Introduction
Post-transplantation lymphoproliferative disorder
(PTLD) has been extensively described as a serious complication
following allogeneic hematopoietic stem cell transplantation (HSCT)
and solid-organ transplantation (SOT). The incidence of PTLD varies
from 1 to 20%, according to the type of organ transplant (1). It was recently reported that the
incidence rate of monomorphic PTLD following allogeneic SCT was
0.41% (3/730). The time to development of PTLD in the 3 reported
cases was 5.0, 5.8 and 5.9 months after receiving allogeneic SCT
(2).
The majority of PTLD cases are associated with
Epstein-Barr virus (EBV) infection. In the immunosuppressed state
following transplantation, suppression of T-cell activity
interferes with immune surveillance and allows proliferation of
latently infected B lymphocytes; the proliferation of a malignant
B-cell clone results in the development of lymphoma (3,4).
However, it was recently reported that 20–30% of PTLD cases were
EBV-negative. EBV-negative PTLDs occur later compared with
EBV-positive cases, have a higher proportion of monomorphic PTLD,
and exhibit a more aggressive clinical course (5,6). The
changes in the immunosuppressive regimens and new, unidentified
infectious agents may be involved in the pathogenesis of
EBV-negative PTLD (5).
We herein present the case of a recipient who
developed monomorphic PTLD of donor origin 6 months after
haploidentical HSCT (haplo-HSCT); the donor also developed the same
type lymphoma 1 year after the donation.
Case report
A 28-year-old female patient was admitted to the
General Hospital of Jinan Military District (Jinan, China) due to
continuous high fever for 1 week. The patient's hematological data
revealed pancytopenia. Based on the result of the bone marrow (BM)
examination, the patient was diagnosed with refractory anemia with
excess blasts accompanied by trilineage dysplasia, with 11.5% of
myeloblasts. Immunophenotyping of the blasts revealed 55.7, 89.2
and 79.2% of CD13-, CD33- and CD34-positive cells, respectively.
Cytogenetic screening of the blasts revealed no chromosomal
abnormalities. The patient suffered from repeated infectious
episodes, such as upper respiratory tract infection and bacterial
sepsis, and required blood transfusion. Due to the patient's poor
general condition and considering that further chemotherapy may
render her unsuitable for transplantation, she received
transplantation without chemotherapy 3 months after admission. The
conditioning regimen consisted of busulfan, cyclophosphamide,
cytarabine and semustine. Short-term methotrexate, mycophenolate
mofetil (MMF), cyclosporin A (CSA) and antithymocyte globulin (ATG)
were used as graft-versus-host disease (GVHD) prophylaxis. The
patient was an only child and did not have a suitable unrelated
donor; thus, she received a haplo-HSCT from her father (aged 45
years) on February 1, 2013, with 5/6 human leukocyte antigen (HLA)
locus matching. The recipient received mixed allografts of
recombinant human granulocyte colony-stimulating factor (G-CSF; 5
mg/kg/day for 5 days)-mobilized bone marrow and peripheral blood
stem cell harvests. EBV serological testing of the patient and the
donor prior to transplantation was negative. The total infused dose
of nucleated cells was 7.9×108/kg body weight, including
2.1×106/kg CD34-positive cells. If the neutrophil count
was <0.5×109/l, 5 mg/kg/day of G-CSF were
administered to help the recipient recover from granulocyte
depletion. When the neutrophil count reached
>1.5×109/l, the dose of G-CSF was slowly tapered.
Neutrophil (0.5×109/l) and platelet
(20×109/l) engraftment were observed on days 15 and 35,
respectively, after the transplantation. Bone marrow examination on
day 30 revealed a normocellular marrow without myelodysplasia.
Cytogenetic analysis revealed a normal male karyotype of 46, XY.
Fluorescence in situ hybridization (FISH) of the lymphocytes
was also used for chimerism detection, which indicated 98% donor
implant. At the 2-, 3-, 6-, 9- and 12-month follow-up, the
chimerism was complete (100%). On day 181, the patient developed a
skin rash, liver dysfunction and diarrhea, and was diagnosed with
grade II GVHD. The GVHD gradually improved with prednisolone
treatment. Bacterial pneumonia and hemorrhagic cystitis also
occurred, but were controlled with extended-spectrum
beta-lactamases (imipenem), and supportive treatment with
hydration, diuretics and platelets, respectively.
Once the acute GVHD of the recipient was controlled,
the immunosuppressive drugs were tapered. Six months after
haplo-HSCT, a neoplastic lesion sized 1×1.5 cm2 appeared
on the recipient's head (frontotemporal region). The mass had a
smooth surface, hard consistency, and was painless. Biopsy of the
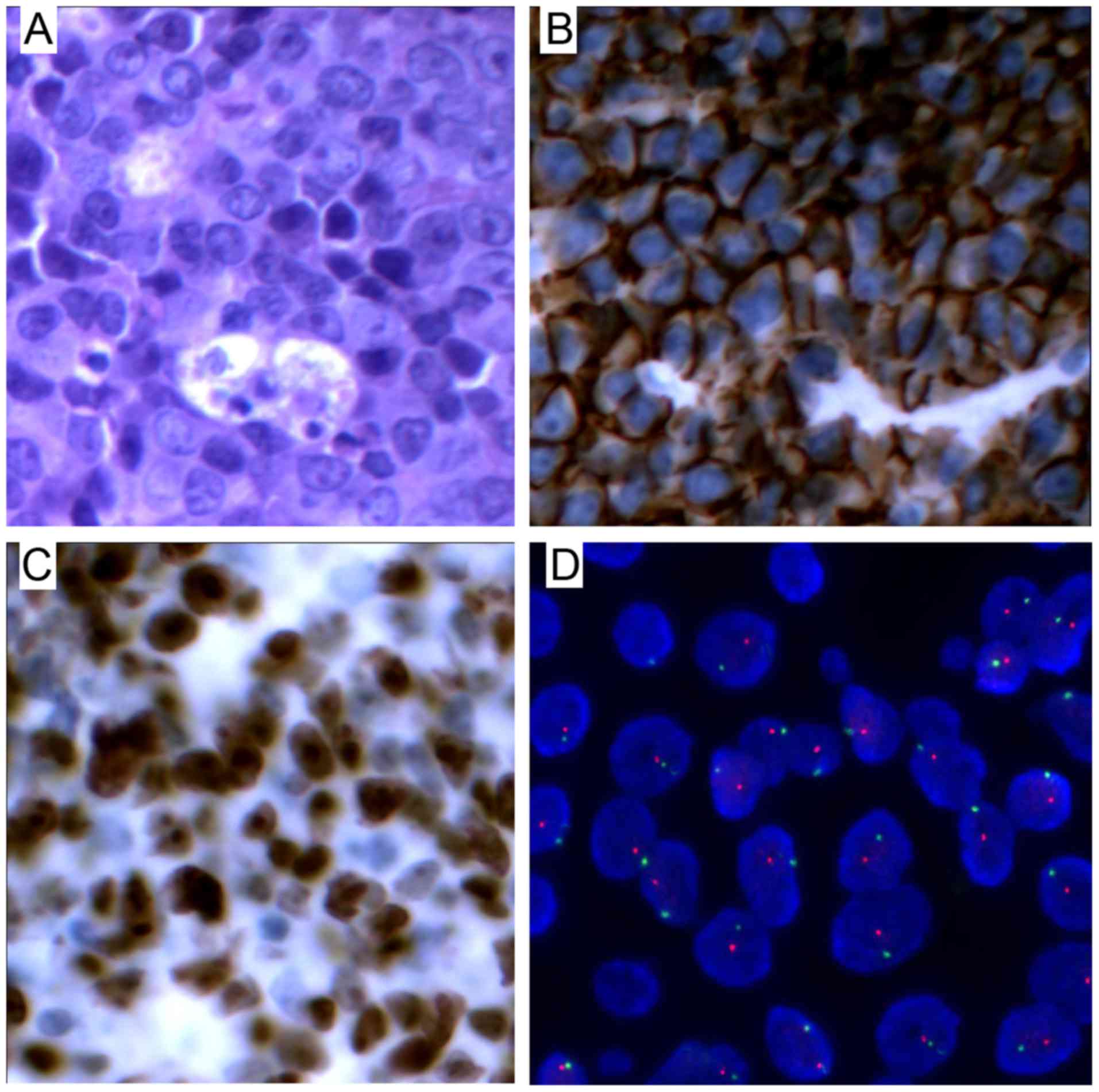
lesion was performed and the histopathological analysis revealed
destruction of the normal structure, with diffuse invasion by cells
exhibiting atypical nuclear bodies. The immunostaining for CD20,
paired Box (PAX)-5 and B-cell lymphoma (Bcl)-6 was positive and the
Ki-67 index was 90%, while cytomegalovirus (CMV) and EBV-encoded
small RNA (EBER) were negative. A clonality assay by immunoglobulin
heavy (IgH) chain gene rearrangement study was also performed by
polymerase chain reaction. The DNA extracted from the pathological
tissues confirmed the monoclonal origin of the neoplasm. FISH study
of a specimen from the patient confirmed the presence of XY male
donor cells and revealed that the lymphoma cells were of donor
origin (Fig. 1). The PTLD involved
monomorphic B cells and the type was diffuse large B-cell lymphoma
(DLBCL). Positron emission tomography-computed tomography (PET-CT)
examination revealed hypermetabolic lesions in multiple lymph
nodes, muscle, right breast and bone. PTLD was diagnosed on August
16, 2013, based on the abovementioned results. The patient was
managed by withdrawal of the immunosuppressant drugs (CSA and MMF)
and administration of chemotherapy, including two courses of
rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone (R-CHOP) and 4 courses of R-COP. PET examination showed
remission and the patient achieved a significant relief of the
signs and symptoms; she remained free of PTLD at the last follow-up
in January 2016, 25 months after the diagnosis of the disease.
Although the donor was disease-free prior to the
haplo-SCT, he was admitted to our hospital on February 22, 2014
(380 days after stem cell donation) due to left eye pain, blurred
vision and fever for 3 days. The head magnetic resonance imaging
examination revealed multiple lesions on the skull. On PET-CT,
hypermetabolic lesions were identified in the prostate, left side
of the seminal vesicle, multiple lymph nodes of the pelvic cavity
and bone. There were no obvious symptoms of frequent micturition,
urgent urination, urinary pain or hematuria. The free
prostate-specific antigen (PSA) and total PSA levels were normal.
The patient underwent transrectal prostate biopsy and the
histopathological analysis revealed DLBCL. The immunostaining for
CD20, PAX-5 and Bcl-6 was positive, the Ki-67 index was 61%,
whereas CMV and EBER were negative. The lactate dehydrogenase level
was 330 U/l and β2-microglobulin level was 2.8 mg/l. The donor was
diagnosed with stage IVB DLBCL; he received 6 courses of R-CHOP
chemotherapy and attained CR on August 13, 2014. He remained
disease-free for 23 months until relapse in July, 2015; after 4
courses of R-CHOP chemotherapy, the patient achieved a second CR,
and then he received an autologous stem cell transplantation in
February, 2016. The conditioning treatment consisted of carmustine,
etoposide, cytarabine and melphalan (BEAM regimen).
Discussion
PTLD is a life-threatening complication following
SOT and allogeneic HSCT (allo-HSCT), which encompasses a
heterogeneous group of lymphoproliferative disorders ranging from
reactive, polyclonal hyperplasia to aggressive non-Hodgkin's
lymphomas. The incidence of PTLD after allo-HSCT is ~1%, and the
majority of cases occur PTLD within the first year of
transplantation. The risk factors of PTLD include
immunodeficiencies caused by high-dose chemotherapy and/or
irradiation, T-cell depleted donor cells, the use of HLA-mismatched
transplants, intensive immunosuppression with T cells to prevent
GVHD, and antibodies, which may increase the risk for PTLD to up to
24% (7).
It was previously demonstrated that 60–70% of PTLD
cases are associated with EBV infection (8). The presence of EBV in lymphoid
proliferation in this setting has been used to support the
diagnosis of PTLD (9,10), determine clonal proliferation
(11), and possibly identify
patients at high risk of developing PTLD (12). Although a number of groups recommend
EBV positivity to be a biomarker for diagnosing PTLD, several
others have reported EBV-negative PTLD cases (5,13).
Compared with EBV-PTLD, the pathogenesis of EBV-negative PTLD is
less well defined. EBV-negative PTLD tends to occur later compared
with EBV-positive cases following transplantation, the majority of
EBV-negative PTLD cases are of monomorphic type, more aggressive
and carry an overall poorer prognosis (6). In all published reports, the average
occurrence time of initial EBV-negative PTLD was at 50 months
post-transplantation compared with 10 months for EBV-positive
cases. It has been hypothesized that EBV-negative PTLD cases are
associated with other viral infections, such as human herpes virus
8 and CMV (14,15), and hit-and-run infection (16) or chronic antigen stimulation by the
graft (17).
In the present case, both the patient and donor were
EBV-negative, the patient suffered from monomorphic PTLD only 6
months after the transplantation, and the donor developed the same
type of lymphoma 1 year after the donation. The interval to
EBV-negative PTLD occurrence was significantly shorter compared
with that previously reported (18).
The immunodeficiency caused by high-dose chemotherapy as part of
the conditioning regimen, ATG, CSA and MMF to deplete donor T cells
and prevent GVHD, haploidentical HSCT and acute GVHD were the
important factors for PTLD. With the exception of the
abovementioned reasons, preconditioning therapy,
chemotherapy-induced recipient stromal abnormalities and
transfection of a leukaemogenic agent (viral or non-viral) from
host to donor cells may also be associated with malignant
transformation of donor progenitor cells (19). Since the donor developed the same
type of lymphoma 1 year after the donation, it is possible that the
patient's PTLD was transmitted from donor to recipient. It was
difficult to distinguish between donor-transmitted and
donor-derived tumors.
Donor-related malignancies are frequently reported
in solid organ transplants. According to a report from transplant
centers, a total of 21 donor-related malignancies from 14 cadaveric
and 3 living donors were reported in a cohort of 34,933 cadaveric
donors and 108,062 recipients (20).
A total of 15 malignancies were donor-transmitted and 6 were
donor-derived. Transmitted tumors were defined as malignancies that
are present in the donors at the time of transplantation, while
derived tumors are de novo tumors that develop in
transplanted donor hematogenous or lymphoid cells after the
transplantation (20). In the
present case, the donor was diagnosed with DLCBL 1 year after the
donation. Although the physical and serological examination did not
reveal any evidence of DLCBL, it cannot be excluded that the donor
was in the period of transition from reactive or polyclonal
hyperplasia to aggressive non-Hodgkin lymphoma at the time of the
transplantation.
This case is noteworthy, as it demonstrates that
lymphoma may be transmitted from the donor to the recipient by
allo-HSCT, which is new evidence for the occurrence of PTLD.
Furthermore, the donor was healthy prior to the donation. Although
there is no direct evidence for the association between HSCT and
the occurrence of lymphoma in the donor and the recipient, it
should be noted that the health of the donor is a crucial
factor.
In summary, we herein reported a case of PTLD
transmitted from donor to recipient during HSCT. Thus, more
sensitive screening assays should be performed to exclude donors
with malignant hematological cancers.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (no. 8150010892) and the
Presidential Foundation of the General Hospital of Jinan Military
District (no. 2014ZD03).
References
|
1
|
Lactase AS: Post-transplant
lymphoproliferative disorders. Oncologist. 11:674–680. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi JH, Park BB, Suh C, Won JH, Lee WS
and Shin HJ: Clinical characteristics of monomorphic
post-transplant lymphoproliferative disorders. J Korean Med Sci.
25:523–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savoie A, Perpête C, Carpentier L, Joncas
J and Alfieri C: Direct correlation between the load of
Epstein-Barr virus-infected lymphocytes in the peripheral blood of
pediatric transplant patients and risk of lymphoproliferative
disease. Blood. 83:2715–2722. 1994.PubMed/NCBI
|
|
4
|
Chang MS and Kim WH: Epstein-Barr virus in
human malignancy: A special reference to Epstein-Barr virus
associated gastric carcinoma. Cancer Res Treat. 37:257–267. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leblond V, Davi F, Charlotte F, Dorent R,
Bitker MO, Sutton L, Gandjbakhch I, Binet JL and Raphael M:
Post-transplant lymphoproliferative disorders not associated with
Epstein-Barr virus: A distinct entity? J Clin Oncol. 16:2052–2059.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson BP, Nalesnik MA, Bahler DW, Locker
J, Fung JJ and Swerdlow SH: Epstein-Barr virus-negative
post-transplant lymphoproliferative disorders: A distinct entity?
Am J Surg Pathol. 24:375–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottschalk S, Rooney CM and Heslop HE:
Post-transplant lymphoproliferative disorders. Annu Rev Med.
56:29–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loren AW, Porter DL, Stadtmauer EA and
Tsai DE: Post-transplant lymphoproliferative disorder: A review.
Bone Marrow Transplant. 31:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frizzera G, Hanto DW, Gajl-Peczalska KJ,
Rosai J, McKenna RW, Sibley RK, Holahan KP and Lindquist LL:
Polymorphic diffuse B-cell hyperplasias and lymphomas in renal
transplant recipients. Cancer Res. 41:4262–4279. 1981.PubMed/NCBI
|
|
10
|
Hanto DW, Frizzera G, Purtillo DT,
Sakamoto K, Sullivan JL, Saemundsen AK, Klein G, Simmons RL and
Najarian JS: Clinical spectrum of lymphoproliferative disorders in
renal transplant recipients and evidence for the role of
Epstein-Barr virus. Cancer Res. 41:4253–4261. 1981.PubMed/NCBI
|
|
11
|
Locker J and Nalesnik M: Molecular genetic
analysis of lymphoid tumors arising after organ transplantation. Am
J Pathol. 135:977–987. 1989.PubMed/NCBI
|
|
12
|
Randhawa PS, Jaffe R, Demetris AJ,
Nalesnik M, Starzl TE, Chen YY and Weiss LM: Expression of
Epstein-Barr virus-encoded small RNA (by the EBER-1 gene) in liver
specimens from transplant recipients with post-transplantation
lymphoproliferative disease. N Engl J Med. 327:1710–1714. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knowles DM, Cesarman E, Chadburn A,
Frizzera G, Chen J, Rose EA and Michler RE: Correlative morphologic
and molecular genetic analysis demonstrates three distinct
categories of post-transplantation lymphoproliferative disorders.
Blood. 85:552–565. 1995.PubMed/NCBI
|
|
14
|
Capello D and Gaidano G: Post-transplant
lymphoproliferative disorders: Role of viral infection, genetic
lesions and antigen stimulation in the pathogenesis of the disease.
Mediterr J Hematol Infect Dis. 1:e20090182009.PubMed/NCBI
|
|
15
|
Mañez R, Breinig MC, Linden P, Wilson J,
Torre-Cisneros J, Kusne S, Dummer S and Ho M: Posttransplant
lymphoproliferative disease in primary Epstein-Barr virus infection
after liver transplantation: The role of cytomegalovirus disease. J
Infect Dis. 176:1462–1467. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jox A, Rohen C, Belge G, Bartnitzke S,
Pawlita M, Diehl V, Bullerdiek J and Wolf J: Integration of
Epstein-Barr virus in Burkitt's lymphoma cells leads to a region of
enhanced chromosome instability. Ann Oncol. 8 Suppl 2:S131–S135.
1997. View Article : Google Scholar
|
|
17
|
Morscio J, Dierickx D, Ferreiro JF,
Herreman A, Van Loo P, Bittoun E, Verhoef G, Matthys P, Cools J,
Wlodarska I, et al: Gene expression profiling reveals clear
differences between EBV-positive and EBV-negative posttransplant
lymphoproliferative disorders. Am J Transplant. 13:1305–1316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evens AM, David KA, Helenowski I, Nelson
B, Kaufman D, Kircher SM, Gimelfarb A, Hattersley E, Mauro LA,
Jovanovic B, et al: Multicenter analysis of 80 solid organ
transplantation recipients with post-transplantation
lymphoproliferative disease: Outcomes and prognostic factors in the
modern era. J Clin Oncol. 28:1038–1046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brunstein CG, Hirsch BA, Hammerschmidt D,
McGlennen RC, Nguyen PL and Verfaillie CM: Leukemia in donor cells
after allogeneic hematopoietic stem cell transplant. Bone Marrow
Transplant. 29:999–1003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kauffman Myron H, McBride MA, Cherikh WS,
Spain PC, Marks WH and Roza AM: Transplant tumor registry: Donor
related malignancies. Transplantation. 74:358–362. 2002. View Article : Google Scholar : PubMed/NCBI
|