Introduction
Colorectal cancer (CRC) is a common and lethal
disease. CRC incidence and mortality rates vary markedly worldwide.
Globally, CRC is the third most commonly diagnosed cancer in men
and the second in women, with an estimated >1.2 million new
cases and 608,700 CRC-related deaths in 2008 (1). Specific genetic changes are considered
to drive the transformation from normal colonic epithelium to
invasive cancer, and these genetic mutations may be inherited or
acquired (2). CRC represents an
ideal model for the study of the molecular pathogenesis of cancer,
due to the accessibility of tissue for biopsy and the clear
progression from normal colonic epithelium to invasive cancer via
an intermediate precursor, the adenomatous polyp (2).
Several blood biomarkers have been investigated in
CRC, including circulating microRNA, endothelial cell specific
molecule-1, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio,
α-2 macroglobulin, KRAS and epidermal growth factor receptor (EGFR)
(3–9).
EGFR is a 170-kDa glycoprotein that belongs to the
transmembrane tyrosine kinase receptor family, and has been
detected in a wide variety of cancer types (10). The activation of EGFR has multiple
consequences, such as cell growth, differentiation and
proliferation; it also promotes malignant transformation,
angiogenesis and metastatic dissemination (10). EGFR has been reported to be
overexpressed in the majority (50–80%) of colorectal tumors, and
its expression has been demonstrated to be associated with poor
outcome in patients with stage IV disease (11–14).
Mourtzikou et al (15) identified that serum (s)EGFR levels
were significantly lower in the patient group when compared with
those in healthy control individuals. In this previous study, there
was no significant association between tumor-node-metastasis (TNM)
stage, histological grade, performance status and EGFR expression
(15). Few studies have reported an
association between histological grade and EGFR overexpression
(16,17), whereas a number of investigators
consider the clinicopathological characteristics of colon carcinoma
not to be affected by EGFR expression (18,19).
However, in certain studies, a higher sEGFR level at baseline was
associated with the best objective response and may be considered a
significant predictor of outcome in patients with advanced CRC
(9).
The present study aimed to determine the sEGFR
levels in healthy volunteers and patients with CRC, to determine
the association between the levels of this tumor marker and
clinicopathological findings, and to investigate its prognostic
value.
Patients and methods
Study design and eligibility
criteria
The serum samples of 140 consecutive patients with
CRC who were referred to Istanbul University Institute of Oncology
and Bakirkoy Dr. Sadi Konuk Training and Research Hospital
(Istanbul, Turkey) between May 2011 and August 2014 were obtained.
The median age of the patients was 60 years (range, 24–84 years).
All the patients were staged using the seventh edition of the
American Joint Committee on Cancer TNM system (20) on a radiological and pathological
basis.
All the patients were treated with a
multidisciplinary approach. Patients with colon cancer who had
undergone surgery including segmental colon resection were treated
with adjuvant chemotherapy (CTx) according to their stage. Patients
with rectal cancer, who received neoadjuvant radiochemotherapy
(RCTx) or radiotherapy (RT), had undergone low anterior resection
or abdominoperineal resection. Certain patients underwent
palliative surgery and stage IV patients received palliative CTx,
with or without targeted therapy (bevacizumab or cetuximab). The
pretreatment evaluation included detailed clinical history and
physical examination, with a series of biochemistry tests and
complete blood cell count. Selection for treatment required an
Eastern Cooperative Oncology Group (ECOG) performance status score
of 0–2 (21), and appropriate bone
marrow (hemoglobin >9 g/dl, absolute neutrophil count
>1,500/µl and platelet count >100,000/µl), cardiac, renal and
hepatic function.
Patients were treated with various CTx regimens,
including single-agent or combination therapy. Regimens of single
or combination CTx were selected according to the performance
status of the patients and extension of disease. Patients received
one of the following treatment regimens: Simplified LV5FU2
(leucovorin 400 mg/m2, followed by 5-fluorouracil as a
400 mg/m2 bolus and a 2,400 mg/m2 infusion
over 46 h every 2 weeks), capecitabine (1,000 mg/m2,
twice daily, oral administration, for 14 days of each 21-day
cycle), modified FOLFOX regimen (simplified LV5FU2 regimen plus
oxaliplatin 85 mg/m2 every 2 weeks), FOLFIRI (simplified
LV5FU2 regimen plus irinotecan 180 mg/m2 every 2 weeks),
XELOX (capecitabine 1,000 mg/m2, twice daily, oral
administration, for 14 days plus oxaliplatin 130 mg/m2
every 3 weeks), or XELIRI (capecitabine 1,000 mg/m2,
twice daily, oral administration, for 14 days plus irinotecan 240
mg m2 every 3 weeks). Bevacizumab was given at a dose
schedule of either 5 mg/kg every 2 weeks or 7.5 mg/kg every 3
weeks. Cetuximab 500 mg/m2 was administered
intravenously every 2 weeks.
All the patients underwent pretreatment imaging of
primary tumors using magnetic resonance imaging (MRI) or computed
tomography (CT) scan. For patients with evaluable imaging studies
prior to and following treatment, the radiological response was
evaluated according to the Response Evaluation Criteria in Solid
Tumors (version 1.1) (22) and
classified as follows: Complete response (CR), partial response
(PR), stable disease (SD) or progressive disease (PD). The tumor
response after 2 months of CTx was used for statistical analysis.
Follow-up programs for metastatic disease consisted of clinical and
laboratory programs and CT scan or MRI, depending on which imaging
methods were used at baseline, and performed at 8-week intervals
during CTx or every 12 weeks for patients receiving no anticancer
treatment. Patients with either a CR or PR were classified as
responders, and patients with an SD or PD were considered
non-responders.
The present study was approved by the Institutional
Review Board (IRB) of Istanbul University, Institute of Oncology
(Istanbul, Turkey). Baseline demographic, clinical and laboratory
data, including age, sex, performance status, tumor marker levels,
KRAS mutation status and treatment details, were obtained
retrospectively for all patients using uniform database templates
to ensure consistent data collection. The patient comorbidities
included cardiac and metabolic diseases.
The control group consisted of 40 age- and
sex-matched healthy females with no previous history of malignancy
or autoimmune disorders. Blood samples were obtained from patients
with CRC at first admission, prior to the administration of any
therapy. Blood samples of healthy controls were collected in dry
tubes and the sera separated from cellular elements by
centrifugation (at 1,431 × g for 10 min) within 30 min following
collection. Blood samples were stored at −80°C prior to analysis.
All the samples were collected under the approval of the IRB and
all the patients provided written informed consent.
Measurement of sEGFR levels
An EGFR ELISA kit (Shanghai Yehua Biological
Technology Co. Ltd, Shanghai, China), which uses a double-antibody
sandwich ELISA to determine the level of human EGFR in samples, was
used according to the manufacturer's protocol. Serum samples and
standards were added to the wells, which were pre-coated with human
EGFR monoclonal antibody. Streptavidin-horseradish peroxidase was
added to form immune complexes and allowed to incubate at 37°C for
1 h. Unbound material was washed away, and chromogen solution was
added and incubated at 37°C for 10 min in the dark for the
conversion of the colorless solution to a blue solution, the
intensity of which is proportional to the amount of EGFR in the
sample. Upon the addition of the acidic stop solution, the color
was converted to yellow. The colored reaction product was measured
using an automated ELISA reader (ChroMate® 4300;
Awareness Technology, Inc., Palm City, FL, USA) at 450 nm. The
results were expressed as ng/ml.
Statistical analysis
SPSS for Windows version 21.0 (IBM Corp., Armonk,
NY, USA) was employed for data analysis. Continuous variables were
categorized using median values as cut-off point. The Chi-square
test or one-way analysis of variance were used for group comparison
of categorical variables, and the Mann-Whitney U test or
Kruskall-Wallis test were used for comparison of continuous
variables. The Spearman's rank order correlation was used for
correlation analysis. Progression-free survival (PFS) was
calculated from the date of admission to the date of first
radiological progression, with or without elevated serum tumor
marker. Overall survival (OS) was calculated from the date of first
admission to the clinic to disease-associated mortality or date of
last contact with the patient or any family member. The
Kaplan-Meier method was used for the estimation of survival
distribution, and variations in PFS and OS were assessed using the
log-rank test. All statistical tests were two-sided and P≤0.05 was
considered to indicate a statistically significant difference.
Results
In total, 140 patients who were pathologically
diagnosed with CRC between May 2011 and August 2014 were included
in the present study. The baseline demographic and
histopathological/laboratory characteristics of patients are
presented in Tables I and II. The median age of the patients was 60
years (range, 24–84 years). Males constituted the majority of the
group (n=96, 69%). A total of 43 of the patients had a family
history of cancer, including 12 with a history of lung cancer and
14 with a history of CRC. The tumor localization was to the rectum
in 42% (n=59) and the colon in 58% (n=81) of the patients (right
colon, n=17; hepatic flexure, n=5; transverse colon, n=5;
descending colon, n=13; splenic flexure, n=1; sigmoid colon, n=37;
recto-sigmoid junction, n=6; and multiple synchronous colon tumors,
n=3). The most frequent metastatic sites were the liver (n=40,
67.8%) and the peritoneum (n=17, 28.8%). The rates of synchronous
(n=34) and metachronous metastases (n=25) were 57.6 and 42.4%,
respectively.
 | Table I.Patient clinicopathological
characteristics. |
Table I.
Patient clinicopathological
characteristics.
| Characteristics | No. of patients |
|---|
| Total | 140 |
| Age, years, median
(range) | 60 (24–84) |
| Sex, male/female | 96/44 |
| Performance
statusa, 0/1/2/3 | 68/61/7/1 |
| Smokinga, yes/no | 61/66 |
| Alcohol
intakea, yes/no | 26/99 |
|
Comorbiditiesa yes/no | 56/79 |
| Obstruction,
yes/no | 17/123 |
| Surgery type |
|
|
Colectomy | 56 |
| Low
anterior resection | 36 |
|
Abdominoperineal
resection | 13 |
|
Palliative surgery | 11 |
| pT stageb, 0/1/2/3/4 | 9/2/12/45/10 |
| pN stageb, 0/1/2 | 42/18/14 |
| Stage of disease,
2/3/4 | 17/64/59 |
| Site of lesion,
colon/rectum | 81/59 |
| Response to
CTxc,
CR/PR/SD/PD/unknown | 2/15/10/24/4 |
| Targeted therapy,
bevacizumab/cetuximab | 36/15 |
| Metastasis,
yes/nod | 59/81 |
 | Table II.Histopathological characteristics and
laboratory parameters. |
Table II.
Histopathological characteristics and
laboratory parameters.
| Variables | No. of patients |
|---|
| Histology,
adenocarcinoma/mucinous | 129/11 |
| Gradea, 1/2/3 | 8/56/6 |
| Angiolymphatic
invasionb, yes/no | 30/18 |
| Vascular
invasionb, yes/no | 16/30 |
| Perineural
invasionb, yes/no | 18/28 |
| Regression
scorec, 1/2/3/4 | 1/12/4/8 |
| KRAS
mutation statusd,
mutant/wild-type | 24/28 |
| Lactate
dehydrogenase, IU/mla |
|
| Normal
(<450) | 97 |
| High
(>450) | 16 |
| Albumin,
g/dla |
|
| Normal
(>4) | 54 |
| Low
(<4) | 58 |
| Carcinoembryonic
antigen, ng/mla |
|
| Normal
(<5) | 78 |
| High
(>5) | 17 |
| Carbohydrate
antigen 19-9, U/mla |
|
| Normal
(<38) | 81 |
| High
(>38) | 28 |
Of the 37 patients with rectal cancer, 28 received
fluoropyrimidine-based RCTx, whereas 9 received short-course RT. A
total of 71 patients who had adjuvant CTx received one of the
following treatment regimens: Simplified LV5FU2 or capecitabine
(n=14), mFOLFOX (n=26) or XELOX (n=31). Oxaliplatin- and
irinotecan-based combination CTx regimens and single-agent
fluoropyrimidine were used in 24, 22 and 9 patients, respectively.
Bevacizumab was administered to 36 patients, whereas 15 patients
received cetuximab as a targeted agent. A response to CTx was
observed in 31% of the 55 metastatic patients who received
palliative CTx.
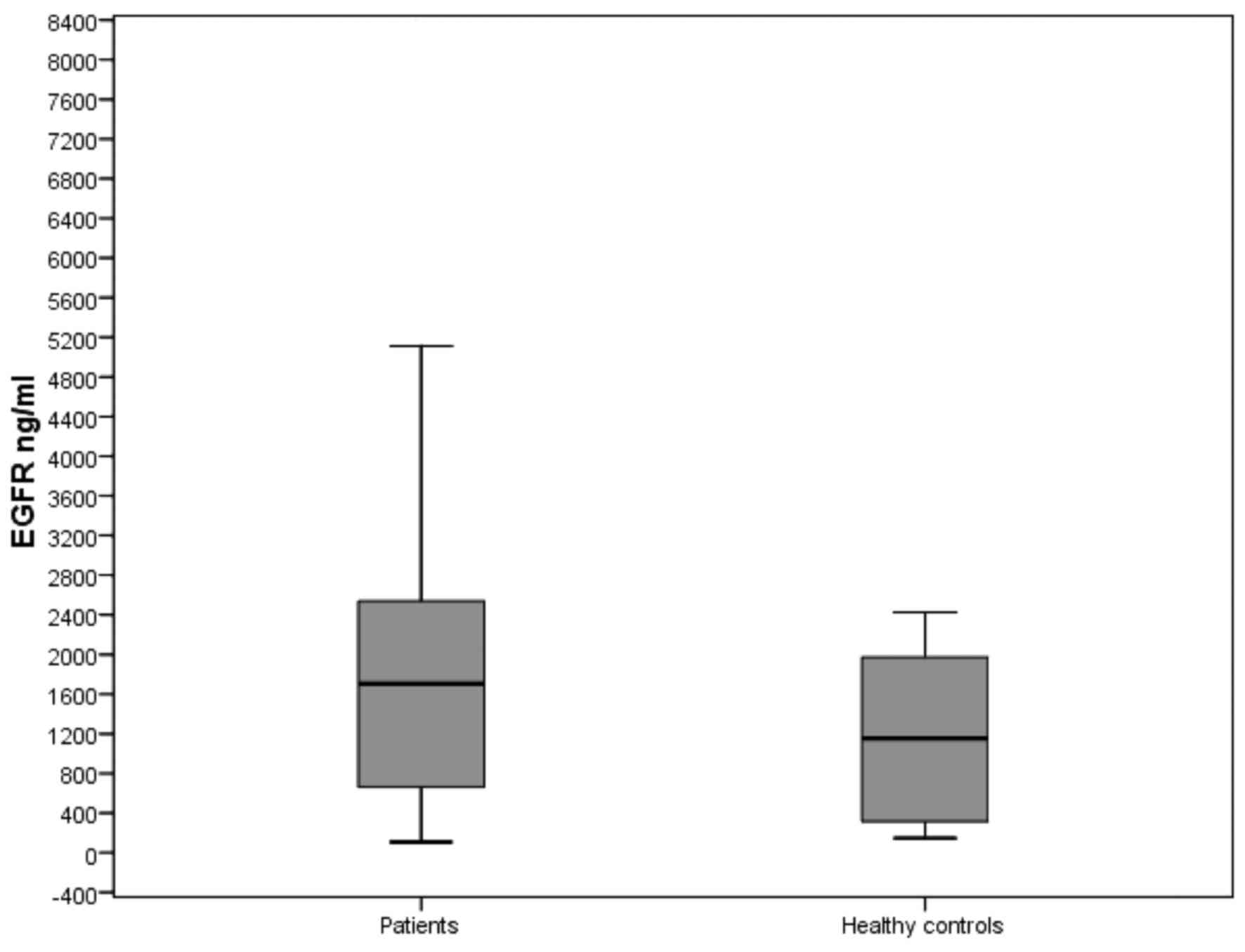
The levels of sEGFR in patients with CRC and healthy
controls are presented in Table
III. The baseline sEGFR levels were significantly higher
compared with the control group (1704.39 vs. 1154.77 ng/ml,
respectively; P=0.002; Fig. 1).
 | Table III.Serum marker levels in patients with
colorectal cancer and healthy controls. |
Table III.
Serum marker levels in patients with
colorectal cancer and healthy controls.
|
| Patients
(n=140) | Controls
(n=40) |
|---|
|
|
|
|
|---|
| Marker | Median | Range | Median | Range | P-value |
|---|
| sEGFR level
(ng/ml) | 1704.39 |
107.57–75,230.81 | 1154.77 |
146.02–2,425.55 | 0.002 |
The associations between the levels of sEGFR and
clinicopathological factors are presented in Tables IV and V. No surgical resection, metastatic status,
higher pathological tumor stage, poorer regression score (3–4) and
higher lactate dehydrogenase (LDH) levels were significantly
associated with higher sEGFR concentrations (all P-values
<0.05).
 | Table IV.Results of comparisons between the
serum assays and various demographic and disease
characteristics. |
Table IV.
Results of comparisons between the
serum assays and various demographic and disease
characteristics.
| Variables | n | Median EGFR, ng/ml
(range) | P-value |
|---|
| Age, years |
|
| 0.33 |
|
<50 | 22 | 2,024.03
(108.99–75,230.81) |
|
|
≥50 | 118 | 1,438.93
(107.57–74,615.28) |
|
| Sex |
|
| 0.81 |
|
Male | 96 | 1,444.55
(107.57–75,230.81) |
|
|
Female | 44 | 1,843.02
(108.99–74,615.28) |
|
| PS |
|
| 0.11 |
| 0 | 68 | 1,035.47
(107.57–50,143.55) |
|
|
1–3 | 69 | 1,971.00
(108.99–75,230.81) |
|
| Smoking |
|
| 0.54 |
|
Yes | 61 | 1,397.52
(107.57–74,615.28) |
|
| No | 66 | 1,602.51
(108.99–75,230.81) |
|
| Alcohol intake |
|
| 0.87 |
|
Yes | 26 | 1,147.23
(107.57–49,116.45) |
|
| No | 99 | 1,491.57
(108.99–75,230.81) |
|
| Comorbidity |
|
| 0.35 |
|
Yes | 56 | 1906.43
(107.57–75230.81) |
|
| No | 79 | 1,251.54
(316.09–74,615.28) |
|
| Obstruction |
|
| 0.38 |
|
Yes | 17 | 1,713.44
(108.99–75,230.81) |
|
| No | 123 | 1,491.57
(107.57–12,141.99) |
|
| Surgery |
|
| 0.03b |
|
Yes | 116 | 1,422.22
(107.57–75,230.81) |
|
| No | 24 | 2,379.78
(421.16–67,643.89) |
|
| pT stage |
|
| 0.05b |
|
0–2 | 23 | 775.65
(316.09–14,169.16) |
|
|
3–4 | 55 | 1,695.33
(107.57–74,615.28) |
|
| pN stage |
|
| 0.42 |
| 0 | 42 | 928.57
(107.57–61,069.96) |
|
|
1–2 | 32 | 1,444.55
(108.99–74,615.28) |
|
| Metastasis |
|
| 0.009b |
|
Yes | 59 | 2,110.26
(146.02–75,230.81) |
|
|
Noa | 81 | 1,020.79
(107.57–74,615.28) |
|
| Response to
CTx |
|
| 0.76 |
| Yes (CR
+ PR) | 17 | 1,938.57
(261.50–49,116.45) |
|
| No (SD
+ PD) | 34 | 2,230.25
(146.02–75,230.81) |
|
| Targeted
therapy |
|
| 0.37 |
|
Bevacizumab | 36 | 1,964.50
(146.02–49,116.45) |
|
|
Cetuximab | 15 | 2,484.01
(289.30–67,643.89) |
|
| Site of lesion |
|
| 0.56 |
|
Colon | 81 | 1,397.52
(146.02–61,069.96) |
|
|
Rectum | 59 | 1,938.57
(107.57–75,230.81) |
|
 | Table V.Results of comparisons between the
serum assays and various histopathological features and laboratory
parameters. |
Table V.
Results of comparisons between the
serum assays and various histopathological features and laboratory
parameters.
| Variables | n | Median EGFR, ng/ml
(range) | P-value |
|---|
| Histology |
|
| 0.39 |
|
Adenocarcinoma | 129 | 1,695.33
(107.57–74,615.28) |
|
|
Mucinous | 11 | 2,123.79
(381.62–75,230.81) |
|
| Grade |
|
| 0.51 |
|
Good |
8 | 660.74
(409.65–8,747.00) |
|
|
Intermediate | 56 | 793.17
(316.09–8,450.66) |
|
|
Poor |
6 | 1,365.48
(107.57–74,615.28) |
|
| Angiolymphatic
invasion |
|
| 0.33 |
|
Yes | 30 | 1,661.01
(107.57–74,615.28) |
|
| No | 18 | 810.37
(313.61–50,143.55) |
|
| Vascular
invasion |
|
| 0.23 |
|
Yes | 30 | 1,661.01
(450.65–74,615.28) |
|
| No | 16 | 887.92
(108.99–74,615.28) |
|
| Perineural
invasion |
|
| 0.19 |
|
Yes | 18 | 1,661.01
(450.65–74,615.28) |
|
| No | 28 | 887.92
(108.99–50,143.55) |
|
| Regression
score |
|
| 0.05a |
|
0–2 | 13 | 771.67
(316.09–2,462.00) |
|
|
3–4 | 12 | 1,971.00
(323.61–61,069.96) |
|
| KRAS
mutation status |
|
| 0.63 |
|
Mutant | 24 | 2,326.84
(146.02–67,643.89) |
|
|
Wild-type | 28 | 2,185.89
(261.50–74,615.28) |
|
| LDH |
|
| 0.05a |
|
Normal | 97 | 1,397.52
(107.57–75,230.81) |
|
|
High | 16 | 2,495.07
(316.09–67,643.89) |
|
| Albumin |
|
| 0.83 |
|
Normal | 54 | 993.87
(261.50–75,230.81) |
|
|
Low | 58 | 2,063.38
(107.57–74,615.28) |
|
| CEA |
|
| 0.56 |
|
Normal | 78 | 1,704.39
(107.57–74,615.28) |
|
|
High | 17 | 1,971.00
(108.99–26,493.59) |
|
| CA19-9 |
|
| 0.45 |
|
Normal | 81 | 1,695.33
(107.57–75,230.81) |
|
|
High | 28 | 2,030.53
(146.02–74,615.28) |
|
The median follow-up time was 14.0 months (range,
1–34 months), 43 patients (31%) experienced disease progression,
and 31 patients (22%) succumbed to the disease. The median PFS and
OS of the whole group were 7.3±1.0 months [95% confidence interval
(CI): 5–9 months] and 26.9±1.1 months (95% CI: 25–29 months),
respectively. The 1-year PFS rate was 26.2% (95% CI: 12.9–39.5);
the 1- and 2-year OS rates were 82.7% (95% CI: 76.23–89.17) and
70.0% (95% CI: 58.83–81.17), respectively. Univariate analyses were
used to evaluate the impact of clinical factors and biomarkers on
prognosis. The Kaplan-Meier method and the log-rank test were
performed for univariate analysis of PFS and OS. A significant
association was observed between other clinicopathological
variables, including presence of metastasis (P≤0.05), no surgical
resection (P=0.01), CTx unresponsiveness (P=0.001), high serum
levels of carcinoembryonic antigen (CEA) (P=0.04) and carbohydrate
antigen (CA) 19-9 (P=0.03), and poorer PFS (Tables VI and VII). There were significant associations
between other clinicopathological variables, including the
localization to the rectum (P=0.03), presence of metastasis
(P<0.001), vascular invasion (P=0.02), perineural invasion
(P=0.03), poor grade (P=0.02), low performance status (P=0.04), no
surgical resection (P<0.001), CTx unresponsiveness (P=0.002),
high serum levels of LDH (P=0.02), CEA (P<0.001) and CA 19-9
(P<0.001), low serum levels of albumin (P=0.02) and poor OS
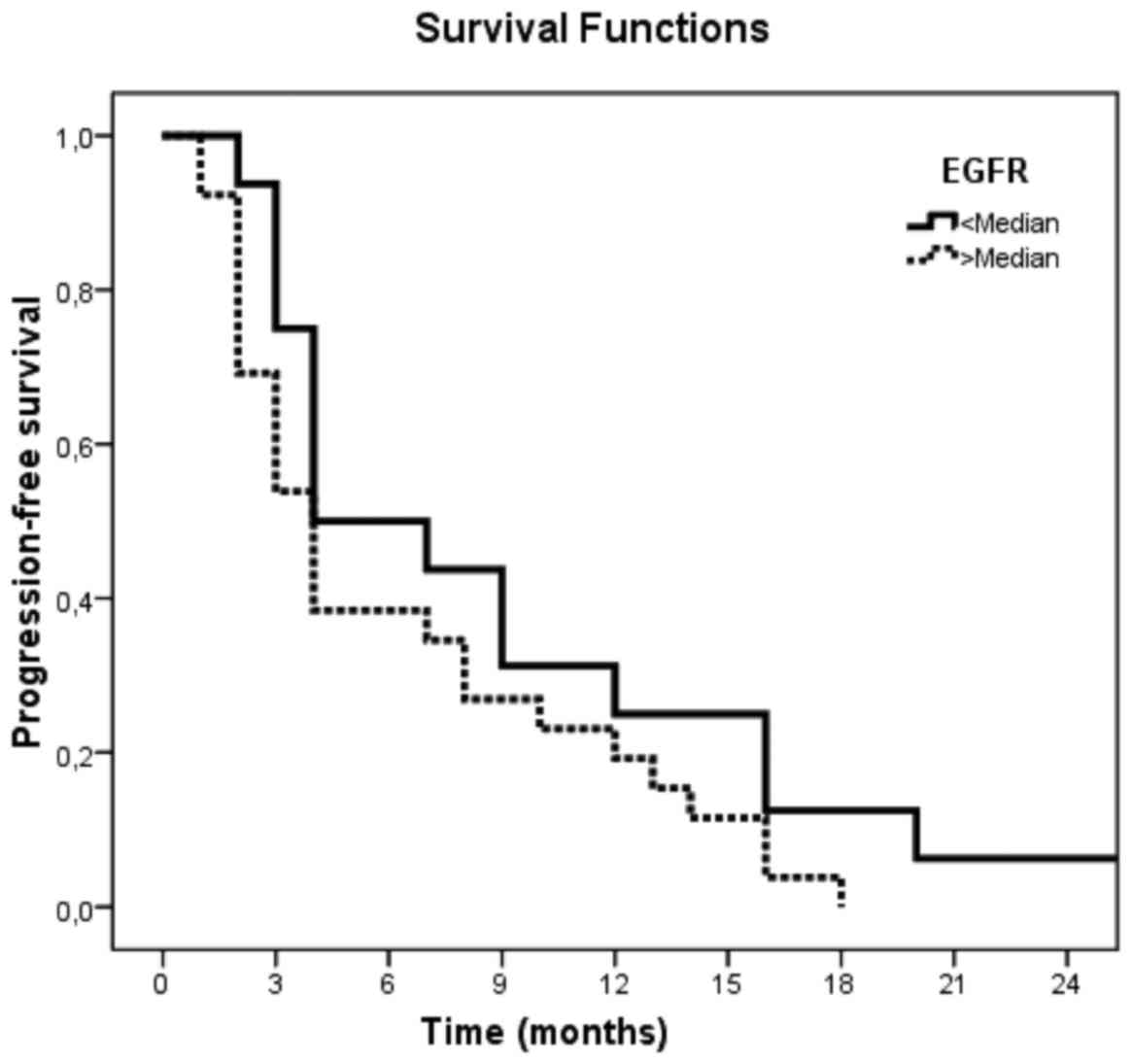
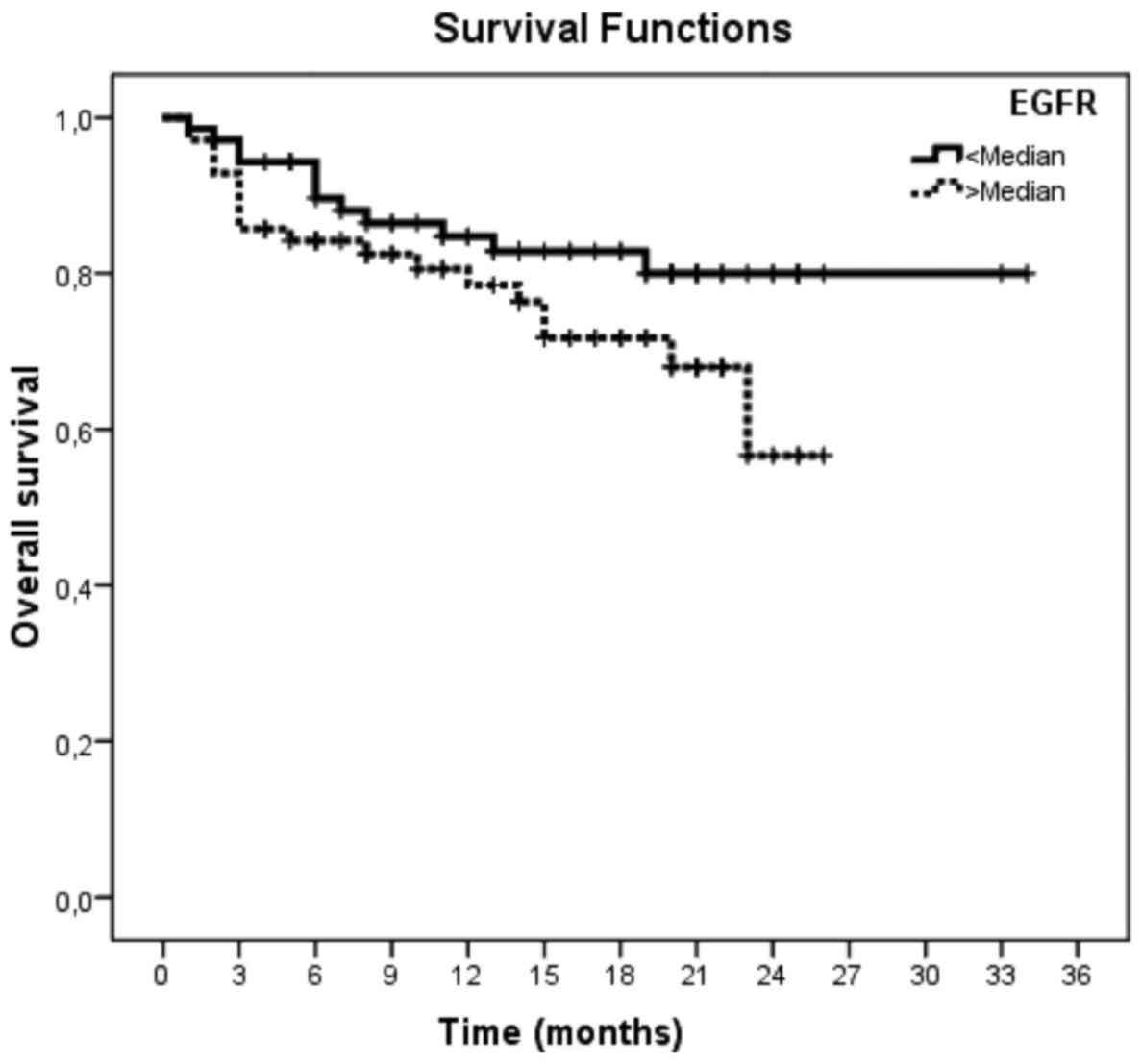
(Tables VIII–X). However, sEGFR levels revealed no
significantly adverse association with PFS and OS (P=0.12 and
P=0.11, respectively; Tables VII
and X; Figs. 2 and 3).
 | Table VI.Univariate analyses of
progression-free survival according to patient and disease
characteristics. |
Table VI.
Univariate analyses of
progression-free survival according to patient and disease
characteristics.
|
|
| Progression-free
survival (months) |
|---|
|
|
|
|
|---|
| Variables | Event no./total
no. | Median survival
(±SE) | 1-year survival, %
(±SE) | P-value |
|---|
| All patients | 43/140 | 7.3 (1.0) | 26.2 (6.8) |
|
| Age, years |
|
|
| 0.45 |
|
<50 | 6/22 | 8.3 (2.2) | Not reached |
|
|
≥50 | 37/118 | 7.2 (1.1) | 25.0 (7.2) |
|
| Sex |
|
|
| 0.46 |
|
Male | 29/96 | 7.5 (1.1) | 28.6 (8.5) |
|
|
Female | 14/44 | 7.1 (2.1) | Not reached |
|
| PS |
|
|
| 0.30 |
| 0 | 11/68 | 8.7 (2.1) | Not reached |
|
|
1–3 | 32/69 | 6.9 (1.2) | 24.1 (7.9) |
|
| Obstruction |
|
|
| 0.43 |
|
Yes | 6/17 | 6.3 (1.9) | Not reached |
|
| No | 33/123 | 7.4 (1.1) | 24.2 (7.5) |
|
| Surgery |
|
|
| 0.01b |
|
Yes | 32/116 | 8.3 (1.2) | 31.3 (8.2) |
|
| No | 11/24 | 4.2 (1.3) | Not reached |
|
| pT stage |
|
|
| 0.85 |
|
0–2 | 2/23 | 11.0 (3.2) | Not reached |
|
|
3–4 | 8/55 | 10.0 (6.0) | Not reached |
|
| pN stage |
|
|
| 0.20 |
| 0 | 4/42 | 6.5 (3.2) | Not reached |
|
|
1–2 | 6/32 | 13.7 (3.7) | Not reached |
|
| Metastasis |
|
|
| 0.05b |
|
Yes | 33/59 | 6.3 (0.9) | 21.9 (7.3) |
|
|
Noa | 10/81 | NR | Not reached |
|
| Response to
CTx |
|
|
| 0.001b |
| Yes (CR
+ PR) | 4/17 | 14.8 (2.3) | Not reached |
|
| No (SD
+ PD) | 27/34 | 4.1 (0.6) | Not reached |
|
| Targeted
therapy |
|
|
| 0.06 |
|
Bevacizumab | 21/36 | 7.3 (1.2) | 28.6 (9.9) |
|
|
Cetuximab | 4/15 | 3.5 (1.2) | Not reached |
|
| Site of lesion |
|
|
| 0.18 |
|
Colon | 19/81 | 8.3 (1.4) | 33.3 (11.1) |
|
|
Rectum | 24/59 | 6.6 (1.3) | 20.8 (8.3) |
|
| Histology |
|
|
| 0.79 |
|
Adenocarcinoma | 37/129 | 8.2 (2.6) | 24.3 (7.1) |
|
|
Mucinous | 5/11 | 7.2 (1.1) | Not reached |
|
| Grade |
|
|
| 0.79 |
|
Good | 1/8 | NR | 9.0 (0.0) |
|
|
Intermediate | 13/56 | NR | 7.5 (2.2) |
|
|
Poor | 2/6 | NR | 5.5 (2.5) |
|
| Regression
score |
|
|
| 0.90 |
|
0–2 | 2/12 | 9.5 (6.5) | Not reached |
|
|
3–4 | 0/13 | 4.0 (0.0) | Not reached |
|
| KRAS
mutation status |
|
|
| 0.14 |
|
Mutant | 14/24 | 4.9 (1.2) | Not reached |
|
|
Wild-type | 14/28 | 7.6 (1.7) | Not reached |
|
 | Table VII.Univariate analyses of
progression-free survival according to laboratory parameters. |
Table VII.
Univariate analyses of
progression-free survival according to laboratory parameters.
|
|
| Progression-free
survival (months) |
|---|
|
|
|
|
|---|
| Variables | Event no./total
no. | Median survival
(±SE) | 1-year survival, %
(±SE) | P-value |
|---|
| LDH |
|
|
|
|
|
Normal | 27/97 | 7.1 (1.1) | 25.9 (8.4) | 0.14 |
|
High | 5/16 | 12.6 (5.0) | NR |
|
| Albumin |
|
|
|
|
|
Normal | 12/54 | 7.6 (1.6) | 26.3 (10.7) | 0.57 |
|
Low | 19/58 | 8.9 (2.1) | 41.7 (14.2) |
|
| CEA |
|
|
|
|
|
Normal | 16/78 | 8.9 (1.5) | 43.8 (12.4) | 0.04a |
|
High | 9/17 | 5.2 (2.1) | NR |
|
| CA19-9 |
|
|
|
|
|
Normal | 18/81 | 9.1 (1.3) | 38.9 (11.5) | 0.03a |
|
High | 19/28 | 6.5 (1.7) | 21.1 (9.4) |
|
| EGFR |
|
|
|
|
|
<Median | 17/43 | 9.0 (1.3) | 31.3 (11.6) | 0.12 |
|
>Median | 26/43 | 6.3 (1.1) | 23.1 (8.3) |
|
 | Table VIII.Univariate analyses of overall
survival according to patient and disease characteristics. |
Table VIII.
Univariate analyses of overall
survival according to patient and disease characteristics.
|
|
| Overall survival
(months) |
|---|
|
|
|
|
|---|
| Variables | Event no./total
no. | Median survival (±
standard error) | 1-year survival, %
(± standard error) | P-value |
|---|
| All patients | 31/140 | 26.9 (1.1) | 82.7 (3.3) |
|
| Age, years |
|
|
| 0.30 |
|
<50 | 4/22 | 22.1 (1.4) | 90.9 (6.1) |
|
|
≥50 | 27/118 | 26.8 (1.2) | 81.1 (3.8) |
|
| Sex |
|
|
| 0.76 |
|
Male | 20/96 | 26.3 (1.3) | 83.3 (4.0) |
|
|
Female | 11/44 | 26.7 (1.9) | 81.5 (5.9) |
|
| PS |
|
|
| 0.02b |
| 0 | 9/68 | 25.4 (1.7) | 87.5 (4.2) |
|
|
1–3 | 22/69 | 23.1 (0.9) | 77.3 (5.2) |
|
| Obstruction |
|
|
| 0.50 |
|
Yes | 5/17 | 20.7 (2.0) | 81.1 (9.9) |
|
| No | 23/123 | 27.5 (1.3) | 83.1 (3.6) |
|
| Surgery |
|
|
|
<0.001b |
|
Yes | 20/116 | 28.6 (1.1) | 88.0 (3.1) |
|
| No | 11/24 | 13.3 (2.0) | 56.9 (10.4) |
|
| pT stage |
|
|
| 0.28 |
|
0–2 | 0/23 | NR | 100.0 (0.0) |
|
|
3–4 | 3/55 | NR | 98.2 (1.8) |
|
| pN stage |
|
|
| 0.43 |
| 0 | 1/42 | 32.3 (0.7) | 97.6 (2.4) |
|
|
1–2 | 2/32 | 32.3 (1.2) | 100.0 (0.0) |
|
| Metastasis |
|
|
|
<0.001b |
|
Yes | 27/59 | 15.9 (1.4) | 61.1 (6.8) |
|
|
Noa | 4/81 | NR | 97.5 (1.7) |
|
| Response to
CTx |
|
|
| 0.002b |
| Yes (CR
+ PR) | 2/17 | 23.6 (1.6) | 93.3 (6.4) |
|
| No (SD
+ PD) | 19/34 | 11.9 (1.4) | 47.6 (9.4) |
|
| Targeted
therapy |
|
|
| 0.55 |
|
Bevacizumab | 13/36 | 17.8 (1.7) | 69.9 (8.6) |
|
|
Cetuximab | 7/15 | 15.2 (2.8) | 52.5 (13.1) |
|
| Site of lesion |
|
|
| 0.03b |
|
Colon | 8/81 | 29.2 (1.2) | 91.0 (3.8) |
|
|
Rectum | 23/59 | 24.7 (1.6) | 76.6 (4.9) |
|
 | Table X.Univariate analyses of overall
survival according to laboratory parameters. |
Table X.
Univariate analyses of overall
survival according to laboratory parameters.
|
|
| Overall survival
(months) |
|---|
|
|
|
|
|---|
| Variables | Event no./total
no. | Median survival
(±SE) | 1-year survival, %
(±SE) | P-value |
|---|
| LDH |
|
|
|
|
|
Normal | 21/97 | 21.5 (0.9) | 84.6 (3.8) | 0.02a |
|
High | 7/16 | 20.5 (3.8) | 62.5 (12.1) |
|
| Albumin |
|
|
|
|
|
Normal | 7/54 | 23.2 (1.0) | 89.8 (4.3) | 0.02a |
|
Low | 20/58 | 23.4 (1.9) | 73.7 (5.8) |
|
| CEA |
|
|
|
|
|
Normal | 7/78 | 24.4 (0.6) | 95.7 (2.5) |
<0.001a |
|
High | 6/17 | 17.9 (2.6) | 68.0 (12.2) |
|
| CA19-9 |
|
|
|
|
|
Normal | 10/81 | 23.8 (0.7) | 93.4 (2.9) |
<0.001a |
|
High | 13/28 | 20.0 (2.8) | 61.5 (9.7) |
|
| EGFR |
|
|
|
|
|
<Median | 12/70 | 28.8 (1.4) | 84.7 (4.5) | 0.11 |
|
>Median | 19/70 | 20.1 (1.2) | 80.6 (4.9) |
|
Discussion
CRC is a major public health concern, with
continuously increasing incidence rates (23). In previous years, notable steps
forward in the molecular characterization of advanced CRC have been
taken. A multiplicity of serum markers have been proposed for early
diagnosis of CRC, estimation of the disease extent and monitoring
patient treatment (24,25).
EGFR has been detected in a wide variety of cancer
types, for some of which its overexpression has been suggested to
be a factor associated with poor prognosis and more aggressive
clinical progression (10). EGFR
expression has been demonstrated to be associated with poor outcome
in patients with stage IV CRC (11–14).
However, sEGFR levels and their diagnostic, prognostic and
predictive roles in CRC have not been investigated in detail.
For non-small-cell lung carcinoma patients, higher
sEGFR levels have been found to be significantly associated with a
higher OS, and the pre-treatment sEGFR levels constituted an
independent prognostic factor (26).
For advanced CRC, in the majority of the studies, the
clinicopathological characteristics of colon carcinoma are not
affected by EGFR expression (18,19);
however, in certain studies, a higher sEGFR level at baseline was
associated with the best objective response and may be considered a
significant predictor of outcome in patients with advanced CRC
(9). In the present study, the
baseline sEGFR level was significantly higher compared with the
control group (1704.39 vs. 1154.77 ng ml; P=0.002), whereas no
surgical resection, metastatic stage, higher pathological tumor
stage, poorer regression status (3–4) and
higher LDH levels were found to be correlated with higher sEGFR
concentrations (all P-values <0.05). However, sEGFR levels
exhibited no significantly adverse association with PFS and OS
(P=0.12 and P=0.11, respectively).
A previous study by Mourtzikou et al
(15) revealed that sEGFR levels
were significantly lower in the patient group when compared with
those in healthy control individuals; however, these authors
collected blood samples from 20 patients with CRC at a preoperative
state and from 30 patients undergoing chemotherapy, which may have
affected the study results. In another study performed by Zampino
et al (9), the greater the
sEGFR level at baseline, the lower the risk of no clinical
response; furthermore, a higher sEGFR at baseline was associated
with the best objective response to EGFR-targeted therapy and may
be considered as a significant predictor of outcome in patients
with advanced CRC.
In conclusion, CRC is a major public health concern
and its incidence rates continue to increase. Research into the
biology of CRC has identified a large number of tumor markers that
provide diagnostic, prognostic or predictive information. The
present study demonstrated that sEGFR concentrations may be
diagnostic markers in patients with CRC. However, their predictive
and prognostic values were not determined.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:692011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ionov Y, Peinado MA, Malkhosyan S, Shibata
D and Perucho M: Ubiquitous somatic mutations in simple repeated
sequences reveal a new mechanism for colonic carcinogenesis.
Nature. 363:558–561. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nonaka R, Miyake Y, Hata T, Kagawa Y, Kato
T, Osawa H, Nishimura J, Ikenaga M, Murata K, Uemura M, et al:
Circulating miR-103 and miR-720 as novel serum biomarkers for
patients with colorectal cancer. Int J Oncol. 47:1097–1102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang H, Fu XG and Chen YT: Serum level of
endothelial cell-specific molecule-1 and prognosis of colorectal
cancer. Genet Mol Res. 14:5519–5526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye
X, Chen Y, Chen D and Dettke M: Stage-dependent changes of
preoperative neutrophil to lymphocyte ratio and platelet to
lymphocyte ratio in colorectal cancer. Tumour Biol. 36:9319–9325.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Šunderić M, Šedivá A, Robajac D, Miljuš G,
Gemeiner P, Nedić O and Katrlík J: Lectin-based protein microarray
analysis of differences in serum alpha-2-macroglobulin
glycosylation between patients with colorectal cancer and persoons
without cancer. Biotechnol Appl Biochem. 63:457–464. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Fu XH, Yuan JQ, Yang ZY, Mao C, Dong
XM, Tang JL and Wang SY: Colorectal cancer: Using blood samples and
tumor tissue to detect K-ras mutations. Expert Rev Anticancer Ther.
15:715–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi N, Yamada Y, Furuta K, Nagashima
K, Kubo A, Sasaki Y, Shoji H, Honma Y, Iwasa S, Okita N, et al:
Association between serum ligands and the skin toxicity of
anti-epidermal growth factor receptor antibody in metastatic
colorectal cancer. Cancer Sci. 106:604–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zampino MG, Magni E, Santoro L, Zorzino L,
Dell'Orto P, Sonzogni A, Fazio N, Monfardini L, Chiappa A, Biffi R
and de Braud F: Epidermal growth factor receptor serum (sEGFR)
level may predict response in patients with EGFR-positive advanced
colorectal cancer treated with gefitinib? Cancer Chemother
Pharmacol. 63:139–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spano JP, Lagorce C, Atlan D, Milano G,
Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF,
et al: Impact of EGFR expression on colorectal cancer patient
prognosis and survival. Ann Oncol. 16:102–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheirsilpa A, Ruangvejvorachai P, Karalak
A, Sangprakarn S, Pummai S and Sangrajrang S: Determination of
epidermal growth factor receptor (EGFR) in patients with colorectal
cancer. Cancer Ther. 5:137–142. 2007.
|
|
12
|
Arteaga CL: The Epidermal Growth Factor
Receptor: From Mutant Oncogene in Non-human Cancers to Therapeutic
Target in Human Neoplasia. J Clin Oncol. 19 (18 Suppl):32S–40S.
2001.PubMed/NCBI
|
|
13
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37 Suppl 4:S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein NS and Armin M: Epidermal growth
factor receptor immunohistochemical reactivity in patients with
American Joint Committee on Cancer Stage IV colon adenocarcinoma:
Implications for a standardized scoring system. Cancer.
92:1331–1345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mourtzikou A, Stamouli M, Kroupis C,
Christodoulou S, Skondra M, Kastania A, Pectasides D, Athanasas G
and Dimas C: Evaluation of carcinoembryonic antigen (CEA),
epidermal growth factor receptor (EGFR), epithelial cell adhesion
molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9)
levels in colorectal cancer patients and correlation with
clinicopathological characteristics. Clin Lab. 58:441–448.
2012.PubMed/NCBI
|
|
16
|
Guo GF, Cai YC, Zhang B, Xu RH, Qiu HJ,
Xia LP, Jiang WQ, Hu PL, Chen XX, Zhou FF and Wang F:
Overexpression of SGLT1 and EGFR in colorectal cancers showing a
correlation with the prognosis. Med Oncol. 28 Suppl 1:S197–S203.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKay JA, Murray LJ, Curran S, Ross VG,
Clark C, Murray GI, Cassidy J and McLeod HL: Evaluation of the
epidermal growth factor receptor (EGFR) in colorectal tumors and
lymph node metastases. Eur J Cancer. 38:2258–2264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohhamadi G, Jamialahmadi K, Lary S and
Ghaffarzadegan K: Expression of
membranousepidermalgrowthfactorreceptor in colorectal
adenocarcinoma and its correlation with clinico pathological
features. Pak J Biol Sci. 14:357–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abd El, All HS, Mishriky AM and Mohamed
FA: Epidermal growth factor receptor in colorectal carcinoma:
Correlation with clinico-pathological prognostic factors.
Colorectal Dis. 10:170–178. 2008.PubMed/NCBI
|
|
20
|
AJCC (American Joint Committee on Cancer)
Cancer Staging Manual. 7th. Edge SB, Byrd DR, Compton CC, et al:
Springer; New York: pp. 1432010
|
|
21
|
Karnofsky DA and Burchenal JH: The
Clinical Evaluation of Chemotherapeutic Agents in CancerMacLeod CM:
Evaluation of Chemotherapeutic Agents. Columbia Univ Press; New
York: pp. 1961949
|
|
22
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2015. Cancer J Clin. 65:457–480..
2015. View Article : Google Scholar
|
|
24
|
Sturgeon CM, Duffy MJ, Stenman UH, Lilja
H, Brünner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ,
et al: National Academy of Clinical Biochemistry laboratory
medicine practice guidelines for use of tumor markers in
testicular, prostate, colorectal, breast, and ovarian cancers. Clin
Chem. 54:e11–e79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duffy MJ, vanDalen A, Haglund C, Hansson
L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C
and Topolcan O: Tumour markers in colorectal cancer: European Group
on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer.
43:1348–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romero-Ventosa EY, Blanco-Prieto S,
González-Piñeiro AL, Rodríguez-Berrocal FJ, Piñeiro-Corrales G and
de la Cadena Páez M: Pretreatment levels of the serum biomarkers
CEA, CYFRA 21-1, SCC and the soluble EGFR and its ligands, EGF,
TGF-alpha, HB-EGF in the prediction of outcome in erlotinib treated
non-small-cell lung cancer patients. Springerplus. 4:1712015.
View Article : Google Scholar : PubMed/NCBI
|