Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare
disease of unknown etiology (1–3). This
tumor was previously described as an inflammatory pseudotumor,
inflammatory myofibroblastoma, lymphoplasmacytic histiocytoma and
fibrous pseudotumor until 1994, when myofibroblastic tumor was
established as a distinct low-grade malignancy by the World Health
Organization (1–3). IMT is considered to be a neoplasm of
intermediate biological potential, which may recur and metastasizes
infrequently (1–4). Histologically, IMT is characterized by
myofibroblastic spindle cells mixed with a hyalinized stroma and
various degrees of inflammatory infiltrates (1–3). IMT
occurs principally in children and young adults (4–10) and
may affect any site in the body, although it is most commonly found
in the lungs (4–6).
It is well established that total surgical excision
is the most effective therapeutic option for surgically accessible
IMT (2). However, the treatment
options for relapsed and/or inoperable IMT are extremely limited
due to the lack of reports regarding effective treatments. In
particular, little evidence has been reported in the literature
regarding the efficacy of chemotherapy for IMT (7–14). We
encountered a case of inoperable IMT with pancreatic involvement
following cholecystectomy for IMT originating from the gallblabber.
The patient failed to respond to non-steroidal anti-inflammatory
drugs (NSAIDs) and steroid therapy, but subsequently showed a good
response to vinorelbine (VNB) and methotrexate (MTX) combination
chemotherapy. We herein describe the clinical course and present a
review of the literature on the effectiveness of chemotherapy for
advanced and unresectable IMT.
Case report
A 63-year-old woman with no significant past medical
history was admitted to a local hospital with the complaint of
abdominal fullness and pain. A tumor was detected in the
gallbladder, and the patient underwent surgical resection. Distant
metastasis or invasion into adjacent organs were not observed based
on radiographic findings prior to the operation and intraoperative
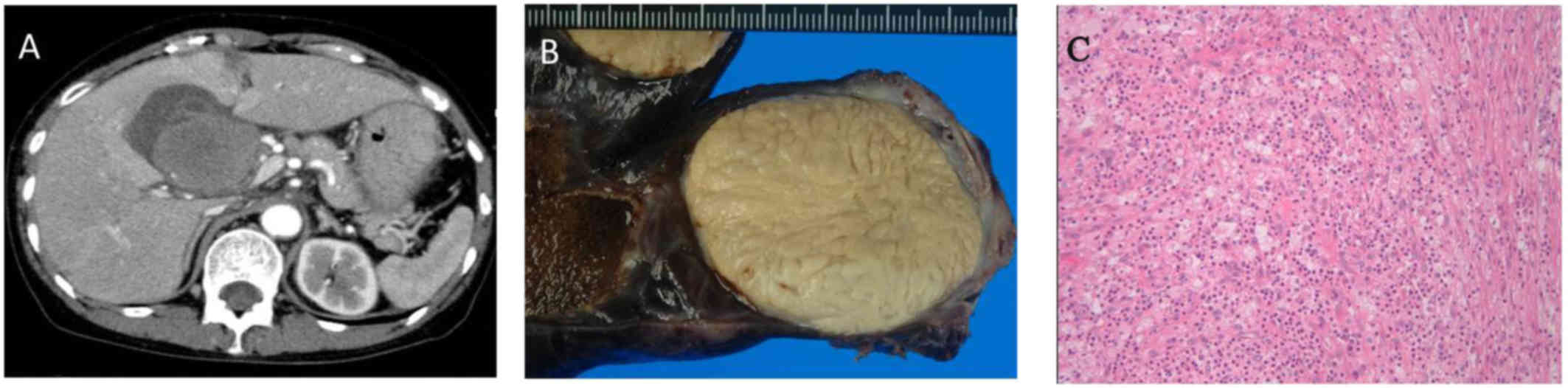
inspection. The pathological diagnosis was IMT (Fig. 1). Thirteen months following
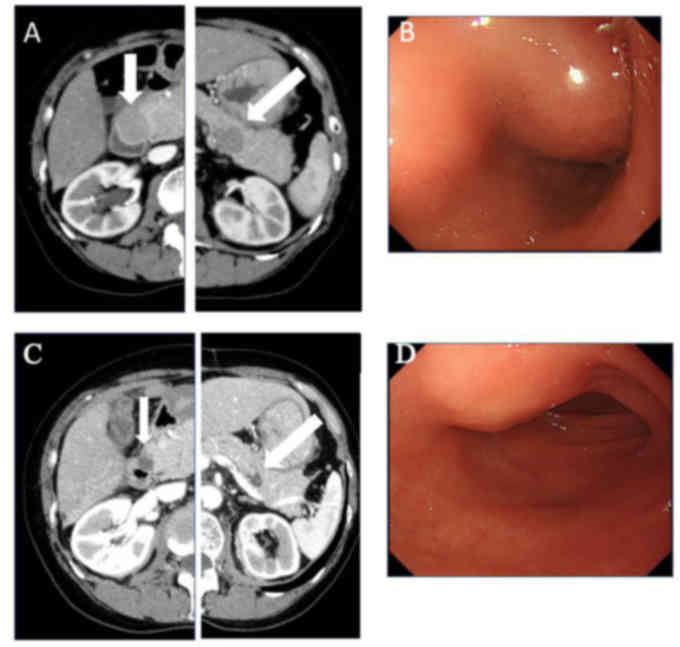
resection, tumors in the pancreatic head and tail (Fig. 2A) were identified on whole-body
computed tomography (CT) during postoperative follow-up. The
patient was subsequently referred to the Department of
Comprehensive Cancer Therapy, Shinshu University School of Medicine
(Matsumoto, Japan) for further examination. Positron emission
tomography with 18F-fluorodeoxyglucose (FDG-PET)
revealed abnormal uptake by both tumors. Endoscopic examination
revealed a submucosal tumor in the duodenum (Fig. 2B). Endoscopic ultrasound-guided
fine-needle aspiration in the pancreatic head tumor was performed
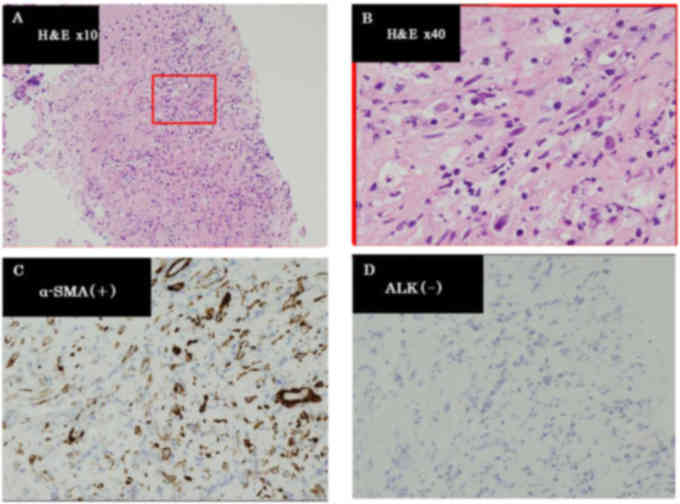
and the histological diagnosis was recurrence of IMT (Fig. 3). The histological findings and
molecular analysis of specimens collected from the pancreas and
gallbladder revealed no pathological evidence of malignancy. In
addition, there was no presence of anaplastic lymphoma kinase (ALK)
protein by immunohistochemical staining or gene rearrangement by
fluorescence in situ hybridization. Treatment with NSAIDs
and methylprednisolone (500 mg/dayx3 day, intravenously) followed
by 1 mg/kg of prednisolone for 1 month was continued, but the
tumors were not reduced in size. Subsequently, treatment with
methotrexate (MTX; 30 mg/m2 on day 1) plus vinorelbine
(VNB; 20 mg/m2 on days 1 and 7) per 3 weeks chemotherapy
was administered. The chemotherapy was continued without any severe
toxicities. The dosage was repeated six times and partial response
was achieved as determined by CT and endoscopic examination
(Fig. 2C and D).
Discussion
We herein describe a case of abdominal IMT that
developed in an elderly female and responded well to VNB and MTX
chemotherapy. IMT is usually encountered in children and
adolescents, mostly occurring between 2 and 16 years of age and
mainly involving the lungs (1–3). Kovach
et al (4) summarized 44 cases
of IMT in their institutes and reported a mean age of 44 years,
ranging from 9 to 88 years. In addition, the tumor location
included several extrapulmonary lesions in addition to the lung
(4). The patient reported herein had
abdominal IMT and was aged 63 years. The association between age of
onset and the involved location of IMT remains unclear. However, it
has been suggested that recurrence was more common if the lesions
were extrapulmonary (4).
Although our patient had an ALK-negative tumor, it
has been demonstrated that ~50% of IMTs harbor ALK gene
rearrangement (2,15). Coffin et al (2) analyzed 59 IMTs and reported that
ALK-negative IMTs had a more aggressive clinical course with a high
risk of distant metastasis. In addition, the absence of ALK
expression was associated with older age (2). Thus, clinical manifestations, including
age, location and ALK negativity in the present case were
consistent with those reported in the literature.
Surgical resection has been considered the preferred
treatment for IMT, and may also be used to confirm this diagnosis
(2,16). However, IMT is considered to be a
neoplasm of intermediate biological potential, which may recur and
metastasize, although infrequently (1–4). In
unresectable cases, radiotherapy and chemotherapy, including
steroids and NSAIDs, were applied (7–14,17,18).
Indeed, several clinical reports indicated the usefulness of
steroids and NSAIDs (11,13,17,18),
although both failed to shrink the tumor in the present case.
Various cytotoxic agents or regimens, including MTX, VNB,
vincristine, cyclophosphamide, doxorubicin, 5-fluorouracil,
cisplatin, carboplatin, paclitaxel, ifosfamide and etoposide, have
been used. However, due to the rarity of IMT, the availability of
data regarding the efficacy of cytotoxic chemotherapeutic agents is
limited. Previously reported treatment regimens, consisting of
cytotoxic chemotherapy alone or in combination with NSAIDs, that
were found to be effective for IMT are summarized in Table I. However, none of the
chemotherapeutic regimens had been evaluated in a case series. In
addition, the majority of data were obtained from pediatric
populations. Thus, there is still a lack of definitive clinical
evidence, particularly for adults. As Favini et al (10) reported a case of IMT that responded
to VNB plus MTX, this chemotherapy regimen was used in the present
case. Chemotherapy was performed without any severe toxicities and
the patient responded well. Although the efficacy of this
chemotherapy was evaluated in aggressive fibromatosis in cases
series (19,20), our experience, including the case
reported by Favini et al (10), may provide a treatment choice for
patients with unresectable IMT. With regard to other optimal
regimens, Kubo et al (12)
described the case of an adult IMT patient (aged 26 years) who
responded to carboplatin+paclitaxel chemotherapy. As this
combination is the most commonly used chemotherapy regimen in
various malignancies, carboplatin+paclitaxel may be an optimal
choice for adult and inoperable IMT. An optimal agent or
combination chemotherapy has not yet been determined for
unresectable IMT. Further clinical experience and studies are
required to determine the usefulness of chemotherapy for inoperable
IMT.
 | Table I.Previous case reports successfully
treated with cytotoxic chemotherapy. |
Table I.
Previous case reports successfully
treated with cytotoxic chemotherapy.
| Case | Age, years | Location | Agent/regimen | Response | NSAIDs | Surgery | (Refs.) |
|---|
| 1 | 7 | Abdomen |
Vincristine+etoposide→cisplatin,
adriamycin, methotrexate | PR | + | + | (8) |
| 2 | 10 | Abdomen |
Methotrexate+vinblastine, adriamycin,
ifosfamide | PR |
| + | (9) |
| 3 | 12 | Conjuctiva |
Methotrexate+vinorelbine | PR |
|
| (10) |
| 4 | 14 | Peritoneum |
Cisplatin+methotrexate | CR | + | + | (11) |
| 5 | 26 | Mediastinum |
Carboplatin+paclitaxel | CR |
|
| (12) |
| 6 | 64 | Frontal bone | Methotrexate | PR | + |
| (13) |
| 7 | 64 | Abdomen |
Doxorubicin+ifosfamide | PR |
|
| (14) |
In summary, our observations in the present case
suggest that intermediate-dose chemotherapy using vinorelbine and
methotrexate is a feasible therapeutic option in adult patients
with unresectable IMT.
References
|
1
|
Fletcher CDM, Unni KK and Mertens F:
Inflammatory myofibroblastic tumor. In: World Health Organization
Classification of TumoursPathology and Genetics of Tumours of Soft
Tissue and Bone. IARC Press; Lyon: pp. 91–93. 2002
|
|
2
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mergan F, Jaubert F, Sauvat F, Hartmann O,
Lortat-Jacob S, Révillon Y, Nihoul-Fékété C and Sarnacki S:
Inflammatory myofibroblastic tumor in children: Clinical review
with anaplastic lymphoma kinase, Epstein-Barr virus, and human
herpesvirus 8 detection analysis. J Pediatr Surg. 40:1581–1586.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kovach SJ, Fischer AC, Katzman PJ, Salloum
RM, Ettinghausen SE, Madeb R and Koniaris LG: Inflammatory
myofibroblastic tumors. J Surg Oncol. 94:385–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tunçözgür B, Ustünsoy H, Bakir K, Uçak R
and Elbeyli L: Inflammatory pseudotumor of the lung. Thorac
Cardiovasc Surg. 48:112–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanders BM, West KW, Gingalewski C, Engum
S, Davis M and Grosfeld JL: Inflammatory pseudotumor of the
alimentary tract: Clinical and surgical experience. J Pediatr Surg.
36:169–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dishop MK, Warner BW, Dehner LP, Kriss VM,
Greenwood MF, Geil JD and Moscow JA: Successful treatment of
inflammatory myofibroblastic tumor with malignant transformation by
surgical resection and chemotherapy. J Pediatr Hematol Oncol.
25:153–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertocchini A, Lo Zupone C, Callea F,
Gennari F, Serra A, Monti L and de Ville de Goyet J: Unresectable
multifocal omental and peritoneal inflammatory myofibroblastic
tumor in a child: Revisiting the role of adjuvant therapy. J
Pediatr Surg. 46:e17–e21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Favini F, Resti AG, Collini P, Casanova M,
Meazza C, Trecate G and Ferrari A: Inflammatory myofibroblastic
tumor of the conjunctiva: Response to chemotherapy with low-dose
methotrexate and vinorelbine. Pediatr Blood Cancer. 54:483–485.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao YL, Wang ZJ, Han JG and Wei P:
Inflammatory myofibroblastic tumor successfully treated with
chemotherapy and nonsteroidals: A case report. World J
Gastroenterol. 18:7100–7103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubo N, Harada T, Anai S, Otsubo K,
Yoneshima Y, Ijichi K, Koga T, Takayama K and Nakanishi Y:
Carboplatin plus paclitaxel in the successful treatment of advanced
inflammatory myofibroblastic tumor. Intern Med. 51:2399–2401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kusunoki-Nakamoto F, Matsukawa T, Tanaka
M, Miyagawa T, Yamamoto T, Shimizu J, Ikemura M, Shibahara J and
Tsuji S: Successful treatment of an unresectable inflammatory
myofibroblastic tumor of the frontal bone using a cyclooxygenase-2
inhibitor and methotrexate. Intern Med. 52:623–628. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inadomi K, Kumagai H, Takayoshi K, Ariyama
H, Kusaba H, Nishie A, Yamamoto H, Takase K, Tanaka M, Sagara K, et
al: Successful combination chemotherapy for metastatic inflammatory
myofibroblastic tumor: A case report. Oncol Lett. 10:2981–2985.
2015.PubMed/NCBI
|
|
15
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janik JS, Janik JP, Lovell MA, Hendrickson
RJ, Bensard DD and Greffe BS: Recurrent inflammatory pseudotumors
in children. J Pediatr Surg. 38:1491–1495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berger A, Kim C, Hagstrom N and Ferrer F:
Successful preoperative treatment of pediatric bladder inflammatory
myofibroblastic tumor with anti-inflammatory therapy. Urology.
70(372): e13–e15. 2007.
|
|
18
|
Chan PW, Omar KZ and Ramanujam TM:
Successful treatment of unresectable inflammatory pseudotumor of
the lung with COX-2 inhibitor. Pediatr Pulmonol. 36:167–169. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azzarelli A, Gronchi A, Bertulli R, Tesoro
JD, Baratti D, Pennacchioli E, Dileo P, Rasponi A, Ferrari A,
Pilotti S and Casali PG: Low-dose chemotherapy with methotrexate
and vinblastine for patients with advanced aggressive fibromatosis.
Cancer. 92:1259–1264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meazza C, Bisogno G, Gronchi A, Fiore M,
Cecchetto G, Alaggio R, Milano GM, Casanova M, Carli M and Ferrari
A: Aggressive fibromatosis in children and adolescents: The Italian
experience. Cancer. 116:233–240. 2010.PubMed/NCBI
|