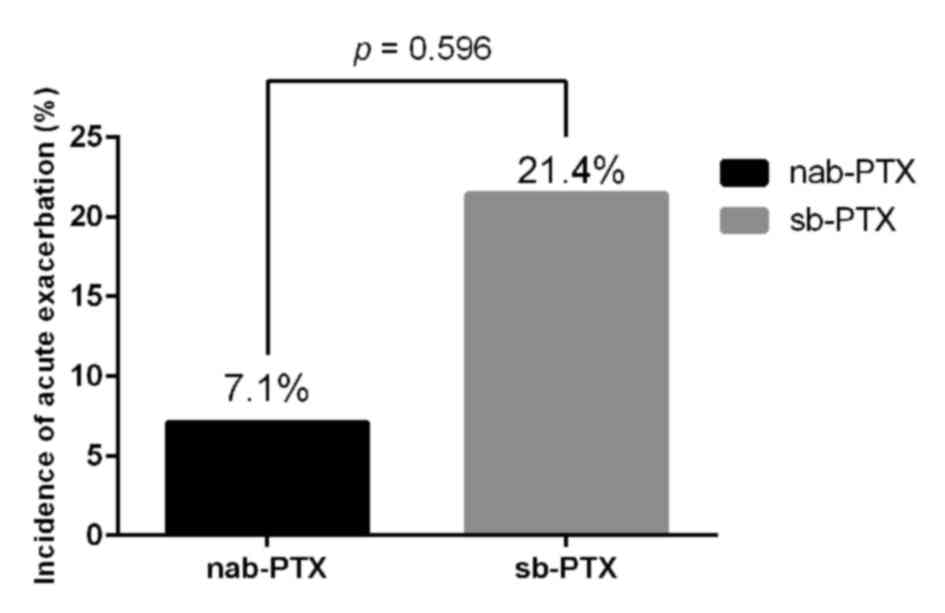
Acute exacerbation of ILD occurred in 1 patient
(7.1%) in the nab-paclitaxel group and 3 patients (21.4%) in the
sb-paclitaxel group; the difference was not statistically
significant (Fig. 1). All the
patients had a performance status of 0 prior to treatment. CT
images of a patient receiving nab-paclitaxel prior to treatment and
following acute exacerbation of ILD are shown in Fig. 2. Acute exacerbation of ILD was
managed with corticosteroid therapy and only 1 patient in the
sb-paclitaxel group succumbed to acute exacerbation of ILD. The
patients' characteristics are summarized in Table II.
 | Table II.Characteristics of patients with acute
exacerbation of ILD. |
Table II.
Characteristics of patients with acute
exacerbation of ILD.
| No. | Chemotherapy | Sex/age, years | Histology | KL.6 (U/ml) | SP.D (ng/ml) | ILD pattern | Best overall
response | Time to AE after
first administration (days) | AE outcome |
|---|
| 1 | Nab-PTX | F/75 | Adenocarcinoma | 859 | 55.5 | UIP | SD | 133 | Improved |
| 2 | Sb-PTX | M/75 | Small-cell
carcinoma | 968 | NA | UIP | PR | 47 | Improved |
| 3 | Sb-PTX + CBDCA | M/72 | Adenocarcinoma | 1,560 | 104 | UIP | SD | 79 | Improved |
| 4 | Sb-PTX + CBDCA | M/61 | Adenocarcinoma | 3,500 | 225 | UIP | SD | 56 | Succumbed to the
disease |
Discussion
To the best of our knowledge, this is the first
study to evaluate the incidence of acute exacerbation in lung
cancer patients with ILD treated with nab-paclitaxel. ILD is one of
the risk factors for the development of lung cancer (7). Acute exacerbation of ILD has been
reported to occur in patients with advanced lung cancer in
association with various chemotherapies. The incidence of acute
exacerbation of ILD has been found to be significantly higher with
gefitinib compared with cytotoxic thermotherapy (8). Although there are currently no
available studies on the safety of anaplastic lymphoma kinase
inhibitors in NSCLC patients with ILD, a case report demonstrated
the risk of acute exacerbation of ILD treated with crizotinib
(9). Therefore, molecularly targeted
agents are not recommended in patients with preexisting ILD. Immune
checkpoint inhibitors are a novel treatment for NSCLC and they may
be successful in improving progression-free and overall survival,
both as first-line treatment and after failure of platinum-based
doublet chemotherapy. However, there are also no studies on immune
checkpoint inhibitors in association with preexisting ILD, since
they are considered as risk factors for acute exacerbation. Hence,
cytotoxic chemotherapy may still be of value in lung cancer
patients with ILD.
There are several reports evaluating the safety of
cytotoxic chemotherapy in lung cancer patients with ILD (1,10,11).
However, the safety of nab-paclitaxel has not been previously
investigated, apart from one case report (12). The incidence of acute exacerbation of
ILD with nab-paclitaxel in the present study was 7.7%. Although
there was no significant difference in the incidence of ILD between
nab-paclitaxel and sb-paclitaxel, it was relatively lower with
nab-paclitaxel compared with sb-paclitaxel, and almost equal to the
previously reported incidence of acute exacerbation in association
with sb-paclitaxel (2,3). In addition, Cremophor EL®
(BASF, Ludwigshafen am Rhein, Germany), which is contained in
sb-paclitaxel for dissolution, is known to increase
hypersensitivity, which may lead to acute exacerbation of ILD
(13).
It is important to evaluate the risk of acute
exacerbation prior to chemotherapy. A UIP pattern on CT was
reported to be a risk factor for chemotherapy-induced acute
exacerbation of ILD (1,11,14).
Another report demonstrated that lower forced vital capacity is a
risk factor, but a UIP pattern is not (4). The rate of the UIP pattern may differ
among institutions, since inter-observer agreement for a UIP
pattern was only moderate among thoracic radiologists (15). In the present study, there were no
significant differences in patient characteristics including these
known risk factors; thus, the safety of nab-paclitaxel may be
comparable to that of sb-paclitaxel in lung cancer patients with
ILD.
The present study had several limitations. First,
this was a retrospective chart review study and certain data, such
as surfactant protein D levels and diffusing capacity of the lung
for carbon monoxide, could not be obtained. It is important to
evaluate ILD prior to treatment. Although a precise evaluation of
ILD was not performed, the study population was likely uniform
based on the good performance status and lack of previous treatment
for ILD. Second, the number of cases was too small to perform
multivariate analysis of risk factors for acute exacerbation of
ILD. To elucidate the risk of acute exacerbation, studies including
a larger patient sample from several institutions are required. In
addition, it may be preferable to limit studies to NSCLC or
small-cell lung cancer to compare the incidence of acute
exacerbation of ILD. A multicenter study may enable the collection
of more cases. Finally, acute exacerbation of ILD was diagnosed
only based on clinical and radiological findings. Bronchoscopy for
differential diagnosis was not performed in the patients included
in the present study, and other causes, such as lymphangitic
carcinomatosis, cannot be definitively excluded.
In conclusion, the findings of the present study
indicate that nab-paclitaxel is a safe option for patients with
advanced lung carcinoma with ILD and support conducting a
prospective clinical trial to confirm the clinical benefits of this
agent.
References
|
1
|
Minegishi Y, Takenaka K, Mizutani H, Sudoh
J, Noro R, Okano T, Azuma A, Yoshimura A, Ando M, Tsuboi E, et al:
Exacerbation of idiopathic interstitial pneumonias associated with
lung cancer therapy. Intern Med. 48:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minegishi Y, Sudoh J, Kuribayasi H,
Mizutani H, Seike M, Azuma A, Yoshimura A, Kudoh S and Gemma A: The
safety and efficacy of weekly paclitaxel in combination with
carboplatin for advanced non-small cell lung cancer with idiopathic
interstitial pneumonias. Lung Cancer. 71:70–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukuya T, Ishiwata T, Hara M, Muraki K,
Shibayama R, Koyama R and Takahashi K: Carboplatin plus weekly
paclitaxel treatment in non-small cell lung cancer patients with
interstitial lung disease. Anticancer Res. 30:4357–4361.
2010.PubMed/NCBI
|
|
4
|
Enomoto Y, Inui N, Kato T, Baba T,
Karayama M, Nakamura Y, Ogura T and Suda T: Low forced vital
capacity predicts cytotoxic chemotherapy-associated acute
exacerbation of interstitial lung disease in patients with lung
cancer. Lung Cancer. 96:63–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small-cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozawa Y, Suda T, Naito T, Enomoto N,
Hashimoto D, Fujisawa T, Nakamura Y, Inui N, Nakamura H and Chida
K: Cumulative incidence of and predictive factors for lung cancer
in IPF. Respirology. 14:723–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudoh S, Kato H, Nishiwaki Y, Fukuoka M,
Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M, et
al: Interstitial lung disease in Japanese patients with lung
cancer: A cohort and nested case-control study. Am J Respir Crit
Care Med. 177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe N, Nakahara Y, Taniguchi H,
Kimura T, Kondoh Y, Kataoka K and Sakamoto K: Crizotinib-induced
acute interstitial lung disease in a patient with EML4-ALK positive
non-small cell lung cancer and chronic interstitial pneumonia. Acta
Oncol. 53:158–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe N, Taniguchi H, Kondoh Y, Kimura
T, Kataoka K, Nishiyama O, Kondo M and Hasegawa Y: Efficacy of
chemotherapy for advanced non-small cell lung cancer with
idiopathic pulmonary fibrosis. Respiration. 85:326–331. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kenmotsu H, Naito T, Kimura M, Ono A,
Shukuya T, Nakamura Y, Tsuya A, Kaira K, Murakami H, Takahashi T,
et al: The risk of cytotoxic chemotherapy-related exacerbation of
interstitial lung disease with lung cancer. J Thorac Oncol.
6:1242–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma Y, Tamiya M, Shiroyama T, Osa A,
Takeoka S, Morishita N, Suzuki H, Okamoto N, Hirashima T and Kawase
I: Nanoparticle albumin-bound paclitaxel+carboplatin therapy for
small cell lung cancer combined with squamous cell carcinoma and
interstitial lung disease. Intern Med. 54:2911–2913. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta N, Hatoum H and Dy GK: First line
treatment of advanced non-small-cell lung cancer-specific focus on
albumin bound paclitaxel. Int J Nanomedicine. 9:209–221.
2014.PubMed/NCBI
|
|
14
|
Asai N, Katsuda E, Hamanaka R, Kosaka K,
Matsubara A, Nishimura M, Tanaka H, Yokoe N, Takahashi A, Yamaguchi
E and Kubo A: The ATS/ERS/JRS/ALAT statement ‘IPF by HRCT’ could
predict acute exacerbation of interstitial lung disease in
non-small cell lung cancer. Tumori. 103:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh SL, Calandriello L, Sverzellati N,
Wells AU and Hansell DM: UIP Observer Consort: Interobserver
agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on
CT. Thorax. 71:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|