Introduction
The rate of locoregional recurrence after definitive
chemoradiotherapy for esophageal carcinoma is ~40–60% (1). A proportion of such patients are
referred for salvage therapy. In general, a patient with
multiple-site recurrence is treated by single-agent or combination
chemotherapy. Platinum agents have become the key drugs in systemic
esophageal cancer chemotherapy. For stage IV esophagogastric
cancer, combination chemotherapy with epirubicin plus cisplatin and
5-fluorouracil (ECF regimen) has resulted in a 1-year survival rate
of 37% and a median survival duration of 9.9 months (2). However, with this triplet drug regimen,
complete remission or long-term survival is extremely rare.
Patients with a limited number of relapse sites,
referred to as oligometastases or oligorecurrence (3), are considered to be suitable candidates
for definitive local therapy with the goal of improving prognosis
(4). For locoregionally recurrent
lesions after definitive chemoradiotherapy, salvage surgery is the
only established treatment strategy that may offer any chance of
long-term survival (5). Swisher
et al reported that salvage esophagectomy resulted in 5-year
survival of 25% in selected patients (6). However, this procedure should be
considered for carefully selected patients, due to the high
incidence of morbidity and mortality (7).
Re-irradiation following previous definitive
chemoradiotherapy is generally contraindicated, considering the
radiation tolerance of the organs at risk, including the lung,
trachea, esophagus and spinal cord. Since recovery from the effects
of the initial radiation therapy occurs with time, the optimal
prescription dose for re-irradiation depends on multiple
determinants, including the initial treatment, histology of the
primary tumor, and location of recurrence.
We herein report the results of 6 patients who
underwent salvage re-irradiation therapy with tolerable toxicity
for locally recurrent esophageal cancer.
Case reports
The consecutive cases of 6 patients who underwent
salvage re-irradiation (5 of whom also received concurrent
chemotherapy) for esophageal cancer recurrence following definitive
chemoradiotherapy between January 2011 and June 2016 at the
Department of Radiology of the University of Tokyo Hospital were
retrospectively analysed. Written informed consent was obtained
from all subjects, following careful explanation regarding the
possibility of severe treatment-related toxicity. All enrolled
cases satisfied the following eligibility criteria: i) A history of
previous chemoradiotherapy for esophageal carcinoma; ii) limited
number of locoregional recurrence sites, including supraclavicular
lymph nodes that could be included within the radiotherapy fields;
iii) no evidence of distant metastasis; iv) Karnofsky Performance
Status (KPS) score at recurrence diagnosis >70; and v) >6
months between initial and salvage irradiation. The median survival
time (MST) was measured from the first day of re-irradiation.
Clinical stage was determined according to the 7th edition of the
American Joint Committee on Cancer TNM staging system (8). Treatment response was assessed
according to the Response Evaluation Criteria in Solid Tumors
guidelines, version 1.1 (https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf).
Acute or late adverse events (AEs) were graded using the Common
Terminology Criteria for Adverse Events, version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf).
All radiotherapy was delivered using a 3-dimensional
conformal radiation therapy technique with 6 or 10 MV photon
energies. The clinical target volume (CTV) of radiotherapy was the
macroscopic tumor and enlarged lymph nodes, if any, surrounded by
sufficient proximal and distal margins. The CTV was delineated on
planning computed tomography (CT) images, with reference to
positron emission tomography (PET) image data, if available. The
CTV to the planning target volume (PTV) margin was 0.5–1.5 cm. As
this was re-irradiation planning, the multiple beam arrangement
technique was used to avoid irradiation of the spinal cord.
Follow-up examinations included a physical examination and
assessment of laboratory values and imaging examinations every 1–3
months.
In our institution, concurrent chemotherapy consists
of nedaplatin and oral S-1 administration or intravenous
5-fluorouracil infusion. S-1 is an oral prodrug of 5-fluorouracil,
which can be administered in an outpatient setting, and nedaplatin
produces less nausea, vomiting and nephrotoxicity compared with
other platinum-containing drugs (9).
Tsuda et al evaluated the feasibility of S-1 and nedaplatin
in combination with radiotherapy, and reported that the toxicity
was tolerable in esophageal cancer treatment (10). Yamashita et al also reported
the efficacy and feasibility of nedaplatin plus S-1 in definitive
or salvage concurrent chemoradiotherapy for early, advanced and
relapsed esophageal cancer (11).
All the patients were male and the median age at the
time of initial radiation treatment was 65 years (range, 52–68
years). The patient characteristics and initial treatment
modalities are summarized in Table
I. Patient D suffered progressive disease after the third cycle
of chemotherapy in initial treatment with the appearance of a new
out-of-field lesion in the esophagus; he underwent salvage
esophagectomy with a gastric conduit reconstruction. Patient E had
undergone endoscopic submucosal dissection (ESD) 6 months prior to
chemoradiotherapy. The ESD result had revealed invasion of the
second submucosal layer, but the patient declined adjuvant therapy
at that time. Recurrence in a superior mediastinal lymph node
developed 6 months later, and the patient was clinically staged as
rTxN1M0; he then underwent definitive chemoradiotherapy, resulting
in complete response.
 | Table I.Patient characteristics at initial
treatment and treatment modality. |
Table I.
Patient characteristics at initial
treatment and treatment modality.
| Patient ID | Age at initial
therapy, years | Gender | Primary site | TNM | Pathology | Prescription
dose | Chemotherapy | Cycles | First response | Site of
recurrence | Reason for selecting
re-RT | Time to re-RT from
initial treatment (months) |
|---|
| A | 67 | Male | Mt | cT1N0M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + 5FU 800 mg/m2 | 4 | CR | Ut | Main bronchus
invasion | 59.2 |
| B | 62 | Male | Mt | cT3N2M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + S-1 120 mg/body | 4 | CR | SMLN | Patient refusal | 39.9 |
| C | 63 | Male | Ut-Mt | cT4N1M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + S-1 120 mg/body | 4 | CR | Ce-Ut | Patient refusal | 20.2 |
| D | 52 | Male | Mt | cT2N0M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + S-1 120 mg/body | 3 | PD | SMLN, supraclavicular
lymph node and anastomotic region | Patient refusal | 14.5 |
| E | 53 | Male | Lt | rTxN1M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + S-1 120 mg/body | 2 | CR | SMLN | Incomplete resection
of salvage surgery | 10.9 |
| F | 68 | Male | Mt | cT4bN2M0 | SqCC | 50.4 Gy/28 fr | NDP 80
mg/m2 + S-1 100 mg/body | 4 | CR | SMLN | Aortic invasion | 6.4 |
The median time interval between initial
radiotherapy and re-irradiation was 17.4 months (range, 6.4–59.2
months). A whole-body PET scan was available for all but 1 patient
(patient F), confirming the absence of distant metastatic lesions
and guiding target volume delineation. The re-irradiation delivered
dose ranged from 30 to 50.4 Gy, with fractionation of 1.8–2.0 Gy
per day or 1.2–1.5 Gy twice daily. A total of 5 patients underwent
concurrent chemotherapy with nedaplatin and oral S-1 administration
with radiotherapy, and the remaining patient underwent radiotherapy
alone. The other characteristics at recurrence are summarized in
Table II. Patient E developed
recurrence in a superior mediastinal lymph node after the first
definitive chemoradiotherapy and underwent salvage surgery;
however, the surgery was limited by lesions involving arteries, and
he was referred to our department for re-irradiation as salvage
therapy. Patient F had his fractionation changed from twice daily
to once daily after lymphadenopathy was noted after he had received
18 Gy.
 | Table II.Results and adverse events in salvage
radiotherapy. |
Table II.
Results and adverse events in salvage
radiotherapy.
| Patient ID | KPS score at re-RT
(%) | Prescription dose and
fractionation | Chemotherapy, no. of
cycles | Response to
re-RT | Acute hematological
AEs, grade | Acute
non-hematological AEs, grade | Late AEs | Time to second
recurrence from re-RT (months) | Site of second
recurrence |
|---|
| A | 90 | 45 Gy, 1.8 Gy/fr
(once daily) | NDP 64
mg/m2+ S.1 100 mg/body, 4 | PR | Leukopenia G4, anemia
G3, thrombocytopenia G3 | Mucositis G1 | Dysphagia G1, pleural
effusion G1, pneumonitis G1 | 6 | Mt |
| B | 90 | 50.4 Gy, 1.2 Gy/fr
(twice daily) | NDP 80
mg/m2+ S.1 120 mg/body, 4 | CR | Leukopenia G3, anemia
G2, thrombocytopenia G1 | Dermatitis G1,
dysphagia G1 | Dysphagia G1, pleural
effusion G2 | 15.6 | SMLN, thoracic
vertebra (T9) |
| C | 90 | 50.4 Gy, 1.2 Gy/fr
(twice daily) | NDP 64
mg/m2+S.1 100 mg/body, 4 | CR | Leukopenia G2, anemia
G2, thrombocytopenia G2 | Mucositis G1, fatigue
G2 | Pneumonitis G1 | 30.4 | Ut |
| D | 90 | 50 Gy, 2 Gy/fr (once
daily) | NDP 80
mg/m2 + S.1 120 mg/body, 4 | PD | Leukopenia G3, anemia
G3, thrombocytopenia G1 | Mucositis G1, fatigue
G1 | NE | NE | NE |
| E | 90 | 30 Gy, 1.5 Gy/fr
(twice daily) | None | PR | Leukopenia G1, anemia
G2, thrombocytopenia G1 | Mucositis G1,
dysphagia G1 | Hypothyroidism G2,
dysphagia G3, pleural effusion G1 | 11.5 | Lung |
| F | 80 | 30 Gy, 1.2 Gy/fr
(twice daily: Total 18 Gy) + 2 Gy/fr (once daily: Total 12 Gy) | NDP 80
mg/m2 + S-1 100 mg/body, 1 | PD | Leukopenia G1, anemia
G2, thrombocytopenia G1 | Mucositis G1, nausea
G2 | NE | NE | NE |
Severe (grade 3–5) acute hematological AEs occurred
in 3 patients, including 1 case of grade 4 leukopenia. There were
no acute non-hematological AEs of grade ≥3. No radiation myelitis,
esophageal perforation, or fissure occurred. One patient developed
grade 3 dysphagia and became permanently dependent on a
percutaneous endoscopic gastrostomy tube feeding ~6 months after
the start of re-irradiation. The same patient also developed grade
2 hypothyroidism, requiring levothyroxine starting 3 months after
irradiation to the time of death.
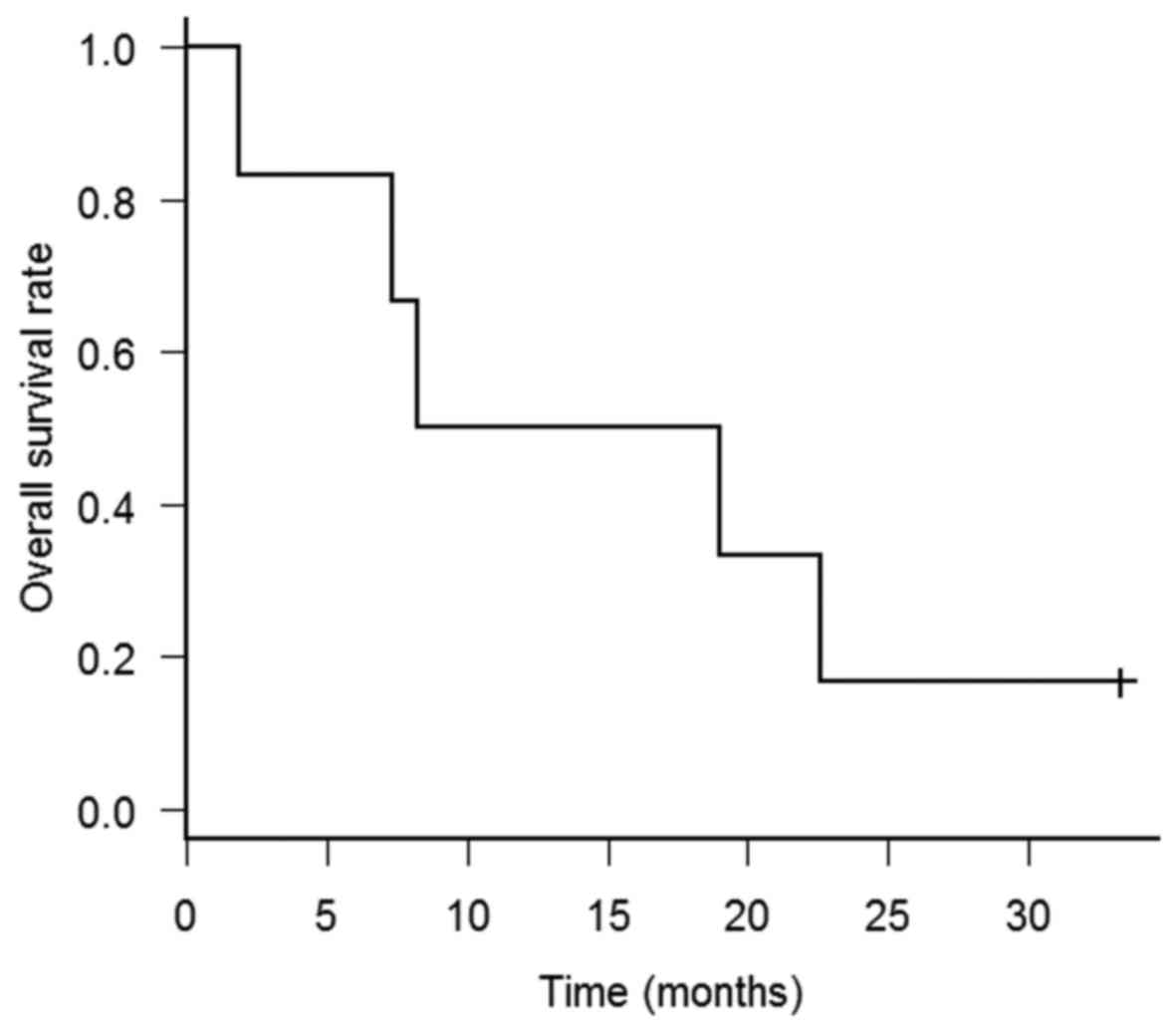
The MST after re-irradiation was 13.6 months (range,
1.9–33.3 months) (Fig. 1). Only 1
patient (patient C) remained alive at the time of the analysis. The
longest disease-free survival from re-irradiation to second
recurrence was 33.3 months. The patient underwent salvage
chemotherapy and was alive without any evidence of disease. Patient
B also remained disease-free for >1 year after re-irradiation.
Fifteen months after the starting date of re-irradiation, the
patient experienced hoarseness and the CT scan revealed superior
mediastinal lymphadenopathy and vertebral metastasis and he
underwent salvage chemotherapy consisting of oral S-1
administration.
Patients D and F exhibited rapid progression of
recurrent disease soon after re-irradiation. Patients A and E
experienced temporary responses to salvage chemoradiotherapy;
however, disease recurrence occurred in the middle thoracic
esophagus and lung, respectively.
Discussion
Esophageal cancer is the seventh most common cause
of cancer-related mortality in Japan, with an estimated 11,746
deaths in 2008 (12). Re-irradiation
salvage therapy, with or without chemotherapy, for recurrent
esophageal carcinoma after definitive chemoradiotherapy yielded
reasonable results in this small group of patients, extending MST
from the expected 9.9 months (2) to
13.6 months. Although treatment toxicity is one of the issues of
most concern at re-irradiation, particularly late AEs, there was no
lethal treatment-related toxicity in the present study, and only 1
of 6 patients (16.7%) developed grade 3 late AEs.
Zhou et al also reported on the efficacy and
feasibility of salvage radiotherapy in patients with locally
recurrent esophageal cancer after definitive chemoradiotherapy
(13). The 3-year overall survival
rate was 21.8%. Radiation pneumonitis grade ≥3 was observed in
5.45%, and esophageal fistula/perforation was observed in 20.0% of
the cases. Fakhrian et al reported on 54 patients with
recurrent esophageal carcinoma undergoing salvage radiotherapy
(14). Median survival time was 12
months (95% confidence interval: 7–17 months) and toxicity was in
an acceptable range. Kim et al described 10 patients who
underwent re-irradiation of recurrent esophageal cancer after
primary definitive radiotherapy (14); 2 patients had a complete response,
but 3 succumbed to esophageal perforation within 2 months of
completion of re-irradiation.
Salvage surgery is a treatment modality for
locoregional recurrence of esophageal cancer. The incidence of
adverse events of salvage surgery following definitive
chemoradiotherapy is higher compared with that of initial surgery.
Tachimori et al reported that patients who underwent salvage
esophagectomy, compared with those who underwent esophagectomy
without preoperative therapy, experienced increased anastomotic
leak rates (31 vs. 25%, respectively), respiratory complication
rates (31 vs. 20%, respectively), and hospital mortality rates (8
vs. 2%, respectively) (15). Chen
et al compared salvage chemoradiotherapy with surgery for
recurrent esophageal squamous cell carcinoma following definitive
radiotherapy (16); they did not
observe a statistically significant survival difference (P=0.697),
but the frequency of complications, including esophageal
fistula/perforation, was higher (19.0%) in the salvage
chemoradiotherapy group.
In the results presented herein, 3 patients who
completed hyperfractionated re-irradiation therapy remained alive 1
year after salvage treatment. Hyperfractionated radiation has been
considered to improve locoregional control rates without increasing
late toxicity in head and neck cancer (17). In patients with recurrent rectal
carcinoma, high doses of hyperfractionated radiation may be
delivered with acceptable risk, without prohibitive long-term side
effects (18). Hyperfractionated
re-irradiation has also been used to successfully manage locally
recurrent lung cancer (19) and head
and neck cancers (20). Moreover,
hyperfractionated radiotherapy resulted in favorable outcomes for
Japanese patients with head and neck carcinoma (21,22).
In conclusion, although the outcome of
re-irradiation for recurrent esophageal cancer was poor, some
patients obtained long-term disease control without any severe
symptoms. Re-irradiation may be a useful modality as salvage
therapy for patients who are not surgical candidates.
Re-irradiation-related AEs were compatible with those of previous
reports. However, the sample size of this study was very limited,
and confirmation with a larger number of patients and a longer
follow-up period is required.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niibe Y and Chang JY: Novel insights of
oligometastases and oligo-recurrence and review of the literature.
Pulm Med. 2012:2610962012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niibe Y and Hayakawa K: Oligometastases
and oligo-recurrence: The new era of cancer therapy. Jpn J Clin
Oncol. 40:107–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeuchi H, Saikawa Y, Oyama T, Ozawa S,
Suda K, Wada N, Takahashi T, Nakamura R, Shigematsu N, Ando N, et
al: Factors influencing the long-term survival in patients with
esophageal cancer who underwent esophagectomy after
chemoradiotherapy. World J Surg. 34:277–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swisher SG, Wynn P, Putnam JB, Mosheim MB,
Correa AM, Komaki RR, Ajani JA, Smythe WR, Vaporciyan AA, Roth JA
and Walsh GL: Salvage esophagectomy for recurrent tumors after
definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg.
123:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Journo XB and Thomas PA: Current
management of esophageal cancer. J Thorac Dis. 6 Suppl 2:253–264.
2014.
|
|
8
|
Ota K: Nedaplatin. Gan To Kagaku Ryoho.
23:379–387. 1996.(In Japanese). PubMed/NCBI
|
|
9
|
Tsuda T, Inaba H, Miyazaki A, Izawa N,
Hirakawa M, Watanabe Y, Kitajima S, Hoshikawa Y, Gomi H, Kimura M
and Itoh F: Prospective study of definitive chemoradiotherapy with
S-1 and nedaplatin in patients with stage II/III (non-T4)
esophageal cancer. Esophagus. 8:45–51. 2011. View Article : Google Scholar
|
|
10
|
Yamashita H, Haga A, Takenaka R, Kiritoshi
T, Okuma K, Ohtomo K and Nakagawa K: Efficacy and feasibility of
ambulatory treatment-based monthly nedaplatin plus S-1 in
definitive or salvage concurrent chemoradiotherapy for early,
advanced, and relapsed esophageal cancer. Radiat Oncol. 11:42016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou ZG, Zhen CJ, Bai WW, Zhang P, Qiao
XY, Liang JL, Gao XS and Wang SS: Salvage radiotherapy in patients
with local recurrent esophageal cancer after radical
radiochemotherapy. Radiat Oncol. 10:542015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fakhrian K, Gamisch N, Schuster T, Thamm
R, Molls M and Geinitz H: Salvage radiotherapy in patients with
recurrent esophageal carcinoma. Strahlenther Onkol. 188:136–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Lee CG, Kim KH, Kim T, Lee J, Cho
Y and Koom WS: Re-irradiation of recurrent esophageal cancer after
primary definitive radiotherapy. Radiat Oncol J. 30:182–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tachimori Y, Kanamori N, Uemura N,
Hokamura N, Igaki H and Kato H: Salvage esophagectomy after
high-dose chemoradiotherapy for esophageal squamous cell carcinoma.
J Thorac Cardiovasc Surg. 137:49–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Lu Y, Wang Y, Yang H, Xia Y, Chen
M, Song H, Li T, Li D, Wang J, et al: Comparison of salvage
chemoradiation versus salvage surgery for recurrent esophageal
squamous cell carcinoma after definitive radiochemotherapy or
radiotherapy alone. Dis Esophagus. 27:134–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beitler JJ, Zhang Q, Fu KK, Trotti A,
Spencer SA, Jones CU, Garden AS, Shenouda G, Harris J and Ang KK:
Final results of local-regional control and late toxicity of RTOG
9003: A randomized trial of altered fractionation radiation for
locally advanced head and neck cancer. Int J Radiat Oncol Biol
Phys. 89:13–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohiuddin M, Marks G and Marks J:
Long-term results of reirradiation for patients with recurrent
rectal carcinoma. Cancer. 95:1144–1150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okamoto Y, Murakami M, Yoden E, Sasaki R,
Okuno Y, Nakajima T and Kuroda Y: Reirradiation for locally
recurrent lung cancer previously treated with radiation therapy.
Int J Radiat Oncol Biol Phys. 52:390–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watkins JM, Shirai KS, Wahlquist AE,
Stuart RK, Chaudhary UB, Garrett-Mayer E, Day TA, Gillespie MB and
Sharma AK: Toxicity and survival outcomes of hyperfractionated
split-course reirradiation and daily concurrent chemotherapy in
locoregionally recurrent, previously irradiated head and neck
cancers. Head Neck. 31:493–502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niibe Y, Karasawa K, Mitsuhashi T and
Tanaka Y: Hyperfractionated radiation therapy for hypopharyngeal
carcinoma compared with conventional radiation therapy: Local
control, laryngeal preservation and overall survival. Jpn J Clin
Oncol. 33:450–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niibe Y, Karasawa K, Mitsuhashi T,
Miyashita H and Tanaka Y: Hyperfractionated radiation therapy for
oropharyngeal carcinoma in a Japanese population. Jpn J Clin Oncol.
34:312–315. 2004. View Article : Google Scholar : PubMed/NCBI
|