Introduction
Primary cardiac neoplasms are rare, with an
incidence of ~0.2%. Primary cardiac angiosarcoma (PCA) comprises 2%
of all primary cardiac neoplasms (including benign tumors) and is
the most common primary malignant cardiac tumor (1,2). PCA
typically presents between the third and fifth decades of life,
most often arising in the right atrium (RA) and infiltrating the
pericardium, which may cause right-sided heart failure or
tamponade, usually with superimposed systemic signs, such as fever,
night sweats, chills, fatigue and weight loss. Pericardiocentesis
yields bloody fluid that often does not contain malignant cells,
even when the tumor cells have invaded the pericardium (3). Diagnostic assessment includes tissue
biopsy followed by histological confirmation, transthoracic
echocardiography (TTE) to determine the tumor dimensions,
pericardial status and cardiac function, computed tomography (CT)
imaging to exclude metastatic disease, magnetic resonance imaging
(MRI) to depict the extracardiac extent of the disease and
delineate the extent of the primary lesion; positron emission
tomography (PET) imaging may also be useful for detecting
metastases when radical surgery is planned (2,4).
However, even radical surgery often yields unsatisfactory results,
as >90% of the patients succumb to the disease within 1 year
(5). Comprehensive treatment
includes neoadjuvant or adjuvant chemotherapy, radiotherapy or
targeted therapy with complete surgical resection; even orthotopic
heart transplantation may prove beneficial for the patients
(6). We herein report a case of
unresectable PCA originating in the RA. The patient received
first-line chemotherapy with weekly paclitaxel, and second-line
therapy with vinorelbine and bevacizumab when the disease
progressed. The relevant literature was also reviewed, to compare
and summarize the treatment of unresectable locally advanced or
metastatic PCA.
Case report
In June 2015, a healthy 41-year-old Chinese woman
complained of a 2-month progressive shortness of breath and chest
discomfort for no apparent reason, which was relieved by rest.
There was no precordialgia, no radiating back pain, no headache or
dizziness, and no edema in the lower extremities. The previous
medical history revealed that the patient had presented with
unexplained pericardial effusion and underwent pericardiocentesis;
however, analysis of the pericardial fluid failed to determine the
etiology. On auscultation, there were no heart murmurs or lung
rales, with the exception of a pericadial rub. The results of the
laboratory tests were as follows: Carbohydrate antigen (CA)125
435.70 U/ml (normal, <35.00 U/ml), CA199 12.70 U/ml (normal,
<35.00 U/ml), carcinoembryonic antigen (CEA)1.69 ng/ml (normal,
<5.00 ng/ml) and neuron-specific enolase 32.51 ng/ml (normal,
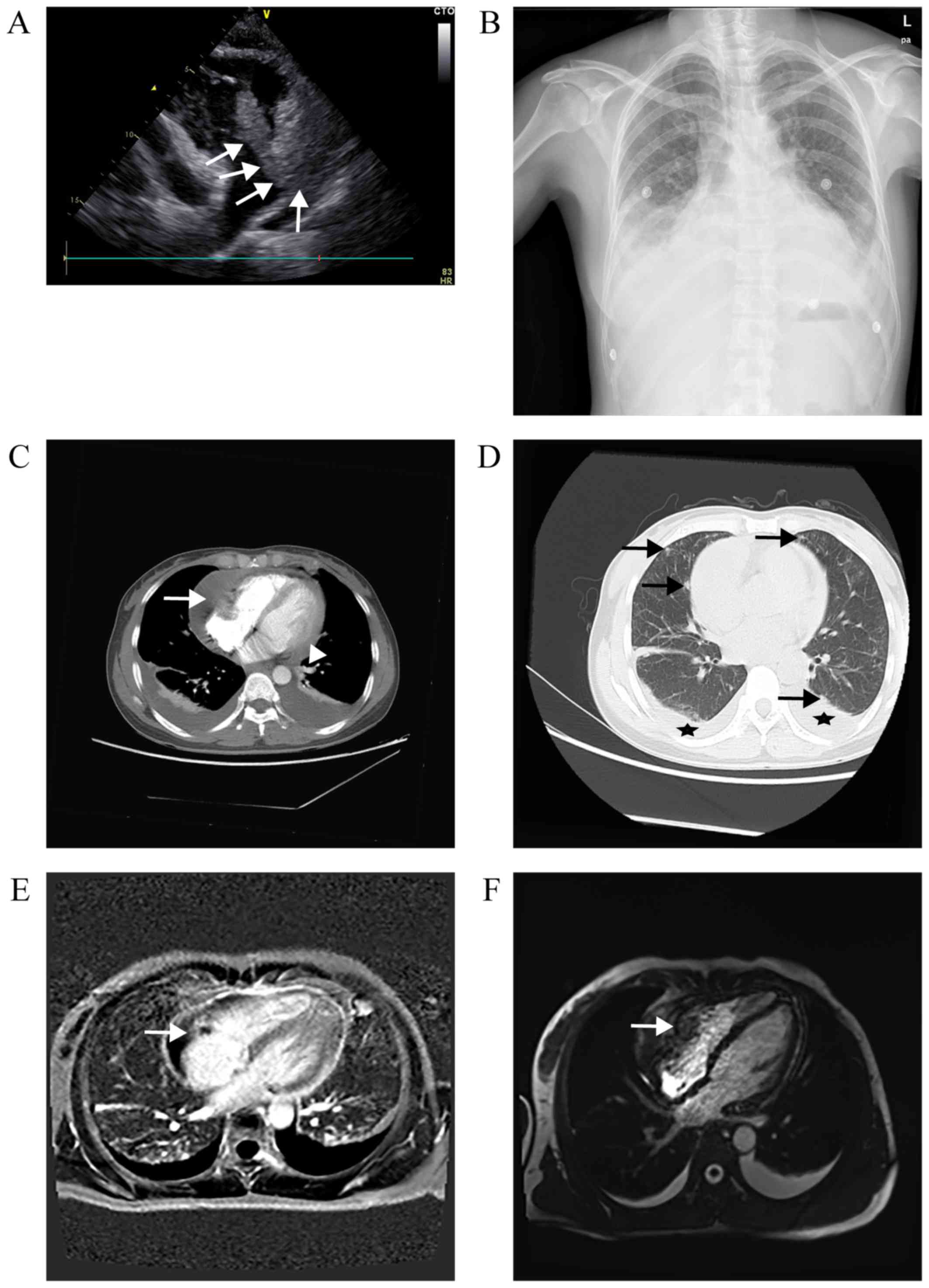
<17.00 ng/ml). TTE revealed a large mass (6.7×3.7 cm),
originating from the free wall of the RA, of which the lower part
entered the right ventricle (RV) during diastole (Fig. 1A), accompanied by a massive
pericardial effusion. Chest X-ray revealed expansion of the heart
shadow (cardiothoracic ratio, 70%) and bilateral pleural effusion
(blunting of the bilateral costophrenic angle; Fig. 1B). A CT scan confirmed the presence
of a heterogeneous irregularly shaped mass infiltrating the RA, and
also detected bilateral pulmonary nodules (Fig. 1C and D). On MRI, the mass exhibited
heterogeneous signal intensity enhancement on T1-weighted images,
and flow void on T2-weighted images (Fig. 1E and F). The
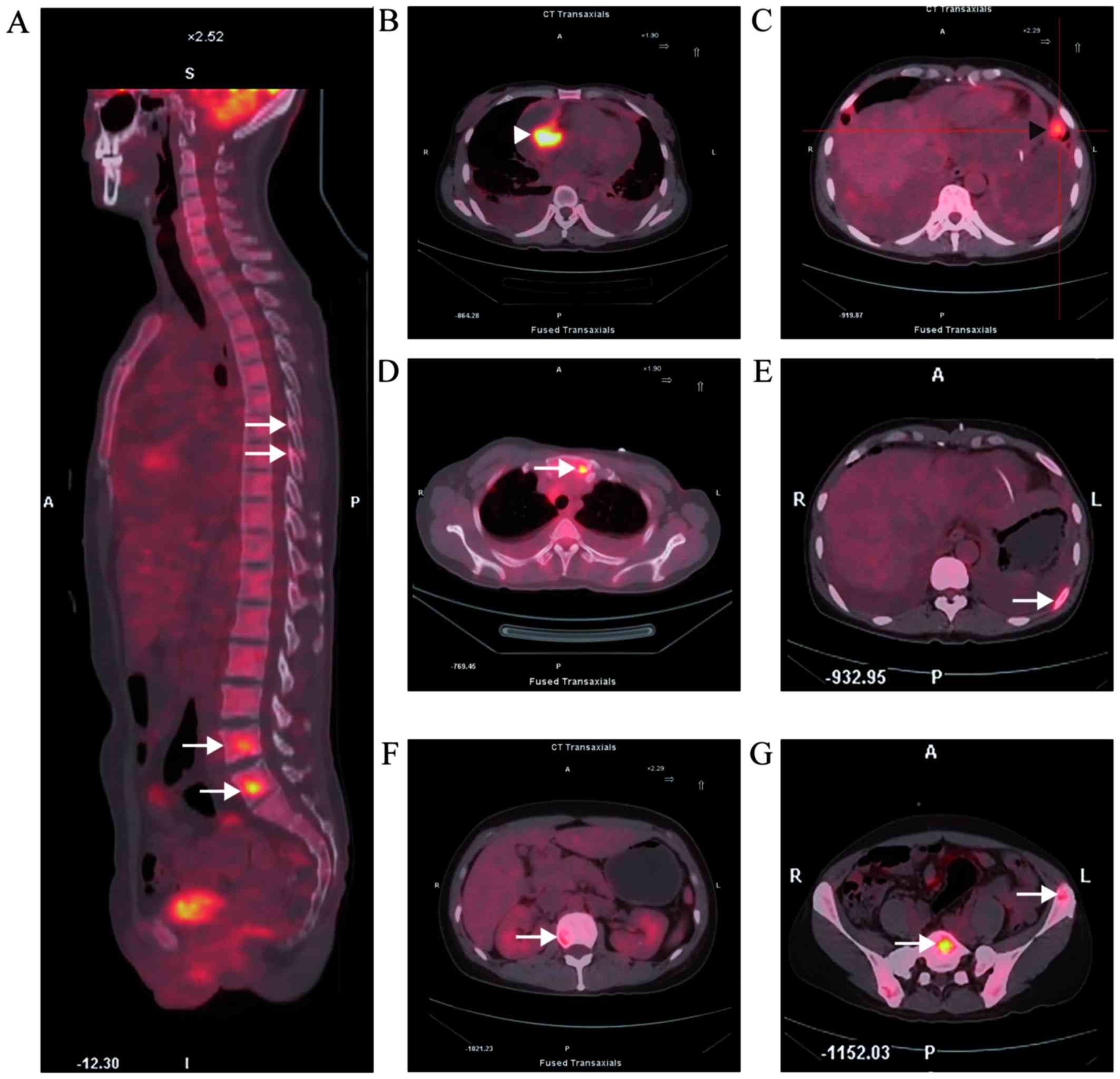
18F-fluorodeoxyglucose uptake in the tumor reached a
standardized uptake value of 13.6 (Fig.
2B). PET-CT also revealed tumor metastasis to multiple organs,
including the lungs and bones (Fig. 2A
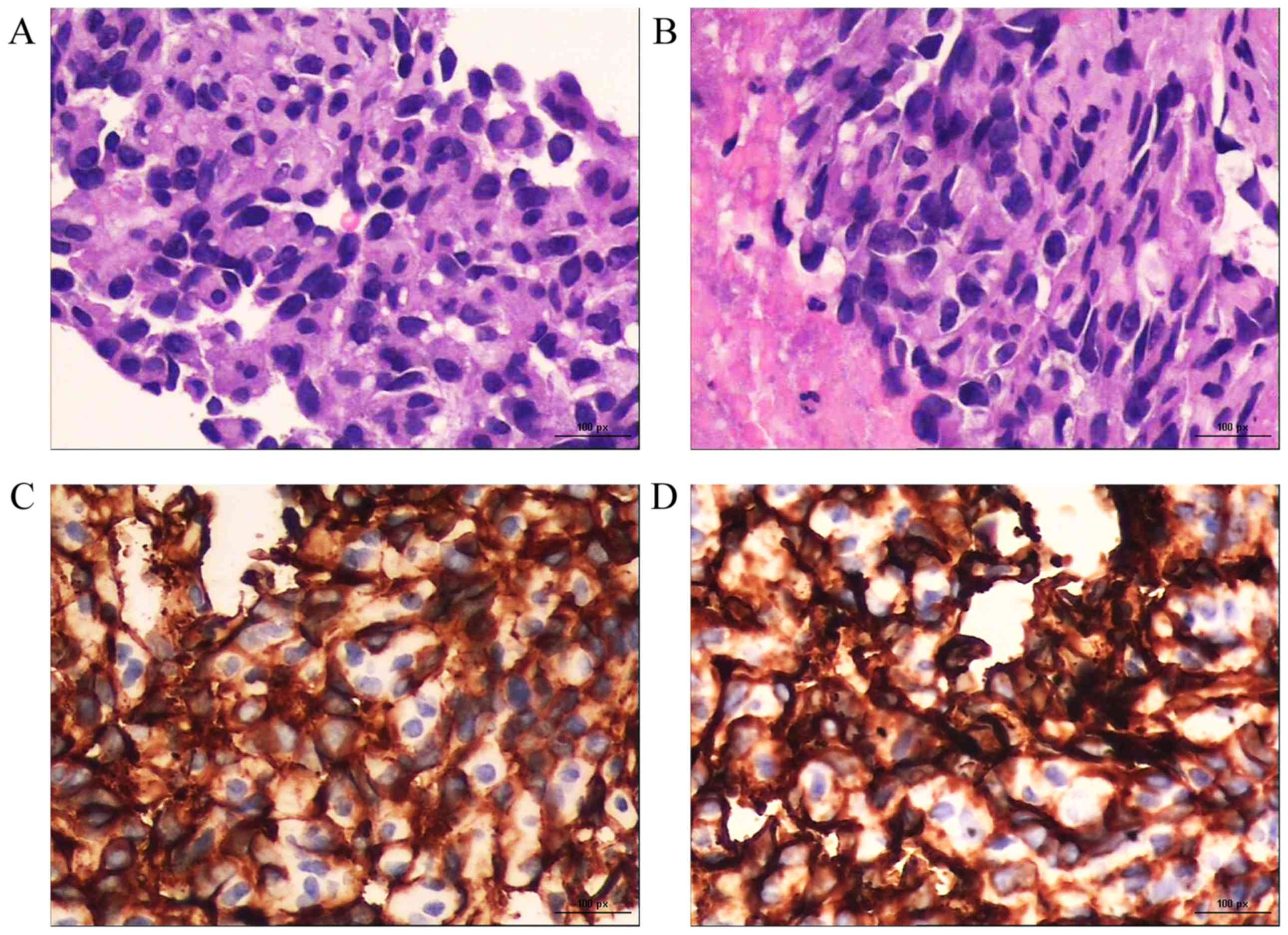
and C-G). CT-guided percutaneous biopsy of a left ilium
metastasis revealed poorly differentiated spindle-shaped tumor
cells with slit-like or irregular vascular channels containing red
blood cells (RBCs; hematoxylin and eosin staining; magnification,
×400). Immunohistochemically, the tumor cells were positive for
CD31 and CD34 (magnification, ×400; Fig.
3). Taken together, these findings confirmed the diagnosis of
metastatic PCA (T2N1M1).
Although the patient's performance status score was
1 on the Eastern Cooperative Oncology Group scale, the tumor had
metastasized to other internal organs and total excision of the
cardiac tumor was anatomically impossible. Therefore, 90
mg/m2 paclitaxel was administered intravenously on days
1, 8 and 15 of a 28-day cycle. Prior to the administration of
paclitaxel, the patient received intravenous premedications,
including dexamethasone 5 mg, cimetidine 400 mg and phenergan 25
mg. Standard antiemetics (mainly palonosetron 0.25 mg) were
prescribed by the treating physician when clinically indicated.
Cycles could not be initiated unless the granulocyte count was
>1500/μl and the platelets were >100,000/μl. The treatment
was well-tolerated by the patient, except for grade II neutropenia
(white blood cells 2.41×109/l, neutrophils
1.06×109/l) and prophylactic granulocyte
colony-stimulating factor was administered at 150 μg. In December
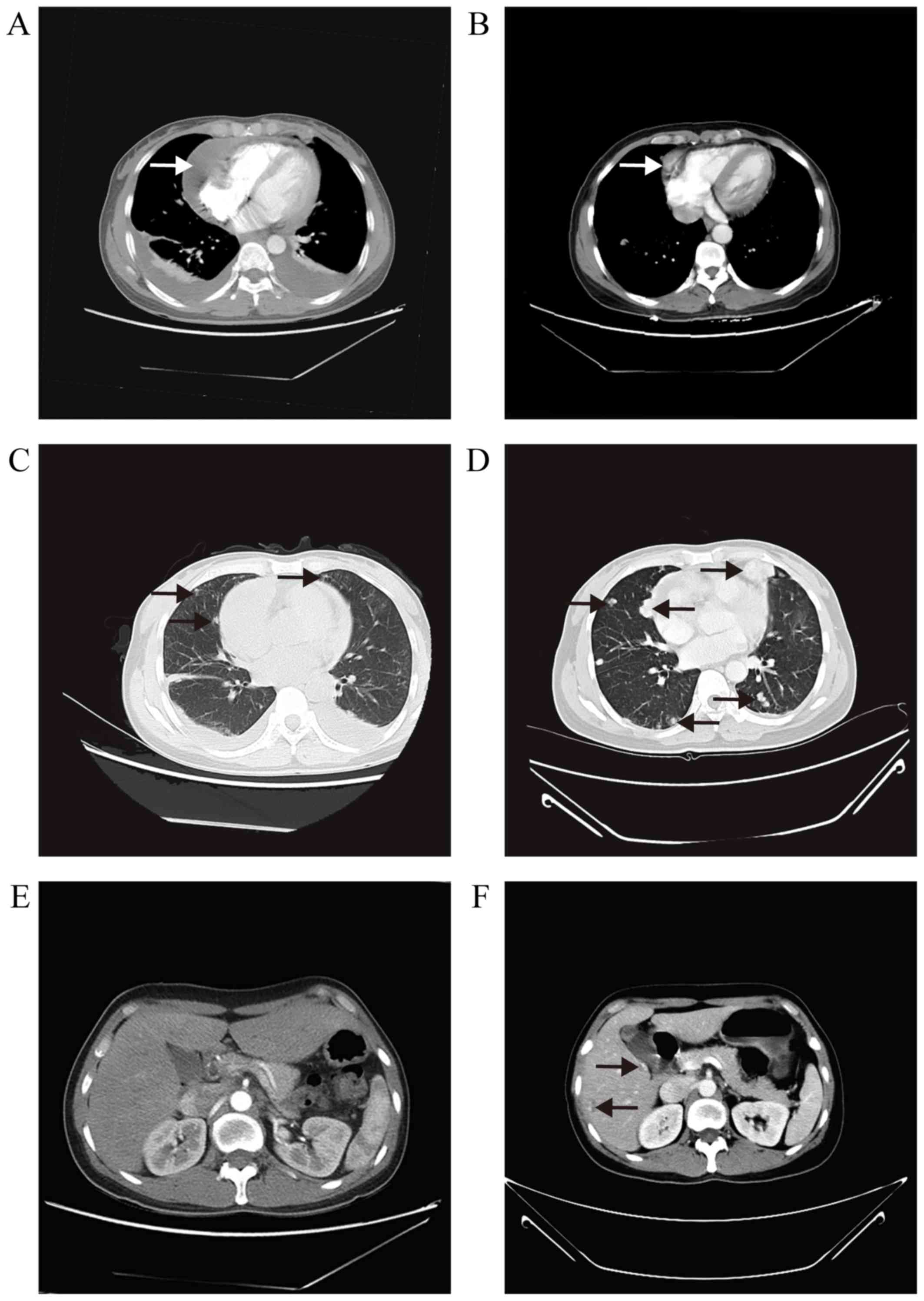
2015, TTE revealed a shrinkage in the cardiac tumor size (1.5×1.1
cm) and absorption of the pericardial effusion. A CT scan, however,
revealed that the volume of the pulmonary nodules had increased and
identified new foci in the liver (Fig.
4). On laboratory tests the CA125 level was 19.00 U/ml, the
CA199 level was 37.60 U/ml and the CEA level was 8.13 ng/ml.
Therefore, vinorelbine was selected as second-line treatment, with
25 mg/m2 vinorelbine administered intravenously on days
1 and 8 of a 21-day cycle. In December 24, bevacizumab was added to
the therapy scheme (vinorelbine 25 mg/m2 on days 1 and 8
of a 21-day cycle following administration of bevacizumab 10 mg/kg
on day 1). The patient complained of abdominal pain; thus,
Oxycontin was administered at 30 mg/12 h to control the symptom. On
January 8, 2016, the patient displayed anemia and respiratory
failure (hemoglobin 56 g/l, RBC count 2.16×1012,
PaO2 9.18 kPa, PaCO2 4.63 kPa,
SpO2 91.5%, actual base excess 18.9 mmol/l, standard
base excess 19.4 mmol/l, D-dimer 40.00 mg/l, and fibrin degradation
products 128.1 mg/l) and was unable to tolerate the chemotherapy;
thus, a blood transfusion was performed, with oxygen inhalation and
diprophylline injection. The patient succumbed to respiratory
failure 7 months after diagnosis.
Discussion
Due to the rapid local relapse and high incidence of
systemic metastasis, PCA has a dismal prognosis, with a mean life
expectancy of only a few months. The literature focusing on the
treatment of unresectable PCA was reviewed. Although the relevant
studies were scarce, several case reports and results from phase II
trials expanded our knowledge of this rare disease. A summary of
most common locations, treatment modalities and outcome is
presented in Table I. The cases
included in the Table I were almost
inoperable, which was undoubtedly among the key factors determining
the patients' prognosis. Although there are currently no
established guidelines for the treatment of angiosarcoma, no
further subgroups have been identified by which adjuvant therapy
could be recommended; as previously reported, chemotherapy,
radiotherapy and targeted therapy are the most common choices for
the treatment of unresectable PCA. In the present case, addition of
radiotherapy to the second-line treatment was initially attempted,
as several cases of PCA exhibited high sensitivity to radiotherapy
(14–16); however, due to the patient's poor
physical condition, radiotherapy had to be abandoned. Weekly
paclitaxel has been reported to be effective in the treatment of
unresectable angiosarcomas (including PCA), with a median
progression-free survival (PFS) of 4 months and a median overall
survival of 8 months (17).
Vinorelbine has demonstrated antitumor activity in angiosarcoma, as
monotherapy or combined with gemcitabine (18,19).
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody
that blocks the activity of vascular endothelial growth factor
(VEGF)-A. A phase II trial concluded that bevacizumab is an
effective and well-tolerated single-agent treatment for metastatic
or locally advanced angiosarcoma (20). Several case reports demonstrated that
combination therapy with bevacizumab and chemotherapy or
radiotherapy may improve quality of life and survival in patients
with metastatic angiosarcoma (21–25). In
addition, another VEGF inhibitor, pazopanib, may prolong the PFS of
metastatic non-adipocytic soft-tissue sarcoma after previous
chemotherapy (26), particularly
when used as maintenance therapy for PCA (16).
 | Table I.Cases of unresectable cardiac
angiosarcoma reported in the English literature identified through
a PubMed search. |
Table I.
Cases of unresectable cardiac
angiosarcoma reported in the English literature identified through
a PubMed search.
| First author,
year | Location | Pericardial
extension | Treatment | Outcome (months) | (Refs.) |
|---|
| Ram Prabu, 2011 | RA | Yes | Weekly paclitaxel (80
mg/m2) | PFS 16a | (5) |
| Suderman, 2011 | LA | Yes | Weekly docetaxel (25
mg/m2) and radiotherapy | PFS 16, OS 22 | (7) |
| Kodali, 2006 | RA | Yes | Doxil 40–50
mg/m2 q/4 weeks (line 1), MAID regimenb (line 2) | PFS 15, OS 16 | (8) |
| Hata, 2011 | RA | No | CRT (30 fractions of
2 Gy) with weekly carboplatin (area under the curve=2) and PTX (60
mg/m2) | PFS 5a | (9) |
| Franceschini,
2013 | RA | Yes | Epirubicin (60
mg/m2), ifosfamide (3,000 mg/m2) and
radiocherapy 60 Gy | PFS 16a | (10) |
| Fehr, 2010 | RA | No | Doxorubicin (75
mg/m2) and ifosfamide (7,500 mg/m2) q/3 weeks
(line 1), radiotherapy (22 fractions of 2 Gy) and weekly paclitaxel
(80 mg/m2) (line 2) | PFS 8.5, OS 12 | (11) |
| Castilla, 2010 | RA | No |
Paclitaxelc | OS 9 | (12) |
| Batzios, 2006 | RV | No | Epirubicin 75
mg/m2, cisplatin 80 mg/m2 and ifosfamide 2
gr/m2, plus uromitexan 800 mgx2 Trastuzumab150 mg once
weekly and imatinib 400 mg | PFS 6, OS 7 | (13) |
| Aoka, 2004 and
interleukin 2 | RA | Yes | Carbon-ion
radiotherapy 64 Gy | PFS 5, OS
18a | (14) |
| Elsayad, 2016 | RA | No | Radiotherapy 55.8 Gy
and weekly paclitaxel (50 mg/m2) (line 1), doxorubicin
and isosfamidec (line
2) and pazopanib (maintenance therapy) | PFS 3, OS
16a | (15,16) |
In conclusion, unresectable cardiac angiosarcomas
are rare but lethal. In such cases, a multimodality approach
including image-guided radiotherapy and targeted therapy may be
considered, as the overall prognosis of these patients is poor.
Further clinical trials focusing on the treatment of unresectable
PCA are warranted.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant nos. 81672327, 81372645,
81502013 and 81602411) and the Program of Shanghai
Academic/Technology Research Leader (grant no. 17XD1402600), the
Fong Shu Fook Tong Foundation and National Key Clinical Discipline
(Oncology), the Shanghai Municipal Education Commission-Gaofeng
Clinical Medicine Grant Support (grant no. 20161410), the Program
for Outstanding Medical Academic Leader and Shanghai Municipal
Commission of Health and Family Planning (grant no. 20154Y496).
References
|
1
|
Kurian KC, Weisshaar D, Parekh H, Berry GJ
and Reitz B: Primary cardiac angiosarcoma: Case report and review
of the literature. Cardiovasc Pathol. 15:110–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kupsky DF, Newman DB, Kumar G, Maleszewski
JJ, Edwards WD and Klarich KW: Echocardiographic features of
cardiac angiosarcomas: The mayo clinic experience (1976–2013).
Echocardiography. 33:186–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araoz PA, Eklund HE, Welch TJ and Breen
JF: CT and MR imaging of primary cardiac malignancies.
Radiographics. 19:1421–1434. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prabu Ram MP, Thulkar S, Ray R and Bakhshi
S: Primary cardiac angiosarcoma with good response to Paclitaxel. J
Thorac Oncol. 6:1778–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pigott C, Welker M, Khosla P and Higgins
RS: Improved outcome with multimodality therapy in primary cardiac
angiosarcoma. Nat Clin Pract Oncol. 5:112–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suderman D, Cooke A, Wong R and Klein J:
Treatment of cardiac angiosarcoma with radiation and docetaxel: A
case report with partial response and prolonged stable disease. J
Thorac Oncol. 6:834–835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kodali D and Seetharaman K: Primary
cardiac angiosarcoma. Sarcoma. 2006:391302006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hata A, Katakami N, Fujita S, Kokubo M and
Imai Y: Angiosarcoma arising from right atrium: Remarkable response
to concurrent chemoradiotherapy with carboplatin and paclitaxel. J
Thorac Oncol. 6:970–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Franceschini D, Scotti V, Simontacchi G,
Meattini I, Paiar F, Greto D, Bonomo P, Franzese C, Di Cataldo V,
Pallotta S and Biti G: Application of helical tomotherapy for the
treatment of a right atrium angiosarcoma: A case report. Tumori.
99:e233–e236. 2013.PubMed/NCBI
|
|
11
|
Fehr M, Kuhn M, Mayer K, Padberg B, Ulmer
U and Cathomas R: Metastatic angiosarcoma arising from the right
atrium: Unusual presentation and excellent response to treatment in
a young patient. J Thorac Oncol. 5:1301–1302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castilla E, Pascual I, Roncalés F, Aguirre
E and Del Río A: Transient response of cardiac angiosarcoma to
paclitaxel. Eur J Cancer Care (Engl). 19:699–700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batzios S, Michalopoulos A, Kaklamanis L,
Stathopoulos J, Christopoulou M, Koutantos J and Stathopoulos GP:
Angiosarcoma of the heart: Case report and review of the
literature. Anticancer Res. 26:4837–4842. 2006.PubMed/NCBI
|
|
14
|
Aoka Y, Kamada T, Kawana M, Yamada Y,
Nishikawa T, Kasanuki H and Tsujii H: Primary cardiac angiosarcoma
treated with carbon-ion radiotherapy. Lancet Oncol. 5:636–638.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elsayad K, Lehrich P, Yppaerilae-Wolters
H, Dieckmann C, Kriz J, Haverkamp U and Eich HT: Primary cardiac
angiosarcoma treated with positron emission tomography/magnetic
resonance imaging-guided adaptive radiotherapy. Can J Cardiol.
32:829.e7–829.e10. 2016. View Article : Google Scholar
|
|
16
|
Elsayad K, Scobioala S, Kriz J, Haverkamp
U and Eich HT: Advances in image-guided radiation therapy for
primary cardiac angiosarcoma: The role of PET-CT and MRI. Oncol Res
Treat. 39:290–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Penel N, Bui BN, Bay JO, Cupissol D,
Ray-Coquard I, Piperno-Neumann S, Kerbrat P, Fournier C, Taieb S,
Jimenez M, et al: Phase II trial of weekly paclitaxel for
unresectable angiosarcoma: The ANGIOTAX study. J Clin Oncol.
26:5269–5274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anderson SE, Keohan ML, D'Adamo DR and
Maki RG: A retrospective analysis of vinorelbine chemotherapy for
patients with previously treated soft-tissue sarcomas. Sarcoma.
2006:159472006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dileo P, Morgan JA, Zahrieh D, Desai J,
Salesi JM, Harmon DC, Quigley MT, Polson K, Demetri GD and George
S: Gemcitabine and vinorelbine combination chemotherapy for
patients with advanced soft tissue sarcomas: Results of a phase II
trial. Cancer. 109:1863–1869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agulnik M, Yarber JL, Okuno SH, von Mehren
M, Jovanovic BD, Brockstein BE, Evens AM and Benjamin RS: An
open-label, multicenter, phase II study of bevacizumab for the
treatment of angiosarcoma and epithelioid hemangioendotheliomas.
Ann Oncol. 24:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang P, Zhu Q and Jiang F: Combination
therapy for scalp angiosarcoma using bevacizumab and chemotherapy:
A case report and review of literature. Chin J Cancer Res.
25:358–361. 2013.PubMed/NCBI
|
|
22
|
De Yao JT, Sun D, Powell AT and Rehmus EH:
Scalp angiosarcoma remission with bevacizumab and radiotherapy
without surgery: A case report and review of the literature.
Sarcoma. 2011:1603692011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koontz BF, Miles EF, Rubio MA, Madden JF,
Fisher SR, Scher RL and Brizel DM: Preoperative radiotherapy and
bevacizumab for angiosarcoma of the head and neck: Two case
studies. Head Neck. 30:262–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeng MR, Fuh B, Blatt J, Gupta A, Merrow
AC, Hammill A and Adams D: Malignant transformation of infantile
hemangioma to angiosarcoma: Response to chemotherapy with
bevacizumab. Pediatr Blood Cancer. 61:2115–2117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nespereira-Jato MV, Peña-Panabad C,
Quindós-Varela M and García-Silva J: Unresectable angiosarcoma
treated with bevacizumab and paclitaxel. Actas Dermosifiliogr.
105:520–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|