Introduction
Plasma cell tumours are lymphoid B-cell neoplasms
that are composed of plasma cells. These tumours may develop in a
disseminated manner, affecting numerous bones (multiple myeloma),
or, more rarely, as a solitary lesion in a single bone (solitary
bone medullary plasmacytoma) or soft tissue
[extramedullary/extraosseous plasmacytoma (EOP)]. EOP is uncommon,
accounting for ~5% of all plasma cell neoplasms, and arises outside
of the bone marrow, unaccompanied by any clinical evidence of
existing multiple myeloma. The median age at diagnosis is ~55
years, and approximately two out of three patients are male
(1,2). In ~80% of cases of EOP, the neoplasm
arises in the upper respiratory tract, including the oropharynx,
nasopharynx, and sinuses; however, EOP may be located at various
other sites, such as the lymph nodes, bladder, digestive system,
breast, thyroid, central nervous system, and skin (1).
The clinical manifestations and symptoms of EOP,
where present, are non-specific, as these depend on the location of
the tumour. EOP manifests as a sessile or pediculate outgrowth,
which may be either circumscribed or infiltrating (1,2).
Following the diagnosis of the tumour locally, it is necessary to
exclude the existence of any systemic processes in order to confirm
the diagnosis of EOP. Following treatment, ~70% of patients remain
in complete remission for at least 10 years. However, in ~25% of
cases, regional recurrences eventually develop, and metastasis to
distant extraosseous sites also occurs occasionally (2). The present report provides a literature
review of cases of parotid plasmacytoma published up until 2016, in
addition to a presentation of one new clinical case taken from the
personal experience of the authors.
Case report
A 47-year-old man was referred to the Maxillofacial
Surgery Clinic at Virgen del Rocio University Hospital (Seville,
Spain) in January 2015, with a 3-month history of a painless lesion
in the retroauricular area that had gradually increased in size.
The patient reported a medical history that included arterial
hypertension and sacrococcygeal trauma. Physical examination
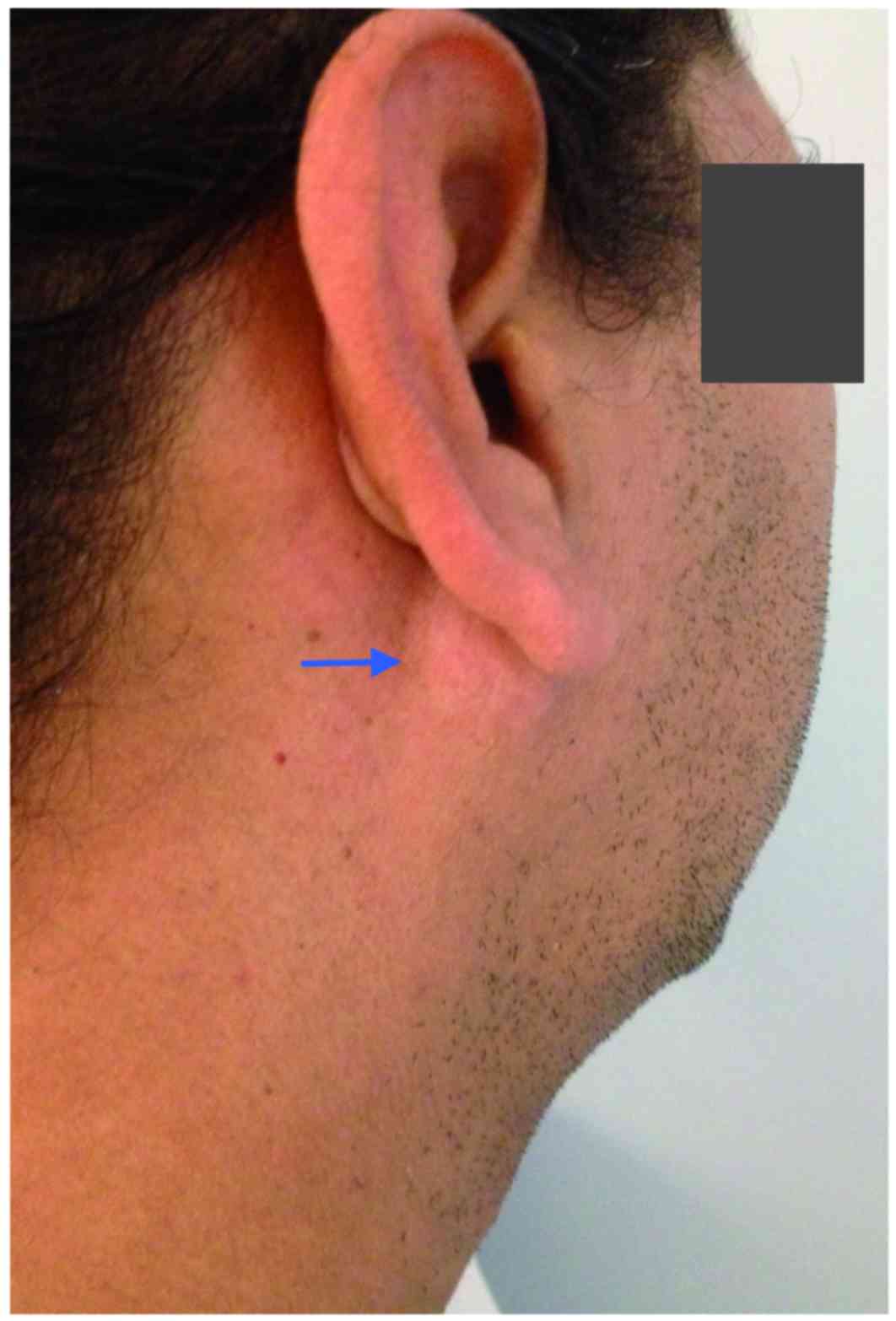
revealed a 3×3-cm, lobulated mass in the right parotid area, which
was moderately tender upon palpation (Fig. 1). The facial nerve was intact, and
there was no evidence of palpable cervical lymph nodes. Clinical
examination was otherwise non-contributory.
On ultrasound, a mass of reduced echogenicity was
detected, without any evidence of cervical lymph node enlargement.
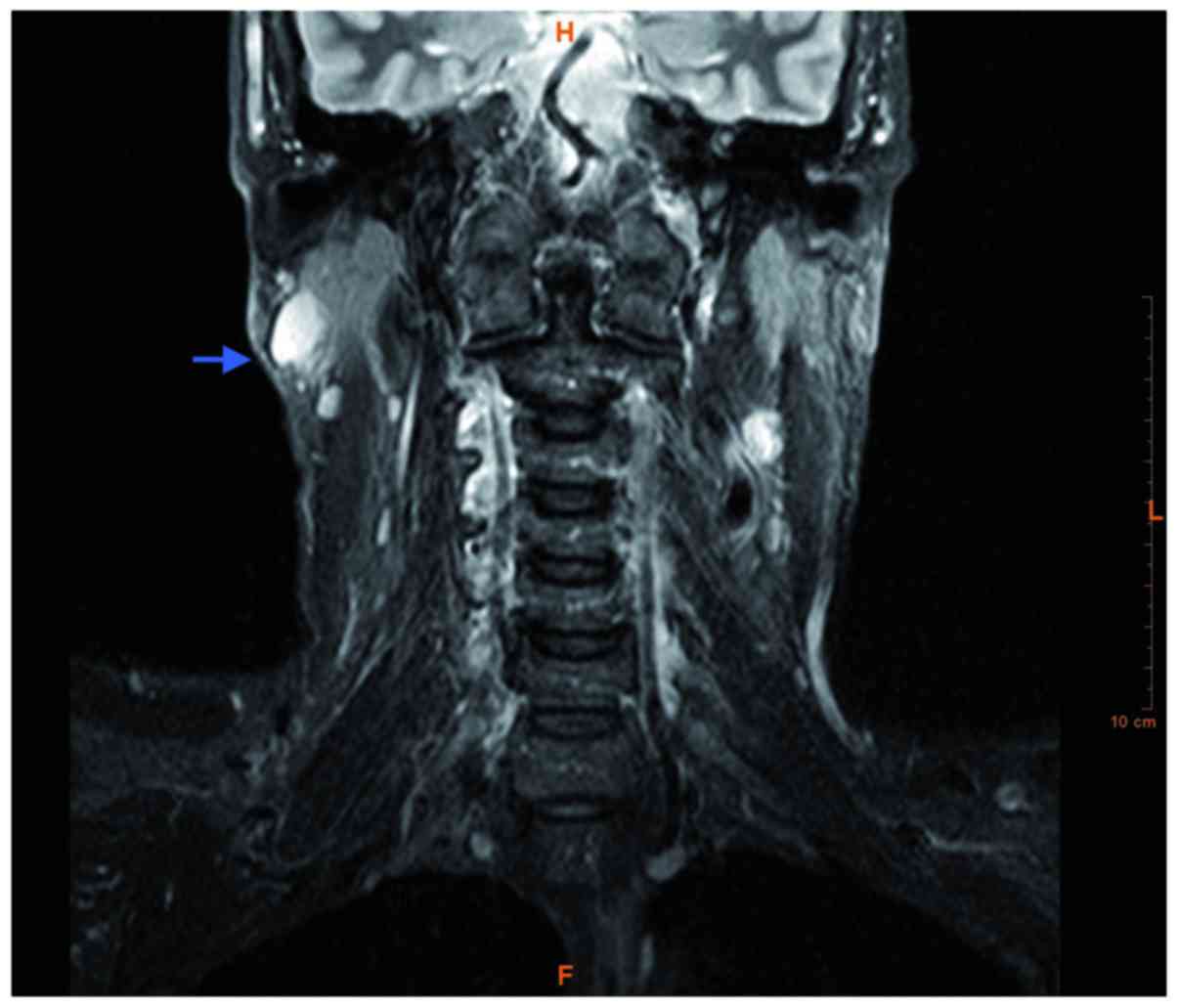
Magnetic resonance imaging of the head and neck revealed a right
parotid tail mass in the superficial portion of the right parotid
gland. The mass measured 3×3×3 cm, was round with well-defined
contours, and appeared hypointense on T1 and T2 sequences, and
hyperintense on T2-short tau inversion recovery sequences (Fig. 2). Ultrasonographically guided
fine-needle aspiration cytology was performed, and the subsequent
cytological analysis demonstrated diffuse infiltration of
neoplastic large monoclonal plasmacytes with variable pleomorphism.
This finding was suggestive of a lymphoproliferative lesion. Under
general anaesthesia, a superficial parotidectomy was performed,
following which the patient had an uneventful course and was
discharged on the third postoperative day, and followed up at the
outpatient clinic. Evaluation of the parotid gland was subsequently
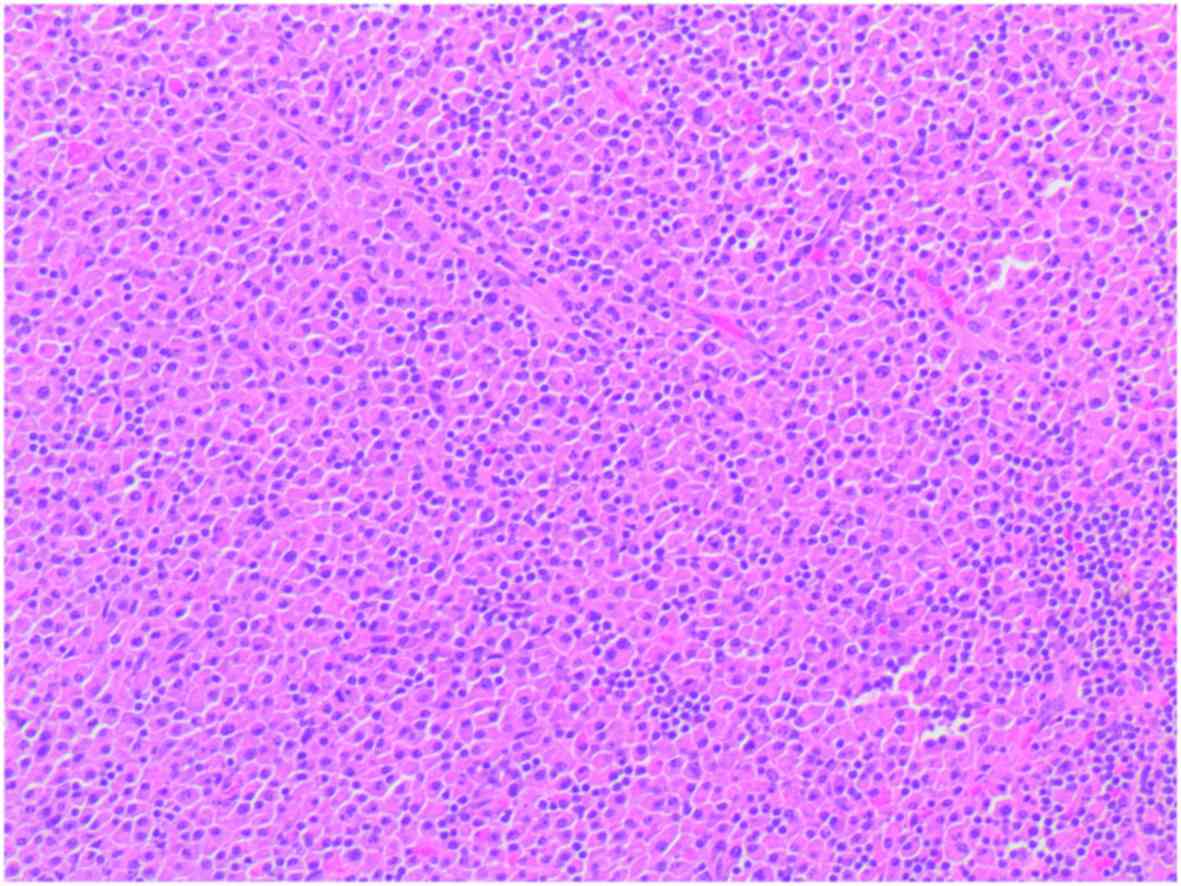
performed. The histological examination of tissue sections stained
with haematoxylin and eosin showing an intraglandular lymph node
with a diffuse plasmacytic proliferation among residual reactive
lymphoid follicles, with minimal pleomorphism (some large nuclei
and binucleated cells) (Fig. 3). An
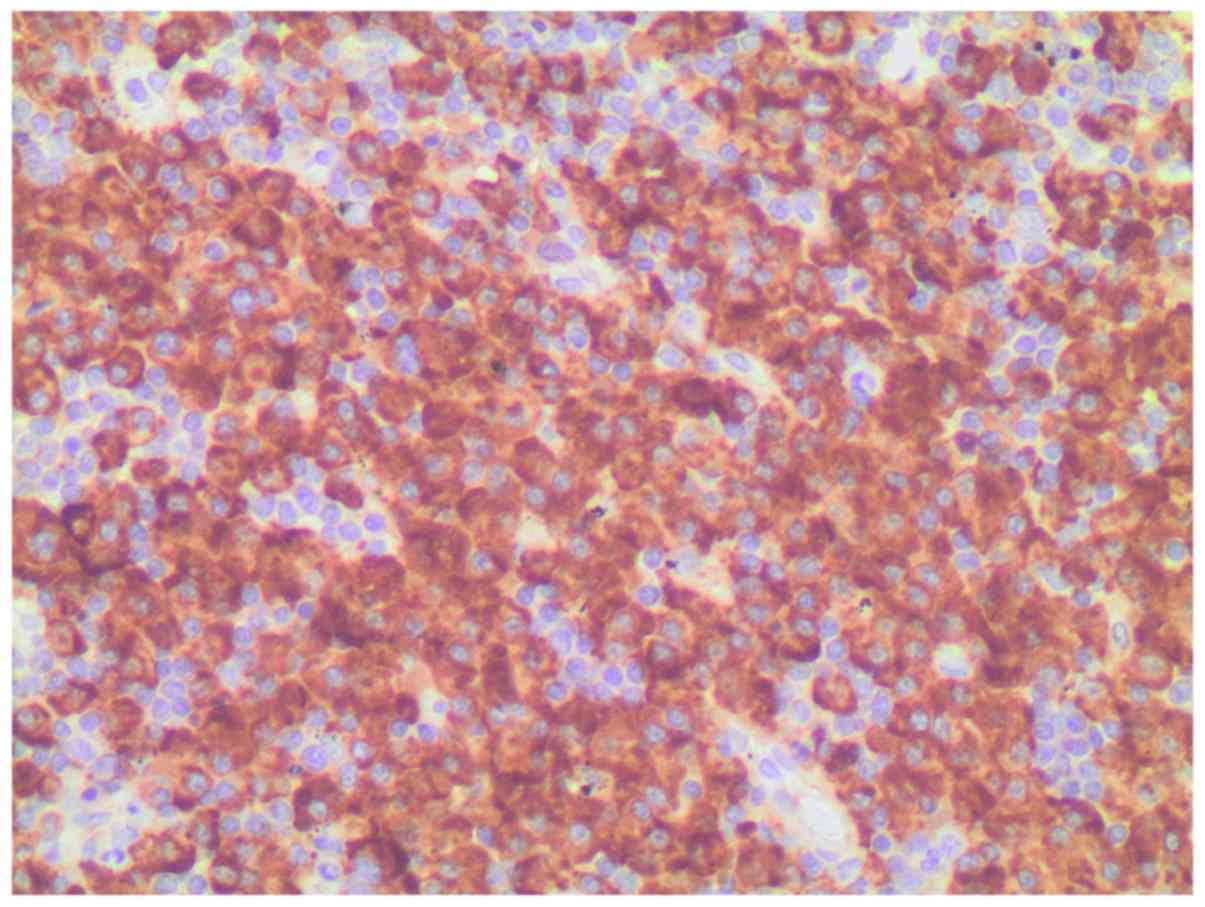
immunohistochemical study revealed monotypic λ light chain
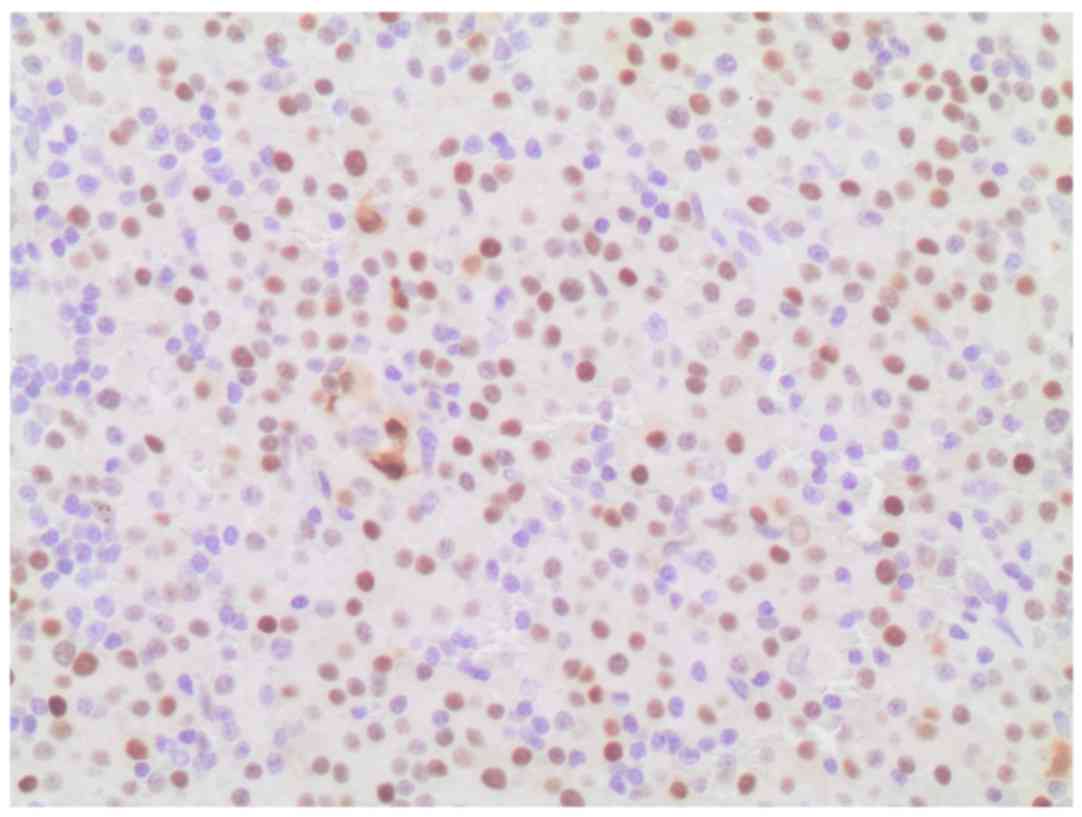
restriction, as well as nuclear immunostaining for cyclin D1
(Figs. 4 and 5), and aberrant expression of CD10
(positive expression, which is not associated with the specific
cell type). CD56 was found to be negative. No monocytic or marginal
lymphoid proliferation was observed; therefore, a diagnosis of
cyclin D1-positive, λ plasmacytic plasmacytoma was determined.
To differentiate between plasmacytoma and myeloma, a
systemic workup was performed. A skeletal radiographic survey
revealed no evidence of extraoral involvement on computed
tomography of the chest, abdomen, or pelvis; and positron emission
tomography with 18F-fludeoxyglucose (FDG) showed a hypermetabolic
lesion (standardised uptake value, 3.9) with a soft tissue
component in the right cervical area, which coincided with the
parotid tail mass detected on the ultrasound study. No FDG-avid
lymphadenopathy or distant metastasis was detected. A bone marrow
biopsy was negative for malignancy, and laboratory analysis did not
reveal anaemia, hypercalcaemia or renal involvement. Assays for
Bence-Jones protein in the urine and for serum myeloma protein were
negative. A final diagnosis of primary extraosseous/extramedullary
plasmacytoma was made. Due to the aggressive nature of the disease,
postoperative radiotherapy of the parotid region and neck,
including the supraclavicular lymph node area, was performed, with
a total dose of 30 Gy. The patient has been followed-up closely for
2 years, during which time he has remained completely devoid of any
clinical symptoms, and shows no evidence of recurrence of the
parotid tumour.
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Discussion
For the present study, a literature search of
Medline/PubMed (http://www.ncbi.nlm.nih.gov/pubMed) that included
articles published up to June 2016 was conducted using the search
term ‘parotid plasmacytoma’. The inclusion criteria were as
follows: English language articles identified in the Medline
database, containing at least one case of plasmacytoma located in
the parotid gland, along with detailed clinical, diagnostic or
therapeutic criteria. The search revealed 18 papers, encompassing a
total of 19 cases, that met these criteria. In total, 20 patients
with plasmacytoma in the parotid gland were evaluated, including
the 19 clinical cases taken from the literature, and the new
clinical case presented in the current study (Table I). The mean age of the patients at
the time of diagnosis, excluding the present case case, was
65.1±10.9 years (range, 38–78 years). The cases comprised 10 female
patients (52.6%) and 9 male patients (47.4%), with parotid
plasmacytoma located unilaterally in all cases: 10 patients showed
left-side involvement, while 9 cases exhibited right-side
involvement. The most frequent signs and symptoms were pain and
swelling on the ipsilateral side. Treatment included chemotherapy
in 3 cases, radiotherapy in 11 cases, and surgical removal in 15
cases.
 | Table I.Overview of published cases of parotid
extraosseous plasmacytoma. |
Table I.
Overview of published cases of parotid
extraosseous plasmacytoma.
| Authors, year | Age/sex | Side | Treatment | (Refs.) |
|---|
| Vainio-Mattila | 74/F | Left | S + RT | (3) |
| Pahor | 61/F | Left | S + CT + RT | (4) |
|
| 69/M | Right | S + CT + RT |
|
| Ferlito et
al | 47/M | Left | S + RT + CT | (5) |
| Kurihara and
Hashimoto | 61/F | Right | S | (6) |
| Edney et
al | 38/M | Right | S | (7) |
| Kanoh et
al | 78/F | Left | S + RT | (8) |
| Ebbers | 68/M | Right | RT | (9) |
| Scholl and Jafek | 60/F | Left | S | (10) |
| Simi et
al | 58/M | Right | S | (11) |
| Rothfield et
al | 53/M | Left | S | (12) |
| Kerr and Dort | 73/F | Right | S + RT | (13) |
| El-Naggar et
al | 73/M | Left | S | (14) |
| Gonzalez-Garcia et
al | 63/M | Right | S + RT | (15) |
| Hari and Roblin | 60/F | Right | S | (16) |
| Ustun et
al | 77/F | Left | S + RT | (17) |
| Kanthan and
Torkian | 73/F | Right | S + RT + CT | (18) |
| Gouveris et
al | 76/M | Left | RT | (19) |
| Alabed et
al | 75/F | Left | RT | (20) |
| Our case | 47/M | Right | S+RT | – |
A plasmacytoma is a solitary mass of neoplastic
monoclonal plasma cells, which can arise in any area of the body.
EOP arises from the plasma cells located in areas of soft tissue,
whereas solitary bone medullary plasmacytoma arises from plasma
cells located in the bone marrow. These two forms of disease
represent different groups of neoplasms with regard to location,
tumour progression, and overall survival rate (1,2). The
diagnosis of EOP, which occurs far less commonly than that of
solitary plasmacytoma of the bone (1), is determined based on the
identification of a localised tumour comprising monoclonal plasma
cells, which are identical to those observed in multiple myeloma;
however, EOP is distinguished by an absence of indicators of the
disseminated form of disease, such as additional lesions detected
on skeletal radiological imaging, the presence of bone marrow
plasmacytosis, and the presence of anaemia, hypercalcaemia, or
renal failure, as was the case for the current patient. Solitary
primary EOP is rare, and is predominantly diagnosed in elderly
individuals (4,13,15). EOP
occurs most often in the head and neck area, and tends to involve
the submucosal tissues of the upper respiratory tract, accounting
for ~0.5% of all neoplasms that occur at this location. Involvement
of the parotid gland is extremely rare, as evidenced by the fact
that only 19 cases (Table I)
(3–20) have been previously documented in the
published literature, including the first case described in 1965 by
Vainio-Mattila (3). Although the
current literature review suggested that parotid gland EOP has a
favourable prognosis, assuming that multiple myeloma has been ruled
out, the clinical behaviour of this disease is not well understood.
The conversion rate of solitary EOP to solitary plasmacytoma of the
bone is 48%, while that of EOP to multiple myeloma is 20%, both of
which are associated with a poorer prognosis (1,2,20). A definitive diagnosis of parotid EOP
cannot be established solely by preoperative investigations, as
demonstrated in the present case; however, it remains important to
recognise this distinctive type of plasmacytoma confined to the
parotid area, in order to avoid confusion with other parotid benign
lesions/reactive enlargements that have a similar appearance.
As mentioned previously, histopathological analysis
in isolation is insufficient to confirm a diagnosis of primary
extramedullary plasmacytoma, since it is necessary to exclude the
possibility of multiple myeloma through skeletal and marrow
examinations, immunoelectrophoresis, quantitative immunoglobulin
testing, and confirmation of the absence of urinary Bence Jones
proteins (17). Cyclin D1 expression
in EOP, as observed in the current patient, has been associated
with an unfavourable prognosis in previous studies (19); however, this did not seem to be true
of the present case. In addition, the distinction between
plasmacytoma and lymphoma with extreme plasma cell differentiation
may be challenging (1,2).
The treatment of solitary extramedullary
plasmacytoma should consist primarily of eradication of the lesion
locally, and surgery therefore appears to be the primary line of
treatment. Radiation therapy has also been used in some cases,
either alone in patients deemed unsuitable for surgery, or as an
adjunct to the surgical removal of the lesion in order to prevent
local relapse, as in the current case. The rates of conversion of
solitary EOP to solitary plasmacytoma of the bone and to multiple
myeloma are 48 and 20%, respectively, and both are associated with
a poorer prognosis (1–20). Although not scientifically validated,
chemotherapy is theoretically advantageous: As well as augmenting
local control of the neoplasm, it may potentially eradicate
sub-clinical disease, delaying or preventing the development of
myeloma (2). Patterns of primary
extramedullary plasmacytoma relapse include local recurrence,
transformation to multiple myeloma, and metastasis. Recurrences are
typically localized and respond well to radiotherapy. Combined
chemotherapy and radiation therapy is recommended for high-risk
surgical patients (ASA physical status classification system
category ≥3), to increase the rates of local control and cure
(2,18–20).
In summary, although patients with parotid
enlargements are commonly observed in everyday clinical practice,
EOP arising in the parotid gland is extremely rare. Fine-needle
aspiration cytology, histopathological examination,
immunohistochemical analysis, and an absence of signs indicating
disseminated disease aid in providing an accurate diagnosis and
treatment. EOP should be considered in the differential diagnosis
of parotid tumours.
References
|
1
|
McKenna RW, Kyle RA, Kuehl WM, Grogan TM,
Harris NL and Coupland RW: Plasma Cell NeoplasmsSwerdlow SH, Campo
E and Harris NL: WHO Classification of Tumours of Haematopoietic
and Lymphoid Tissues. Lyon: IARC Press; pp. 200–213. 2008
|
|
2
|
Bachar G, Goldstein D, Brown D, Tsang R,
Lockwood G, Perez-Ordonez B and Irish J: Solitary extramedullary
plasmacytoma of the head and neck-long-term outcome analysis of 68
cases. Head Neck. 30:1012–1019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vainio-Mattila J: Plasmacytoma of the
parotid gland. Arch Otolaryngol. 82:635–637. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pahor AL: Extramedullary plasmacytoma of
the head and neck, parotid and submandibular salivary glands. J
Laryngol Otol. 91:241–258. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlito A, Polidoro F and Recher G:
Extramedullary plasmacytoma of the parotid gland. Laryngoscope.
90:486–493. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurihara K and Hashimoto N: Extramedullary
plasmacytoma associated with a Castleman's lesion of the cervical
nodes. J Oral Pathol. 12:131–138. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edney JA, Thompson JS, Conley MC and Moore
GE: Plasmacytoma of the parotid gland. J Surg Oncol. 28:165–167.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanoh T, Hattori N, Uchino H, Fujita A,
Ohmura M and Makimoto K: Extramedullary plasmacytoma of the parotid
gland: Report of a case and review of the literature. Tohoku J Exp
Med. 146:469–478. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ebbers J: Plasmacytoma of the parotid
gland. Laryngol Rhinol Otol (Stuttg). 65:127–129. 1986.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scholl P and Jafek BW: Extramedullary
plasmacytoma of the parotid gland. Ear Nose Throat J. 65:564–567.
1986.PubMed/NCBI
|
|
11
|
Simi U, Marchetti G, Bruno R, Di Nasso F
and Cardini M: Plasmacytoma of the parotid gland: Report of a case
and review of the world literature. Acta Otorhinolaryngol Belg.
42:93–96. 1988.PubMed/NCBI
|
|
12
|
Rothfield RE, Johnson JT and Stavrides A:
Extramedullary plasmacytoma of the parotid. Head Neck. 12:352–354.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerr PD and Dort JC: Primary
extramedullary plasmacytoma of the salivary glands. J Laryngol
Otol. 105:687–692. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
el-Naggar AK, Ordonez NG and Batsakis JG:
Parotid gland plasmacytoma with crystalline deposits. Oral Surg
Oral Med Oral Pathol. 71:206–208. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gonzalez-Garcia J, Ghufoor K, Sandhu G,
Thorpe PA and Hadley J: Primary extramedullary plasmacytoma of the
parotid gland: A case report and review of the literature. J
Laryngol Otol. 112:179–181. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hari CK and Roblin DG: Solitary
plasmacytoma of the parotid gland. Int J Clin Pract. 54:197–198.
2000.PubMed/NCBI
|
|
17
|
Ustun MO, Ekinci N and Payzin B:
Extramedullary plasmacytoma of the parotid gland. Report of a case
with extensive amyloid deposition masking the cytologic and
histopathologic picture. Acta Cytol. 45:449–453. 2001.PubMed/NCBI
|
|
18
|
Kanthan R and Torkian B: Solitary
plasmacytoma of the parotid gland with crystalline inclusions: A
case report. World J Surg Oncol. 1:122003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gouveris H, Hansen T and Franke K:
Solitary extramedullary plasmacytoma and granulomatous sialadenitis
of the parotid gland preceding a B-cell non-Hodgkin's lymphoma.
Mund Kiefer Gesichtschir. 10:122–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alabed YZ, Rakheja R and Laufer J:
Solitary extramedullary plasmacytoma of the parotid gland imaged
with 18F-FDG PET/CT. Clin Nucl Med. 39:549–550. 2014. View Article : Google Scholar : PubMed/NCBI
|