Introduction
Bladder cancer is one of the most common types of
urinary tract malignancy worldwide (1). It is clinically characterized by its
progression, recurrence, metastasis and drug resistance (2). Notably, due to the lifetime requirement
for monitoring tumor recurrence, the typical cost of bladder cancer
from diagnosis to mortality is the highest among all cancer types
(3). To better understand the
molecular mechanisms of the disease, research is under way so that
novel treatments may be identified. However, therapeutic methods
have remained essentially unchanged over the past three decades,
indicating an urgent need to further research this malignancy
(4).
Interestingly, it has been reported that the
incidence of bladder cancer is 3–4 times greater in men than in
women (5). Lifestyle or
environmental factors, such as cigarette smoke and industrial
chemicals, are believed to be responsible for the gender-specific
disparity in bladder cancer morbidity and aggressiveness (6). However, it remains a preferential
disease in men even after controlling for these carcinogens
(6). Thus, a hypothesis has been
proposed that the androgen receptor (AR) and related signaling
pathways are involved in the etiology and progression of bladder
cancer (7).
AR signals have been demonstrated to correlate with
bladder cancer development and progression both in vitro and
in vivo (7). However, the
correlation between AR expression and its clinical significance
remains controversial in patients with bladder cancer. Some
evidence has indicated that AR expression is related to bladder
cancer pathology grade, clinical stage or prognosis (8–10). While
a multi-institutional study revealed no correlations (11). Thus, the present meta-analysis aimed
to evaluate the expression and clinical significance of AR in
bladder cancer. To the best of our knowledge, this is the first
meta-analysis to investigate the impact of AR expression on bladder
cancer.
Data collection methods
Publication search strategy
In accordance with the Preferred Reporting Items for
Systematic Reviews and Meta-analyses guidelines (12), a systematic review of literature was
performed in January 2017 using PubMed (ncbi.nlm.nih.gov/pubmed), Web of Knowledge (webofknowledge.com), Embase (embase.com)
and the Cochrane Central Search Library (cochranelibrary.com). Search terms used included
‘androgen receptor,’ ‘AR,’ ‘bladder,’ ‘cancer,’ ‘carcinoma’ and
‘tumor.’ All abstracts and review articles on this topic were
reviewed, and references of original studies were identified by
manual search.
Inclusion criteria and exclusion
criteria
Eligible studies had to meet the following selection
criteria: i) Studies had to evaluate the association between AR and
bladder cancer; ii) the report contained key information about AR
expression and bladder cancer susceptibility, tumor grade, lymph
node metastasis recurrence-free survival (RFS) or progression-free
survival (PFS); iii) studies published in English; and iv)
conference abstracts, reviews and letters to editors were not
included. Studies with overlapping or insufficient data were
excluded.
Data extraction
Two independent reviewers (Jinbo Chen and Yu Cui)
extracted the information from eligible studies to the inclusion
criteria. Disagreement was resolved during a consensus with a third
reviewer (Xiongbing Zu). The literature data and demographic were
extracted individually. Odds ratio (OR) and 95% confidence
intervals (CI) were used to estimate the association between AR
expression and bladder cancer susceptibility, tumor grade and
clinical stage. Hazard ratio (HR) and its 95% CIs were used to
elevate the association between AR expression and RFS and PFS. If
available, the HRs with their 95% CIs and P-values were collected
from the original article. If not, HRs and their 95% CIs were
calculated using the data of observed cancer
progression/recurrences, the data of samples in each group or the
data provided by the authors. If only survival curves were
available, data was extracted from the graphical survival plots and
the HRs were estimated (13,14). The quality of studies was evaluated
using the Newcastle-Ottawa Scale (15). Scores of 7–9 were defined as high
quality study, and a score <7 as low quality study.
Statistical analysis
A meta-analysis was performed to assess the
association between AR expression and bladder cancer
susceptibility, tumor grade, tumor stage, RFS as well as PFS.
Statistical heterogeneity was assessed using a formal Q-statistic
as well as I-squared, with the statistical significance level set
at 0.05 (16). A fixed-effects model
was used when no heterogeneity was found; otherwise, the
random-effects model was used to calculate pooled ORs. Publication
bias was evaluated by Egger's (17)
and Begg's (18) test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were implemented in STATA 11.0 statistical
software (StataCorp LP, College Station, TX, USA).
Results
Literature search
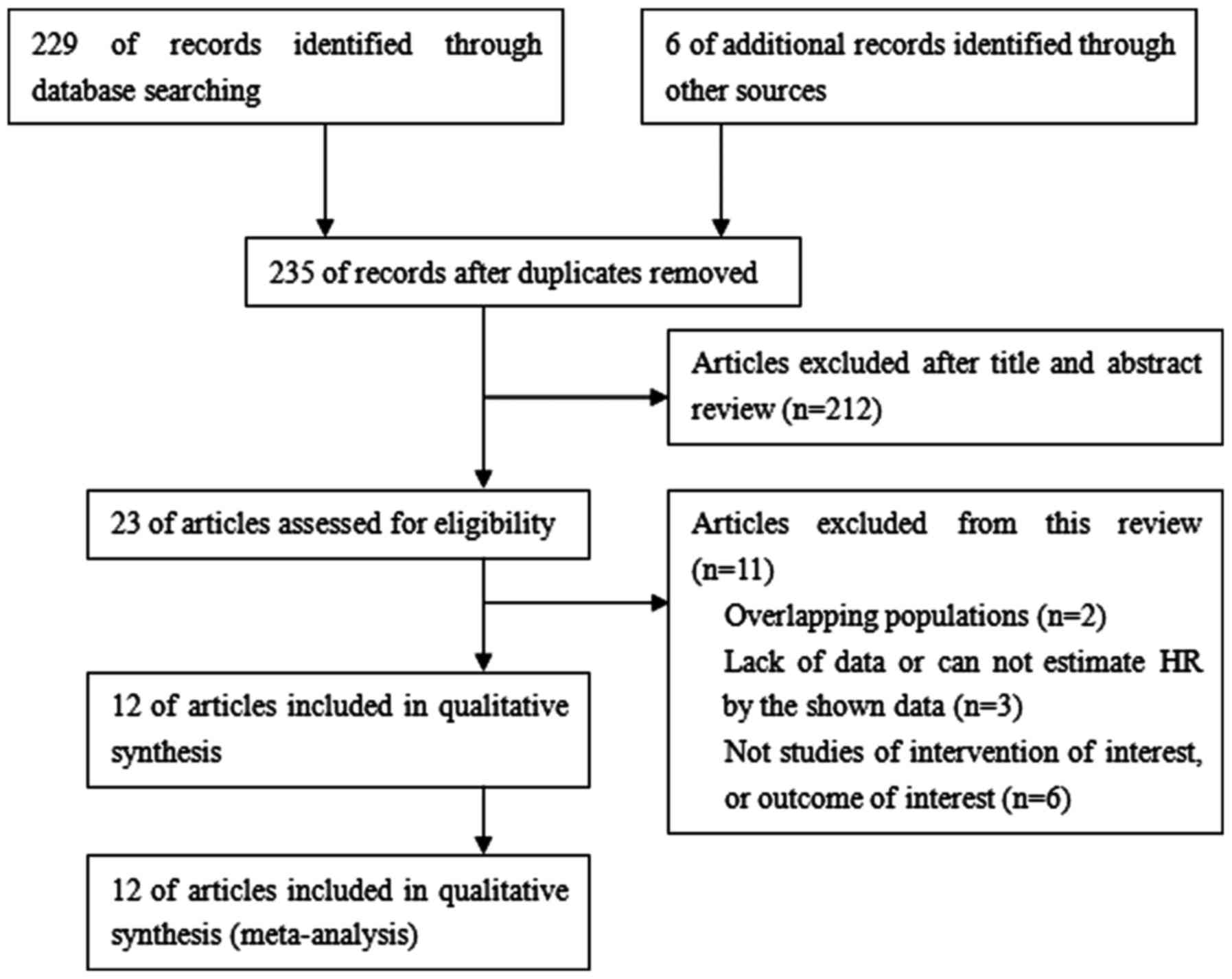
A total of 235 studies were identified from the
database or manual search. According to the selection criteria, 223
studies were excluded, resulting in 12 studies with 1,652 patients
for analysis. A flow chart of article selection summarizes the main
characteristics of included studies, as demonstrated in Fig. 1. Finally, data were available from
five studies on AR expression and bladder cancer susceptibility
(568 tumor cases vs. 523 normal controls), six studies on AR
expression and tumor grade (387 low grade vs. 663 high grade
cases), nine studies on AR expression and tumor stage (582
non-invasive vs. 712 invasive cases), five studies on AR expression
and RFS (414 cases) and four studies on AR expression and PFS (319
cases). The essential information of the included studies was
listed in Table I (8–11,19–26).
 | Table I.Androgen receptor expression and its
association with clinicopathological features and prognosis in
bladder cancer. |
Table I.
Androgen receptor expression and its
association with clinicopathological features and prognosis in
bladder cancer.
|
|
|
|
| Tumor vs.
non-tumor, n (%) | Tumor grade, n
(%) | Tumor stage, n
(%) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Authors, year | Nation | n | Method | Tumor | Non-tumor | Low | High | NI | MI | Prognosis | (Refs.) |
|---|
| Zhuang et
al, 1997 | Finland |
9 | IHC
scorea | 4/9 (44.4) | NA | NA | NA | 1/5 (20.0) | 3/4 (75.0) | NA | (8) |
| Boorjian et
al, 2004 | USA | 49 | IHC
scoreb | 26/49 (53.1) | 32/37 (86.5) | 8/9 (88.9) | 16/33 (48.5) | 21/28 (75.0) | 3/14 (21.4) | NA | (9) |
| Boorjian et
al, 2009 | USA | 55 | IHC
(5%)c | 24/55 (43.6) | NA | NA | NA | 13/22 (59.1) | 11/33 (33.3) | NA | (10) |
| Mir et al,
2011 | USA | 472 | IHC
scoreb,d | 61/472 (12.9) | NA | 11/90 (12.2) | 50/382 (13.1) | 15/167 (9.0) | 46/305 (15.1) | RFS | (11) |
| Tuygun et
al, 2011 | Turkey | 139 | IHC
(10%)c | 71/139 (51.1) | 0/58 (0) | 46/72 (63.9) | 25/67 (37.3) | 64/106 (60.4) | 7/33 (21.2) | RFS/PFS | (19) |
| Zheng et al,
2011 | USA | 24 | IHC
scored | 8/24 (33.3) | NA | NA | NA | 2/5 (40.0) | 6/19 (31.6) | PFS | (20) |
| Miyamoto et
al, 2012 | USA | 188 | IHC
scored | 79/188 (42.0) | 113/141 (80.1) | 31/56 (55.4) | 48/132 (36.4) | 49/97 (50.5) | 30/91 (33.0) | RFS/PFS | (21) |
| Mashhadi et
al, 2014 | Iran | 120 | IHC
(10%)c | 26/120 (21.7) | 0/132 (0) | NA | NA | NA | NA | NA | (22) |
| Nam et al,
2014 | Korea | 169 | IHC
(10%)c | 63/169 (37.3) | NA | 47/120 (39.2) | 16/49 (32.7) | NA | NA | RFS/PFS | (23) |
| Jing et al,
2014 | China | 58 | IHC
scored | 31/58 (53.4) | NA | 22/40 (55.0) | 9/18 (50.0) | 22/45 (48.9) | 9/13 (69.2) | NA | (24) |
| Williams et
al, 2015 | USA | 297 | IHC
scorea | 73/297 (24.6) | NA | NA | NA | 36/107 (33.6) | 37/190 (19.5) | NA | (25) |
| Izumi et al,
2016 | Japan | 72 | IHC
(10%)c | 44/72 (61.1) | 35/42 (83.3) | NA | NA | NA | NA | RFS | (26) |
AR expression and bladder cancer
susceptibility
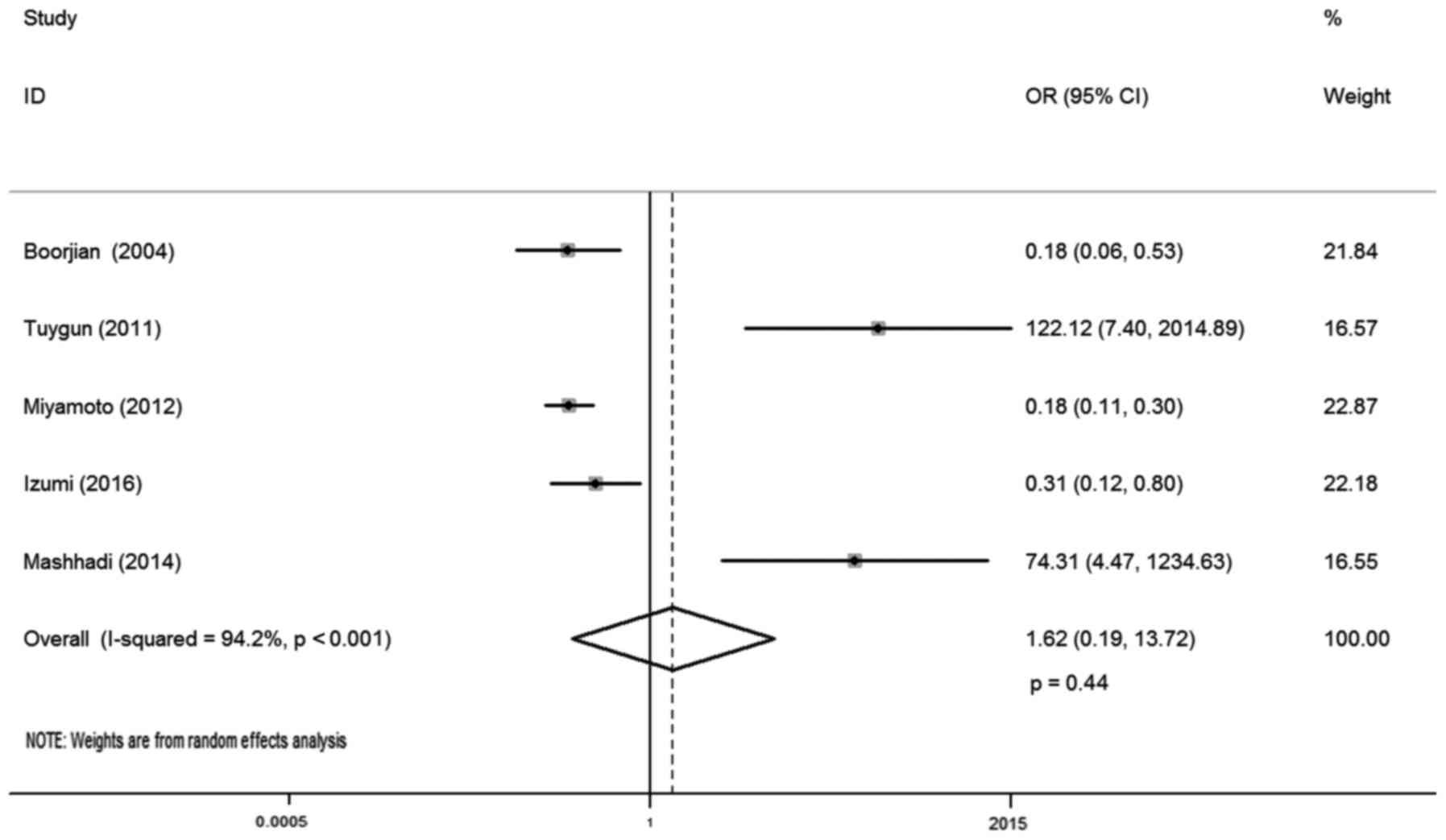
In total, five studies reported AR expression and
bladder cancer susceptibility, involving 1,091 samples (568
urothelial tumor vs. 523 normal urothelium tissues). There was
statistical heterogeneity between these trials (I-squared=94.2%;
P<0.001), so a random-effects model was used in the analysis.
The reason for this may be that AR positive and negative cases in
the control group of these literatures were different. The district
and ethnicity differences may contribute to this heterogeneity, and
so subgroup analysis was conducted according to ethnicity. Overall,
no significant association was observed between AR expression and
bladder cancer susceptibility (OR, 1.62; 95% CI, 0.19–13.72;
P=0.44; Fig. 2). Subgroup analysis
by different ethnicity demonstrated that AR expression had no
significant association with bladder cancer susceptibility both for
Caucasian (OR, 1.13; 95% CI, 0.05–24.47; P=0.98) and Asian patients
(OR, 4.34; 95% CI, 0.01–3544.09; P=0.66; Table II).
 | Table II.Subgroup analysis for androgen
receptor and clinicopathological features and prognosis in bladder
cancer. |
Table II.
Subgroup analysis for androgen
receptor and clinicopathological features and prognosis in bladder
cancer.
|
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|---|
| Category | n | Odds ratio | 95% confidence
interval | P-value | I-squared, % | P-value |
|---|
| Susceptibility due
to ethnicity |
|
|
|
|
|
|
|
Caucasian | 3 | 1.13 |
0.05–24.47 | 0.98 | 95.30 | <0.01 |
|
Asian | 2 | 4.34 |
0.01–3544.09 | 0.66 | 95.10 | <0.01 |
| Tumor grade and
ethnicity |
|
|
|
|
|
|
|
Caucasian | 4 | 1.95 | 1.36–2.81 | <0.01 | 60.90 | 0.05 |
|
Asian | 2 | 1.30 | 0.72–2.35 | 0.39 | 0 | 0.9 |
| Classification
system |
|
|
|
|
|
|
| 2004
WHO | 4 | 1.94 | 1.35–2.80 | <0.01 | 11 | 0.34 |
| 1998
WHO | 2 | 1.25 | 0.68–2.32 | 0.47 | 72.80 | 0.05 |
| Tumor stage and
ethnicity |
|
|
|
|
|
|
|
Caucasian | 8 | 2.06 | 1.02–4.16 | 0.04 | 77 | <0.01 |
|
Asian | 1 | 0.43 | 0.11–1.58 | 0.20 | NA | NA |
| Recurrence-free
survival and ethnicity |
|
|
|
|
|
|
|
Caucasian | 3 | 0.48a | 0.31–0.75 | 0.01 | 0 | 0.42 |
|
Asian | 2 | 1.13a | 0.87–1.47 | 0.74 | 47.70 | 0.125 |
| Progression-free
survival and ethnicity |
|
|
|
|
|
|
|
Caucasian | 3 | 1.31a | 0.92–1.85 | 0.13 | 55 | 0.07 |
|
Asian | 1 | 0.61a | 0.23–1.62 | 0.32 | NA | NA |
AR expression and tumor grade
A total of six studies investigated the relationship
between AR expression and bladder cancer grade, including 1,050
cases. No statistical heterogeneity between trials was identified
(I-squared=43.9%; P=0.113), so a fixed-effects model was used in
the analysis. Overall, the AR expression was positively correlated
with low bladder cancer grade (OR, 1.74; 95% CI, 1.27–2.37;
P<0.01; Fig. 3). Subgroup
analysis by different ethnicities demonstrated that AR expression
correlated with low bladder cancer grade for Caucasian patients
(OR, 1.95; 95% CI, 1.36–2.81; P<0.01). However, such association
was not observed for Asian populations (OR, 1.30; 95% CI,
0.72–2.35; P=0.39). Subgroup analysis by different pathology
classification systems demonstrated that AR expression correlated
with low bladder cancer grade for the 2004 World Health
Organization (WHO) classification system (OR, 1.94; 95% CI,
1.35–2.80; P<0.01). However, such relationship was not found for
the 1998 WHO classification system (OR, 1.25; 95% CI, 0.68–2.32;
P=0.47; Table II).
AR expression and tumor stage
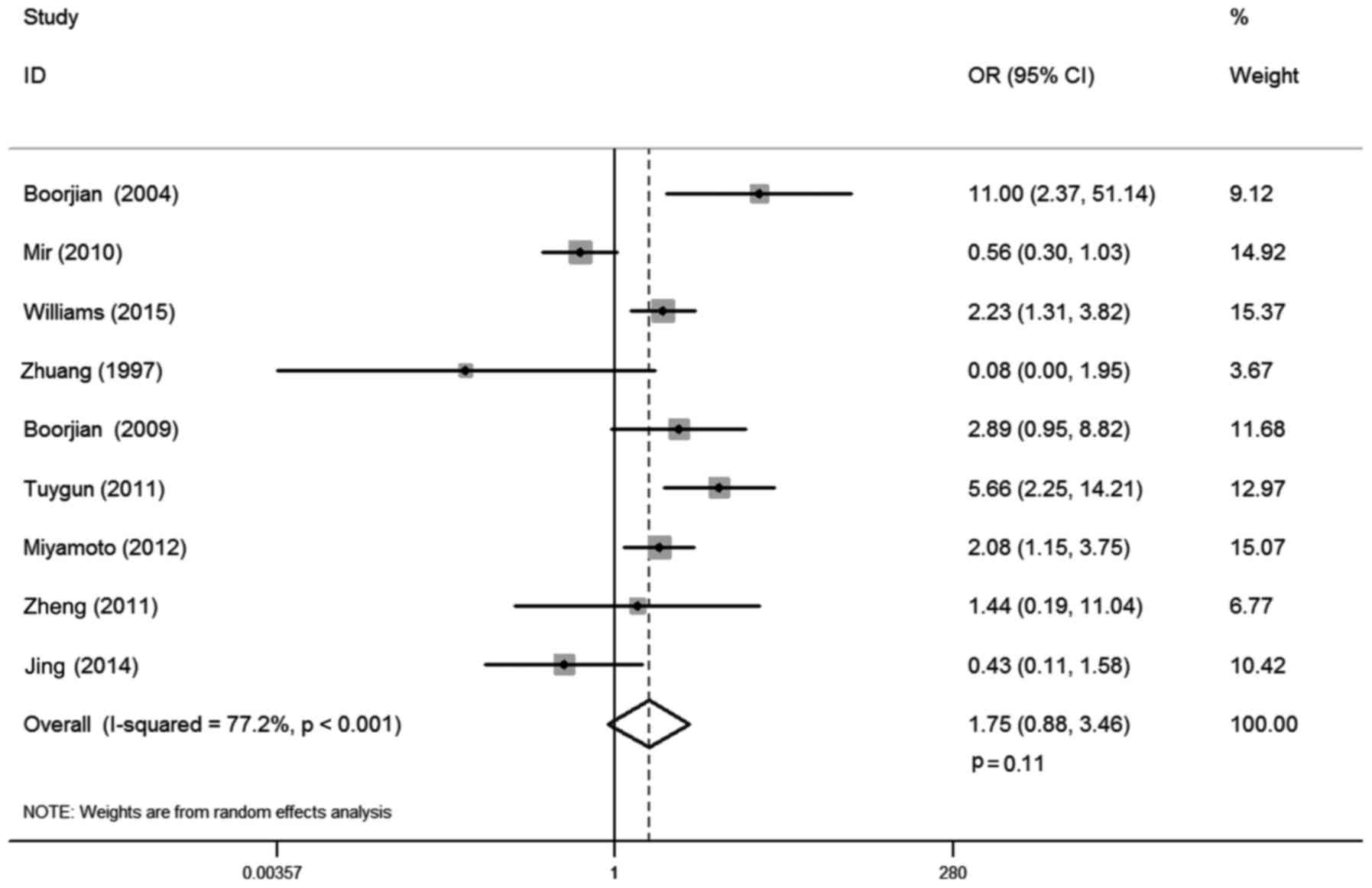
There were 9 studies that reported the correlation
between AR expression and bladder cancer stage, involving 1,294
cases. Statistical heterogeneity was observed between trials
(I-squared=77.2%; P<0.001). Thus, a random-effects model was
used in the analysis. The district and ethnicity differences may
contribute to this heterogeneity, so subgroup analysis according to
ethnicity was conducted. Overall, no significant association was
demonstrated between AR expression and tumor stage (OR, 1.75; 95%
CI, 0.88–3.46; P=0.11; Fig. 4).
Subgroup analysis by ethnicity demonstrated that AR expression was
positively correlated with non-invasive tumors compared with muscle
invasive stage for Caucasian patients (OR, 2.06; 95% CI, 1.02–4.16;
P=0.04), whereas such association was not found in Asian
populations (OR, 0.43; 95% CI, 0.11–1.58; P=0.20; Table II).
AR expression and RFS
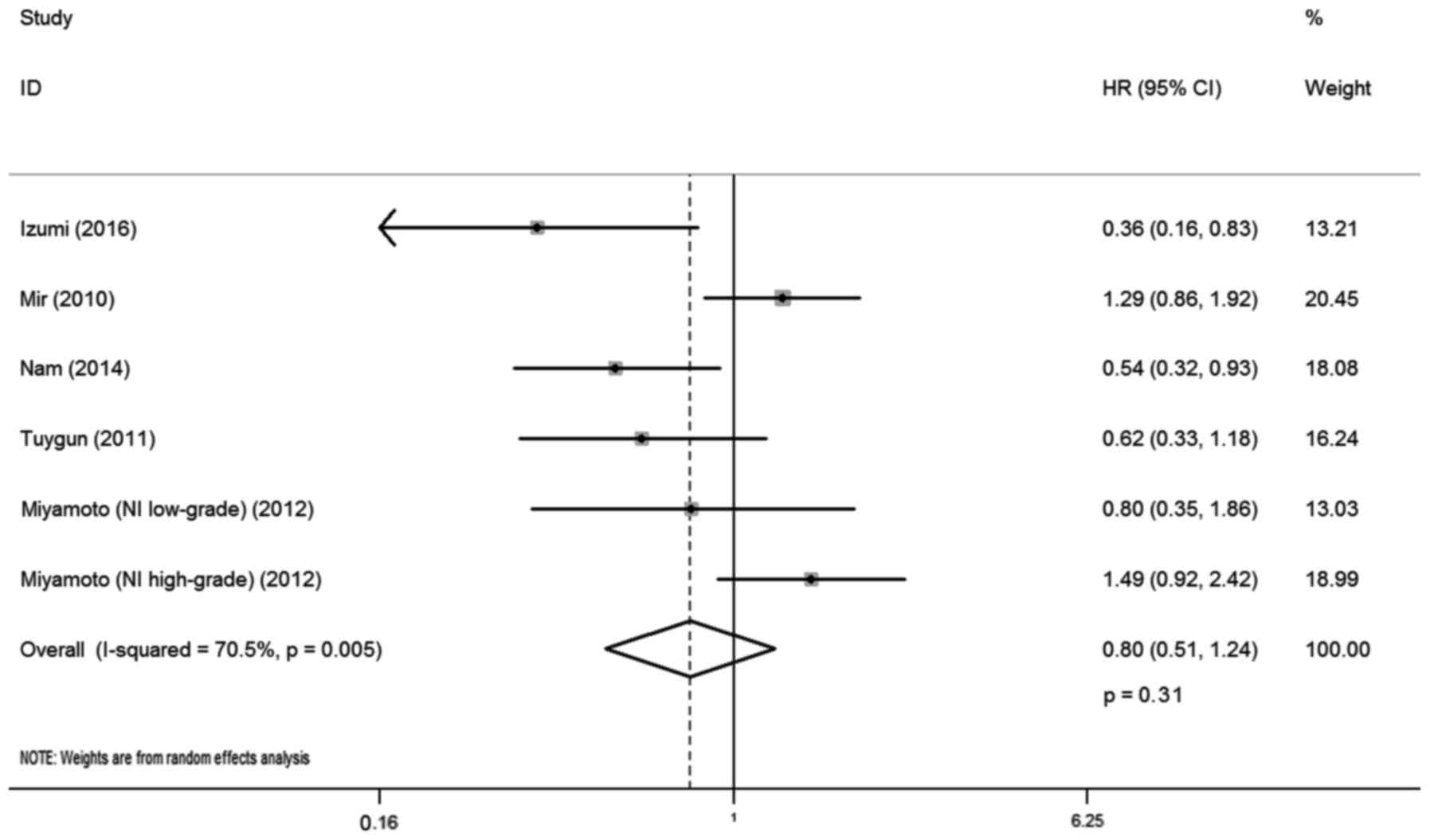
A total of five studies reported the correlation
between AR expression and RFS, involving 414 cases. There was
statistical heterogeneity between trials (I-squared=70.5%;
P=0.005), and so a random-effects model was used in the analysis.
The district and ethnicity differences as well as the limited
number of patients included in the studies may have contributed to
this heterogeneity; therefore, subgroup analysis according to
ethnicity was conducted. Overall, no significant association was
observed between AR expression and RFS (HR, 0.80; 95% CI,
0.51–1.24; P=0.31; Fig. 5). Subgroup
analysis by ethnicity indicated that AR expression was correlated
with lower recurrence rate for Caucasian patients with bladder
cancer (HR, 0.48; 95% CI, 0.31–0.75; P=0.01), whereas such
association was not observed for Asian patients (HR, 1.13; 95% CI,
0.87–1.47; P=0.74).
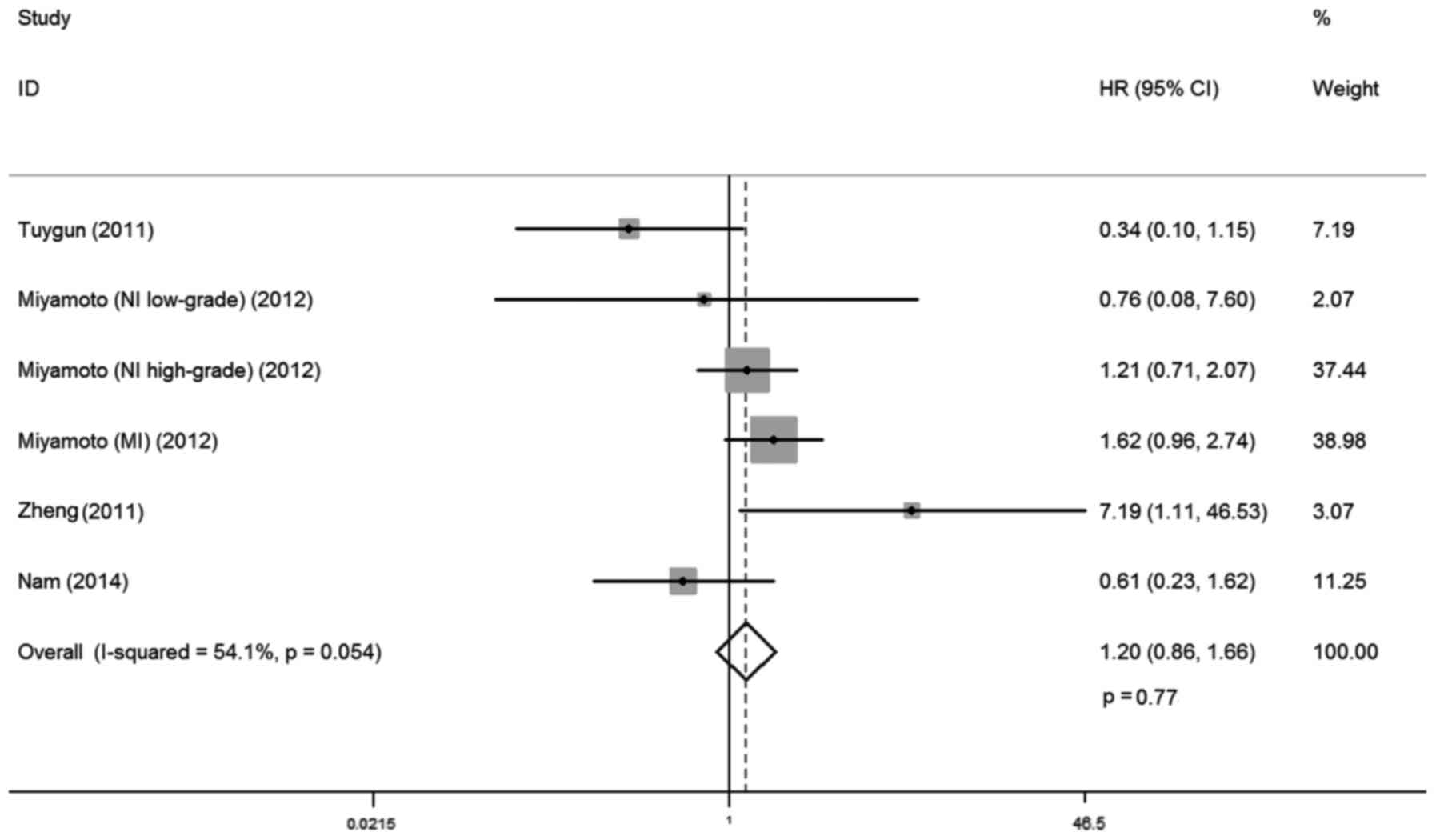
AR expression and PFS
A total of four studies investigated the
relationship between AR expression and PFS, involving 319 cases.
There was no statistical heterogeneity between trials
(I-squared=54.1%; P=0.054), so a fixed-effects model was used in
the analysis. Overall, no significant association was observed
between AR expression and PFS (HR, 1.20; 95% CI, 0.86–1.66; P=0.77;
Fig. 6) Subgroup analysis by
ethnicity demonstrated that AR expression was not correlated with
progression free rates for Caucasian and Asian patients with
bladder cancer (HR, 1.31; 95% CI, 0.92–1.85; P=0.13; and HR, 0.61;
95% CI, 0.23–1.62; P=0.32, respectively; Table II).
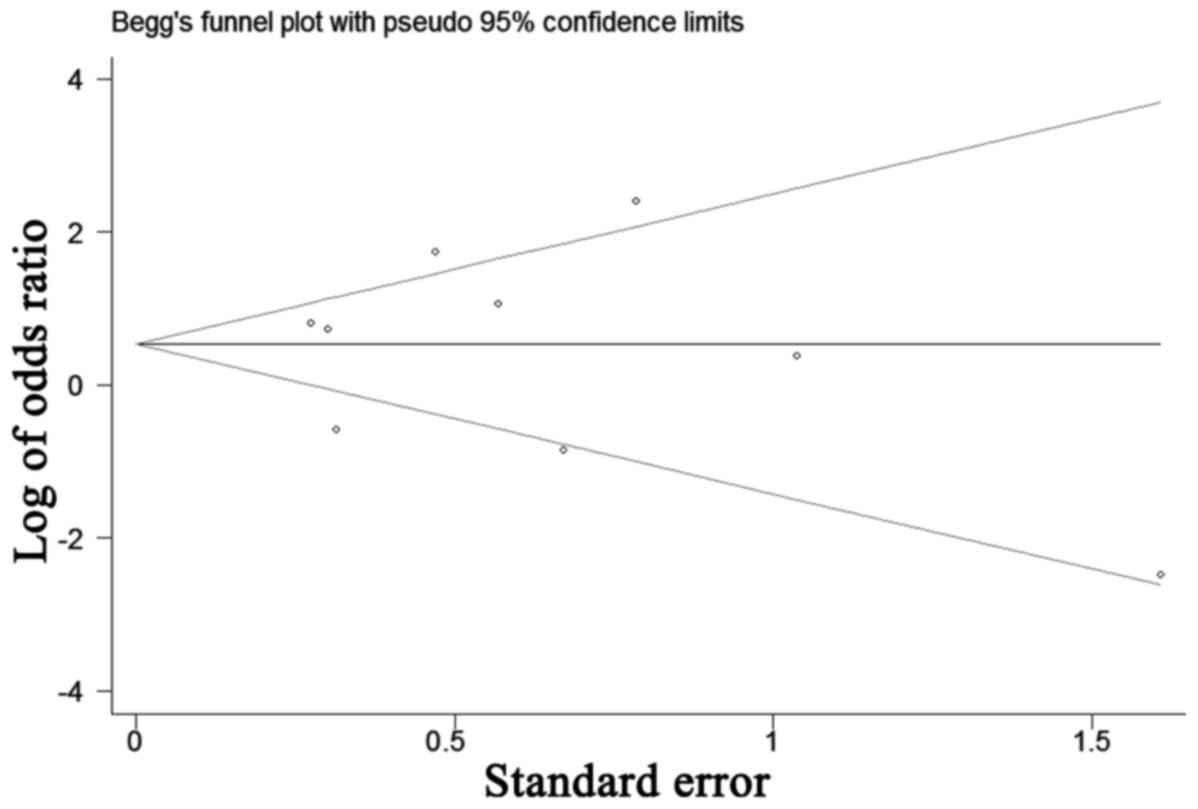
Publication bias
The funnel plot for the relationship between AR
expression and tumor stage was demonstrated in Fig. 7. P-values for Begg's adjusted rank
correlation test was 0.75 and the Egger's regression asymmetry test
was 0.48. The results did not reveal any evidence of publication
bias.
Discussion
The AR gene, located in the X chromosome (q11-12),
is a member of the nuclear receptor superfamily (27). As a transcription factor, it mediates
physiological activities by binding to androgen. It has been
demonstrated that androgen signaling has been linked to regulation
of proliferation, motility and cell death in urinary malignancies
(28,29). Importantly, it has essential roles in
malignancies of the prostate, bladder, kidney, lung, breast and
liver (30–32). To date, aiming to investigate the
possible role of AR in bladder cancer, various studies have defined
AR expression patterns and functions in these patients' samples
(8–11). However, due to diversity in sample
sizes, ethnicity, immunohistochemistry (IHC) techniques, scoring
systems and interventions, the results remain inconsistent.
Therefore, the present in-depth meta-analysis aimed to reveal the
correlation between AR expression and clinicopathological features,
as well as prognosis in patients with bladder cancer.
Notable efforts have been made to study AR
expression in bladder cancer tissues compared with normal bladder
samples. However, there is large discrepancy among the results of
these studies. In the present meta-analysis, the AR positive rate
ranged from 12.9–61.1% in bladder cancer tissues, while this rate
ranged from 0–86.5% in normal bladder urothelium. Studies by Tuygun
et al (19) and Mashhadi
et al (22) revealed that AR
expression was much higher in bladder cancer tissues, compared with
0% positivity in normal tissues. However, data from studies by
Boorjian et al (9), Miyamoto
et al (21) and Izumi et
al (26) demonstrated that the
AR positive rate was lower in bladder cancer tissues than in normal
bladder samples. The present meta-analysis with five studies and
1,091 samples demonstrated no significant association between AR
expression and bladder cancer susceptibility.
Since cancer biomarker expression may increase in
the early stage and decrease in later periods, AR expression in
different tumor grades and stages was further investigated in the
present meta-analysis. Unexpectedly, the correlation results
between AR expression and bladder cancer development have not
reached a consensus. Tuygun et al (19) demonstrated that AR expression
negatively correlated with tumor grade and clinical stage in 139
patients. Also, this relationship was indicated by Miyamoto et
al (21). In contrast, a
multi-institutional study by Mir et al (11) reported that AR expression was not
associated with pathological grade and stage in bladder cancer.
Concluding these data, the present subgroup meta-analysis indicated
that AR expression was positively correlated with low tumor grade
and non-invasive tumor stage for Caucasian patients, while
significant associations for tumor grade and stage were not
observed in Asian patients.
Furthermore, the pathological grading system of
bladder cancer was updated in 2004 (33). Subgroup analysis based on this new
classification system in the present meta-analysis demonstrated
that AR expression had a positive correlation with low bladder
cancer grade. Similarly, subgroup analysis indicated that AR
expression was positively correlated with non-muscle invasive
tumors, compared with muscle invasive tumors in the Caucasian
population. These results revealed that AR expression decreased
with increasing grade and stage, indicating that low AR expression
was associated with bladder cancer development in the specified
population and classification system.
Bladder cancer is a disease of high recurrence and
easy progression (6). Thus, it is
necessary to monitor tumor recurrence throughout a patient's
lifetime (34). Unfortunately, ~70%
of patients presenting with superficial bladder tumor develop
recurrence and 10–20% of these patients progress to muscle invasive
bladder cancer (35). Recently, the
controversial relationship between AR expression and bladder cancer
prognosis has been reported. Studies by Nam et al (23) and Izumi et al (26) indicated that AR-positive patients had
a significantly lower risk of tumor recurrence compared with those
with AR-negative tumors. Contrastingly, Mir et al (11) demonstrated that loss of AR expression
was not associated with clinical outcome, including RFS.
Interestingly, Miyamoto et al (21) divided bladder patients into
non-invasive low grade, non-invasive high grade and muscle invasive
groups, and analyzed AR expression and RFS and PFS in these groups.
The present subgroup analysis demonstrated that AR expression was
positively correlated with lower recurrence rates in Caucasian
patients with bladder cancer; however, its expression had no
significant impact on PFS. As cases included in the present
analysis were limited, more studies are required to clarify AR
expression and its prognostic role in bladder cancer in the
future.
Several limitations should be noted in the present
meta-analysis. First, all data included were from retrospective
studies. This may potentially enlarge certain bias, such as
selection bias. Second, case quantities in some studies were
confined, particularly for the Asian population. Third, publication
on AR expression and prognosis of overall survival was not
sufficient, which restrained our ability to conduct analysis.
Forth, the standard for IHC positive staining between the studies
was not consistent, which may cause some heterogeneity.
Furthermore, several HRs for RFS and PFS were calculated based on
the data extracted from the survival curve, and this may also
induce some errors.
In conclusion, the present study provided evidence
for correlations between AR expression and clinicopathological
features, as well as prognosis in patients with bladder cancer.
Although heterogeneity exists in the included studies, the present
meta-analysis demonstrated that AR expression was correlated with
tumor grade, clinical stage and recurrence rates in the specified
population and classification system. No association was observed
between AR and bladder cancer susceptibility or PFS. Accordingly,
further mechanistic studies are required to determine the precise
functional role of AR signaling in the development and progression
of bladder cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572523) and the
Fundamental Research Funds for the Central Universities of Central
South University (grant no. 2016zzts121).
Glossary
Abbreviations
Abbreviations:
|
AR
|
androgen receptor
|
|
RFS
|
recurrence-free survival
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
OR
|
odds ratio
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
IHC
|
immunohistochemistry
|
|
NI
|
non-invasive
|
|
MI
|
muscle invasive
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herr HW: Transurethral resection of
muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol.
19:89–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sievert KD, Amend B, Nagele U, Schilling
D, Bedke J, Horstmann M, Hennenlotter J, Kruck S and Stenzl A:
Economic aspects of bladder cancer: What are the benefits and
costs? World J Urol. 27:295–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carneiro BA, Meeks JJ, Kuzel TM, Scaranti
M, Abdulkadir SA and Giles FJ: Emerging therapeutic targets in
bladder cancer. Cancer Treat Rev. 41:170–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dobruch J, Daneshmand S, Fisch M, Lotan Y,
Noon AP, Resnick MJ, Shariat SF, Zlotta AR and Boorjian SA: Gender
and bladder cancer: A collaborative review of etiology, biology,
and outcomes. Eur Urol. 69:300–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Izumi K and Miyamoto H: The role of
the androgen receptor in the development and progression of bladder
cancer. Jpn J Clin Oncol. 42:569–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuang YH, Bläuer M, Tammela T and
Tuohimaa P: Immunodetection of androgen receptor in human urinary
bladder cancer. Histopathology. 30:556–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boorjian S, Ugras S, Mongan NP, Gudas LJ,
You X, Tickoo SK and Scherr DS: Androgen receptor expression is
inversely correlated with pathologic tumor stage in bladder cancer.
Urology. 64:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boorjian SA, Heemers HV, Frank I, Farmer
SA, Schmidt LJ, Sebo TJ and Tindall DJ: Expression and significance
of androgen receptor coactivators in urothelial carcinoma of the
bladder. Endocr Relat Cancer. 16:123–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mir C, Shariat SF, van der Kwast TH,
Ashfaq R, Lotan Y, Evans A, Skeldon S, Hanna S, Vajpeyi R, Kuk C,
et al: Loss of androgen receptor expression is not associated with
pathological stage, grade, gender or outcome in bladder cancer: A
large multi-institutional study. BJU Int. 108:24–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Int J Surg. 8:336–341.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egger M, Smith Davey G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuygun C, Kankaya D, Imamoglu A, Sertcelik
A, Zengin K, Oktay M and Sertcelik N: Sex-specific hormone
receptors in urothelial carcinomas of the human urinary bladder: A
comparative analysis of clinicopathological features and survival
outcomes according to receptor expression. Urol Oncol. 29:43–51.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng Y, Izumi K, Yao JL and Miyamoto H:
Dihydrotestosterone upregulates the expression of epidermal growth
factor receptor and ERBB2 in androgen receptor-positive bladder
cancer cells. Endocr Relat Cancer. 18:451–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu
I, Izumi K, Chang C, Messing EM, Netto GJ and Yeh S: Expression of
androgen and Oestrogen receptors and its prognostic significance in
urothelial neoplasm of the urinary bladder. BJU Int. 109:1716–1726.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mashhadi R, Pourmand G, Kosari F, Mehrsai
A, Salem S, Pourmand MR, Alatab S, Khonsari M, Heydari F, Beladi L
and Alizadeh F: Role of steroid hormone receptors in formation and
progression of bladder carcinoma: A case-control study. Urol J.
11:1968–1973. 2014.PubMed/NCBI
|
|
23
|
Nam JK, Park SW, Lee SD and Chung MK:
Prognostic value of sex-hormone receptor expression in
non-muscle-invasive bladder cancer. Yonsei Med J. 55:1214–1221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jing Y, Cui D, Guo W, Jiang J, Jiang B, Lu
Y, Zhao W, Wang X, Jiang Q, Han B and Xia S: Activated androgen
receptor promotes bladder cancer metastasis via Slug mediated
epithelial-mesenchymal transition. Cancer Lett. 348:135–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams EM, Higgins JP, Sangoi AR,
McKenney JK and Troxell ML: Androgen receptor immunohistochemistry
in genitourinary neoplasms. Int Urol Nephrol. 47:81–85. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izumi K, Ito Y, Miyamoto H, Miyoshi Y, Ota
J, Moriyama M, Murai T, Hayashi H, Inayama Y, Ohashi K, et al:
Expression of androgen receptor in non-muscle-invasive bladder
cancer predicts the preventive effect of androgen deprivation
therapy on tumor recurrence. Oncotarget. 7:14153–14160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CS, Kokontis J and Liao ST:
Molecular cloning of human and rat complementary DNA encoding
androgen receptors. Science. 240:324–326. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Culig Z and Santer FR: Androgen receptor
co-activators in the regulation of cellular events in prostate
cancer. World J Urol. 30:297–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lombard AP and Mudryj M: The emerging role
of the androgen receptor in bladder cancer. Endocr Relat Cancer.
22:R265–R277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang C, Lee SO, Yeh S and Chang TM:
Androgen receptor (AR) differential roles in hormone-related tumors
including prostate, bladder, kidney, lung, breast and liver.
Oncogene. 33:3225–3234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan Z, Li C, Fan J, He D and Li L:
Androgen receptor (AR) signaling promotes RCC progression via
increased endothelial cell proliferation and recruitment by
modulating AKT→ NF-κB →CXCL5 signaling. Sci Rep. 6:370852016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyamoto H, Yang Z, Chen YT, Ishiguro H,
Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, et al:
Promotion of bladder cancer development and progression by androgen
receptor signals. J Natl Cance Inst. 99:558–568. 2007. View Article : Google Scholar
|
|
33
|
Lopez-Beltran A and Montironi R:
Non-invasive urothelial neoplasms: According to the most recent WHO
classification. Eur Urol. 46:170–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Qi XJ, Cao YW, Wang YH, Yang XC,
Shao SX and Niu HT: Bladder tumor heterogeneity: The impact on
clinical treatment. Urol Int. 95:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
European Association of Urology: EAU guidelines on muscle-invasive
and metastatic bladder cancer: Summary of the 2013 guidelines. Eur
Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|