Introduction
Colorectal cancer is a common malignancy worldwide
with approximately 1400,000 newly diagnosed cases and 694,000
cancer-related death cases (1). Due
to the increasing life expectancy, earlier diagnosis and improved
surgery, pre- and postoperative oncological treatment and regular
checkups, the proportion of elderly colorectal cancer patients has
been on the increase (2), reaching
up to 75% as reported by Hermans et al (3). Elderly patients of colorectal cancer
frequently experience decreased functional reserve and increased
comorbidities such as cardiovascular and pulmonary diseases, which
make them under-represented in clinical trials (2). In general colorectal cancer patients,
laparoscopy can achieve shorter hospital stay, faster postoperative
recovery, equal local recurrence rate, as well as disease-free and
overall survival (4,5).
In elderly colorectal cancer patients, comparative
clinical trials concerning laparoscopic vs. open surgery are
limited. Several meta-analyses compared the short-term outcomes
between laparoscopy and laparotomy for elderly colorectal cancer
patients and concluded that laparoscopic surgery achieved less
intraoperative blood loss (6),
longer operating time (6), shorter
length of hospital stay (6–8), lower postoperative morbidity and rapid
bowel function recovery (6–8). To the best of our knowledge, no
meta-analysis of laparoscopy vs. laparotomy has been performed with
regard to long-term outcomes for elderly colorectal cancer
patients.
In the present meta-analysis, we collected data from
previous studies to perform a meta-analysis of laparoscopy vs.
laparotomy concerning long-term outcomes for elderly colorectal
cancer patients with the aim of selecting improved surgical
techniques in this age subgroup.
Data collection methods
Search strategy
Studies were searched with regard to the comparison
of long-term outcomes between laparoscopy and open surgery for
elderly colorectal cancer patients from inception to April 20,
2017. The search strategy for Medline and other databases involved
the following key words: ((((‘Colorectal Neoplasms’ [MeSH] OR
‘Rectal Neoplasms’[MeSH] OR ‘Colonic Neoplasms’[MeSH] OR
colectomy[Title] OR sigmoidectomy[Title] OR ‘Colorectal
resection’[Title))] AND (laparoscopically[Title/Abstract] OR
laparoscopic[Title/Abstract] OR laparoscopy[Title/Abstract))] AND
(open[Title/Abstract] OR laparotomy[Title/Abstract))] AND
(elder[Title/Abstract] OR elderly[Title/Abstract] OR
old[Title/Abstract] OR aged[Title/Abstract] OR
octogenarian[Title/Abstract] OR nonagenarian[Title/Abstract] OR
octogenarians[Title/Abstract] OR nonagenarians[Title/Abstract] OR
older[Title/Abstract)]. Randomized control trials (RCTs), two-arm
prospective studies, retrospective studies, and cohort studies were
included. The reference list of potential studies was searched
manually for eligibility by two independent reviewers, and if there
was disagreement regarding inclusion, a third reviewer was
consulted.
Inclusion criteria
Inclusion criteria for the study were: i) Research
population was colorectal cancer patients who were ≥65. ii) The
intervention of the experimental and control groups was laparoscopy
and open surgery, respectively. iii) The endpoints included a 3- or
5-year survival rate. iv) The study design was RCT or other
comparative study.
Exclusion criteria
Exclusion criteria for the study were: i)
Non-elderly colorectal cancer patients. ii) Any study without a
control group. iii) The patients were limited to tumor stage I or
II. iv) No long-term outcomes were described. v) Non-English
articles were excluded.
Data extraction
Data extraction was crosschecked synchronously
between two authors to rule out any discrepancy. The third author
made a final decision for the discrepancy. The following data were
independently extracted for each included study: first author's
surname, publication year, age of patients, tumor location, 3- and
5-year survival rate. If no 3- and 5-year survival rates were
given, it was read from the results of the Kaplan-Meier curve using
Engauge Digitizer version 4.1 (http://sourceforge.net/). If data sets overlapped or
were duplicated, only the most recent data were included. If it was
necessary, the authors were contacted for additional
information.
Evaluation of methodological
quality
The methodological quality of the included cohort
studies was evaluated according to the Newcastle-Ottawa Scale
(NOS). The concrete content was as follows: selection of patients,
comparability, and evaluation of results. The cohort study was
evaluated as low quality when the score was ≤5 and excluded from
our meta-analysis. By contrast, the study was evaluated as high
quality when the score was ≥6 and included in our
meta-analysis.
Endpoint
The primary endpoint included the 3- and 5-year
survival rates.
Statistical analysis
Statistical analysis was carried out using Stata12.0
software (Chicago, IL, USA). Risk ratio (RR) was calculated to
express the effect size of categorical variables such as the 3- and
5-year survival rate. I2 statistic was used to show the
heterogeneity between studies. The random effects model was used
when there was obvious heterogeneity between studies
(I2≥50%). The fixed effect model was used when there was
no obvious heterogeneity between studies
(I2<50%).
The study was approved by Ethics Committee of
Beijing Tiantan Hospital (Beijing, China). Publication consent is
not applicable, since our paper does not contain any individual
persons data. All data supporting the results are available.
Results
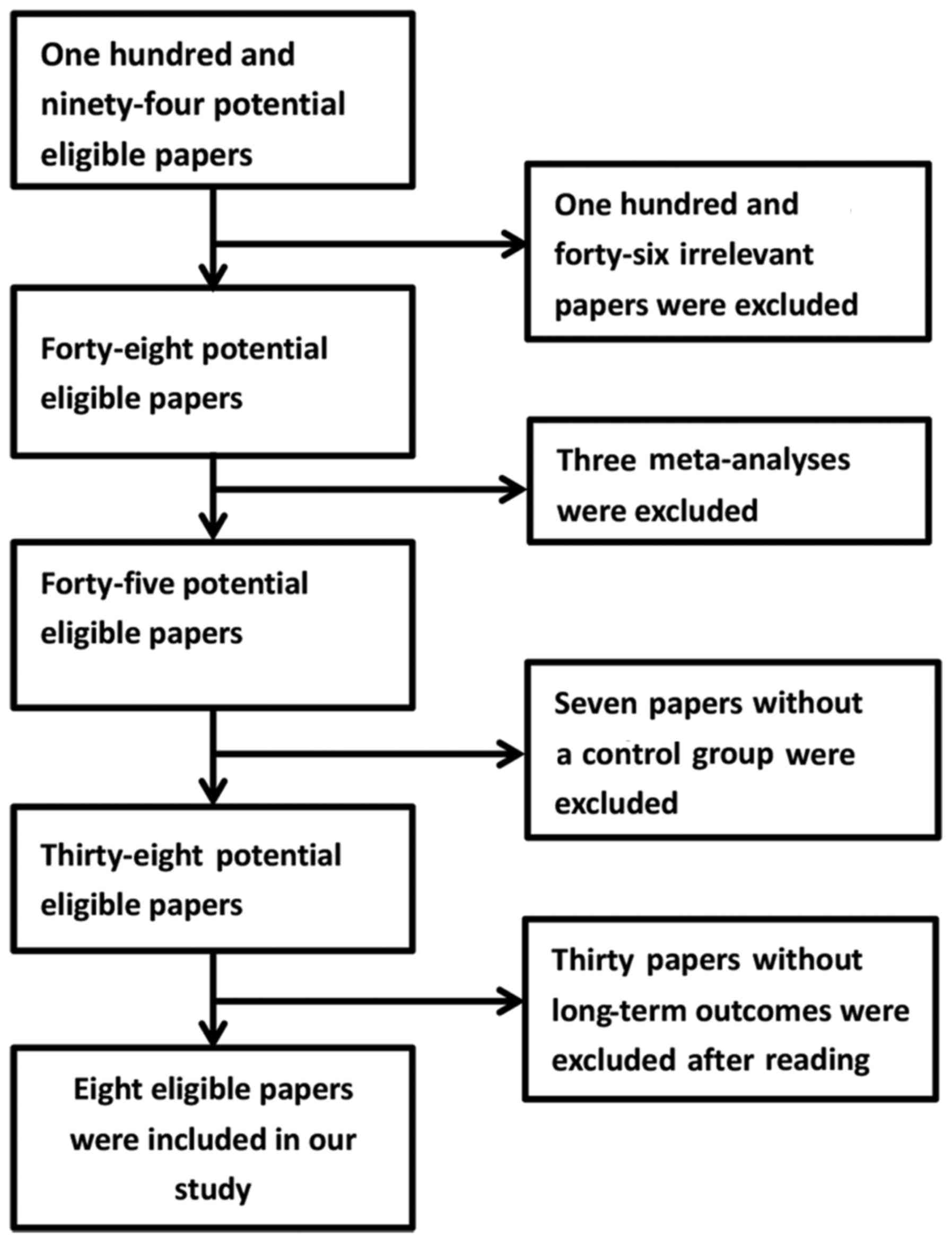
Eight cohort studies (9–16) were
included in our meta-analysis. The selection procedure is shown in
the flowchart of Fig. 1. No missing
studies were found by reviewing the reference list of included
articles. In total, 29,663 patients were incorporated in the 8
studies, in which 1,410 patients were in the laparoscopic group and
the remaining 28,253 patients were in the open surgery group. Two
studies limited the patient age to ≥65 (10,13).
Other studies limited the patient age to ≥70 (9,11,12,14–16).
Basic characteristics and methodological quality of included
studies are shown in Table I.
 | Table I.Basic characteristics and
methodological quality of included studies. |
Table I.
Basic characteristics and
methodological quality of included studies.
|
|
|
|
|
|
| Sample size |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Authors | Year | Age, years | Location | Study design | Total | Lap | Open | Score | (Refs.) |
|---|
| Zeng et
al | 2015 | ≥70 | R | CS | 294 | 112 | 182 | 7 | (16) |
| She et
al | 2013 | ≥75 | C | CS | 434 | 189 | 245 | 6 | (14) |
| Altuntas et
al | 2012 | ≥70 | R | CS | 90 | 56 | 34 | 7 | (9) |
| Robinson et
al | 2011 | ≥65 | CRC | CS | 242 | 47 | 195 | 6 | (13) |
| Cummings et
al | 2012 | ≥65 | C | CS | 27,436 | 424 | 27,012 | 7 | (10) |
| Hinoi et
al | 2015 | ≥80 | CRC | CS | 918 | 459 | 459 | 6 | (11) |
| Moon et
al | 2016 | ≥80 | CRC | CS | 142 | 71 | 71 | 6 | (12) |
| Shigeta et
al | 2016 | ≥80 | CRC | CS | 107 | 52 | 55 | 5 | (15) |
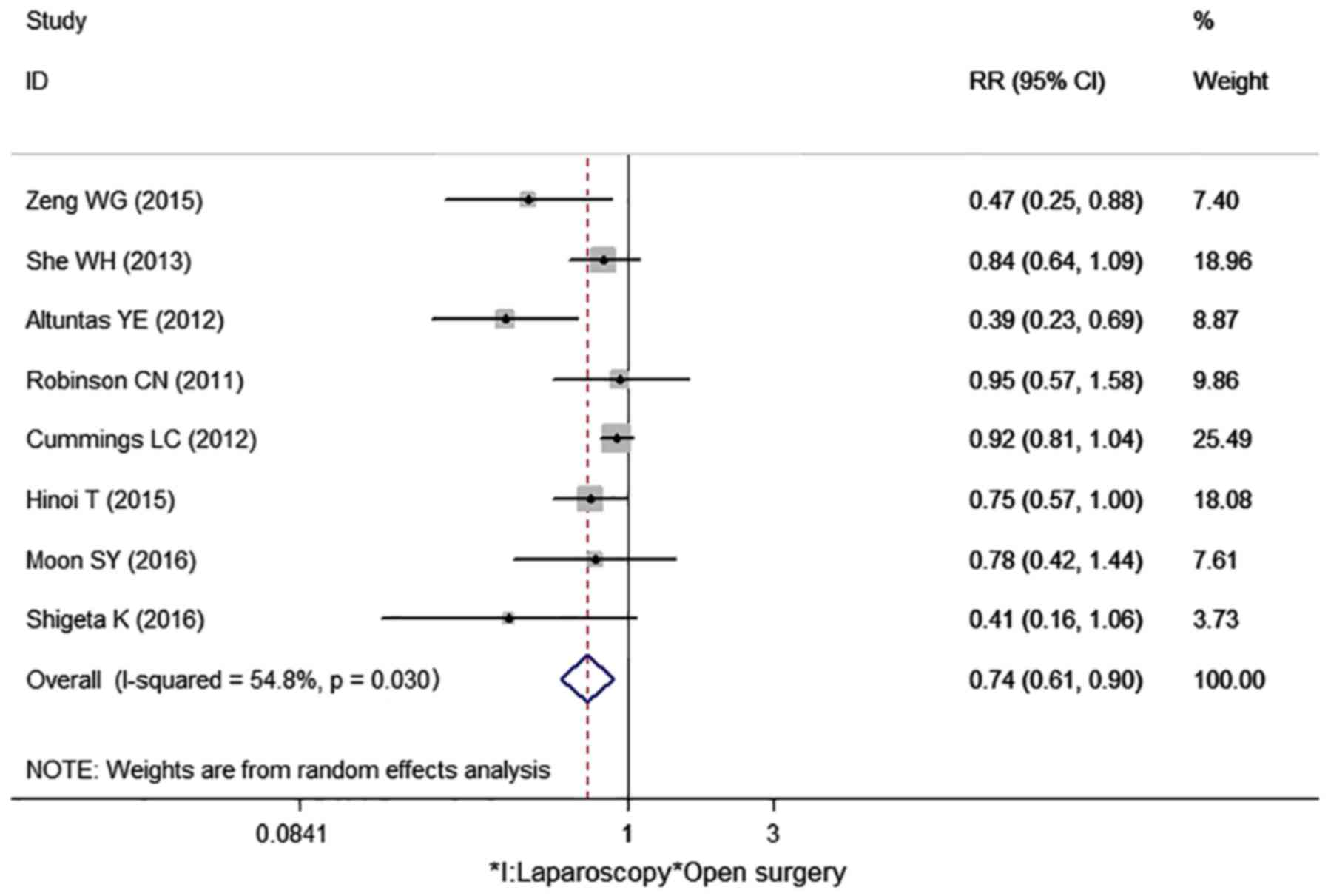
Three-year survival rate
Eight cohort studies reported the 3-year survival
rate in our meta-analysis (Table
II) (9–16). One study showed the 3-year survival
rate directly (12). Other studies
showed the survival curve, by which the 3-year survival rate can be
calculated (9–11,13–16). The
I2 value was 54.8%; thus, the random effects model was
used to pool the 8 studies. The result indicated that laparoscopic
surgery had a higher 3-year survival rate than open surgery
(RR=0.74, 95% CI: 0.61–0.90, P=0.003) (Fig. 2).
 | Table II.Long-term outcomes of 8 cohort
studies. |
Table II.
Long-term outcomes of 8 cohort
studies.
|
| 3-year survival
rate | 5-year survival
rate |
|
|---|
|
|
|
|
|
|---|
|
| LAP | OPEN | LAP | OPEN |
|
|---|
|
|
|
|
|
|
|
|---|
| Authors | Dead | Alive | Dead | Alive | Dead | Alive | Dead | Alive | (Refs.) |
|---|
| Zeng et
al | 11 | 101 | 38 | 144 | NM | NM | NM | NM | (16) |
| She et
al | 60 | 129 | 93 | 152 | 81 | 108 | 127 | 118 | (14) |
| Altuntas et
al | 13 | 43 | 20 | 14 | 24 | 32 | 25 | 9 | (9) |
| Robinson et
al | 13 | 34 | 57 | 138 | 23 | 24 | 76 | 119 | (13) |
| Cummings et
al | 155 | 269 | 10,751 | 16,261 | 212 | 212 | 13,803 | 13,209 | (10) |
| Hinoi et
al | 70 | 389 | 93 | 366 | 123 | 336 | 105 | 354 | (11) |
| Moon et
al | 14 | 57 | 18 | 53 | 21 | 50 | 26 | 45 | (12) |
| Shigeta et
al | 5 | 47 | 13 | 42 | NM | NM | NM | NM | (15) |
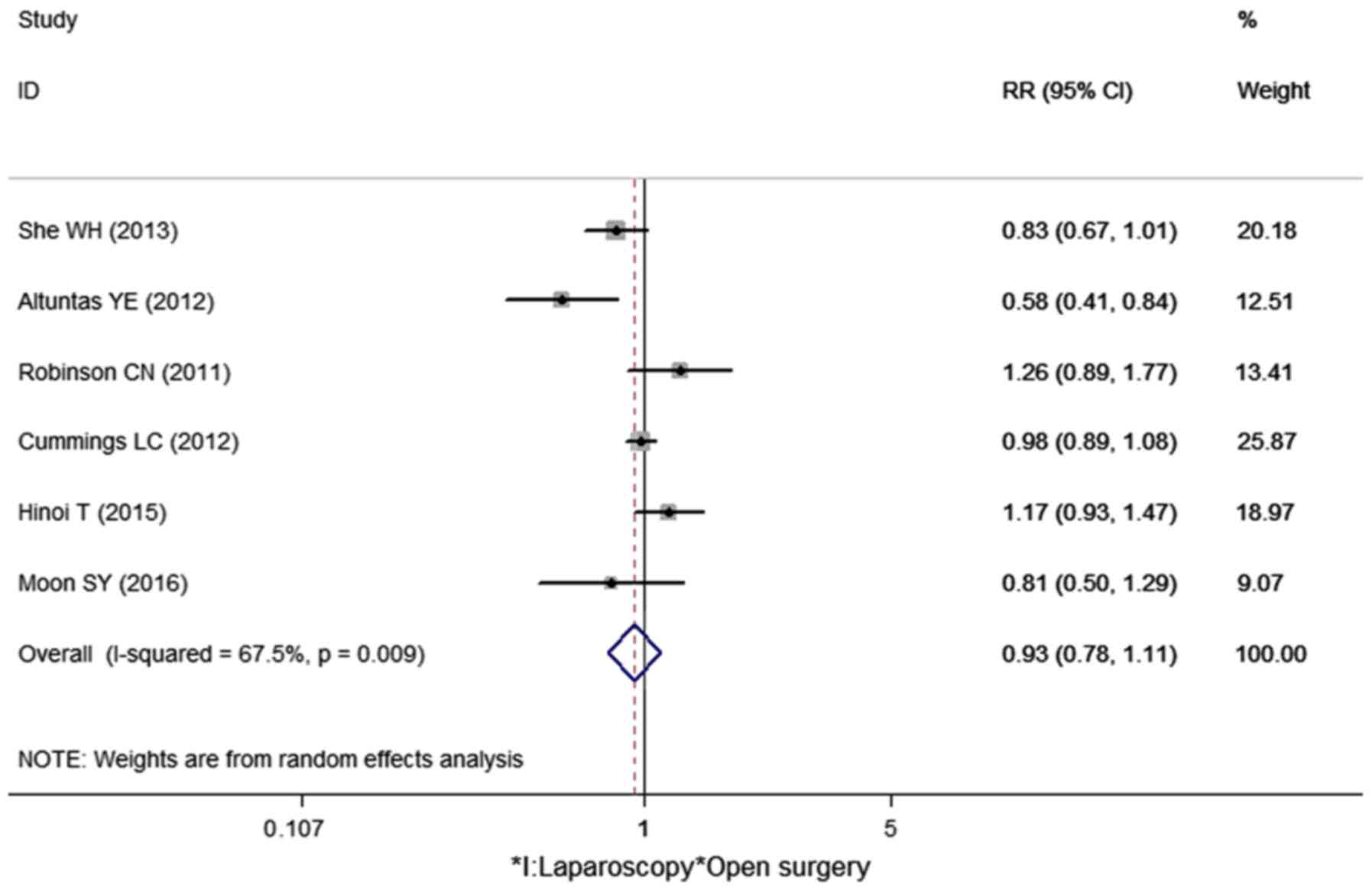
Five-year survival rate
Six cohort studies reported a 5-year survival rate
in our meta-analysis (Table II).
Two studies showed the 5-year survival rate directly (10,14).
Other studies showed the survival curve, by which the 5-year
survival rate can be calculated (9,11–13). The
I2 value was 67.5%; thus, the random effects model was
used to pool the 6 studies. No statistical difference was found
between laparoscopic surgery and open surgery with regard to the
5-year survival rate for the elderly colorectal cancer patients
(RR=0.93, 95% CI: 0.78–1.11, P=0.424) (Fig. 3).
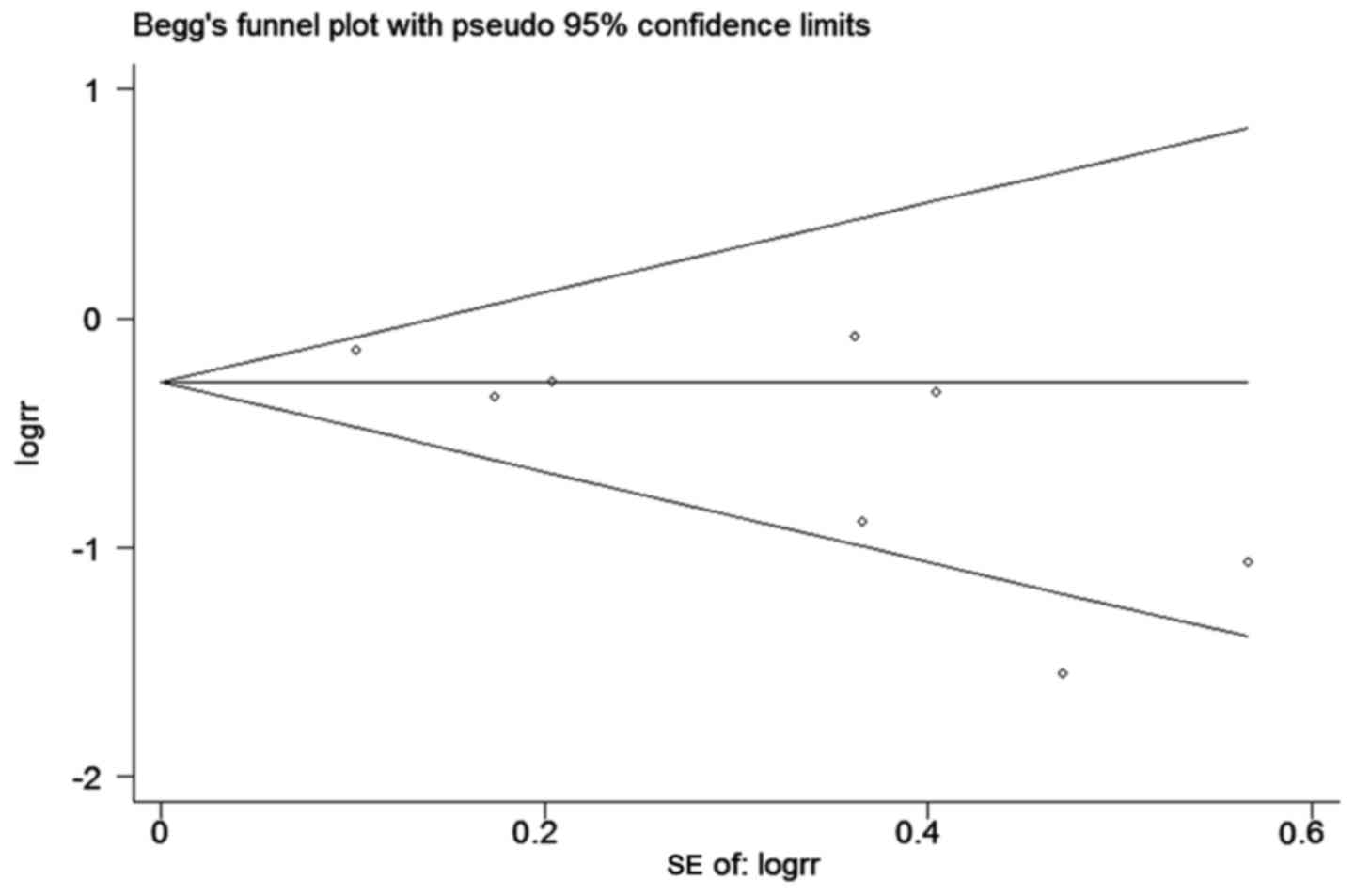
Publication bias
Funnel plot and Begg's test was used to evaluate the
publication bias of the included studies. The shape of the funnel
plot for the meta-analysis of studies on 3-year survival rate
demonstrated symmetry (Pr >|z|=0.108) (Fig. 4).
Discussion
Colorectal cancer has become a disease of older age
(2), which may be explained by the
increased life expectancy of recent years (17). The decreasing functional reserve and
comorbidities in elderly patients highlight the significance of
operation style selection. In general, laparoscopic surgery can
achieve decreased surgical trauma, faster postoperative recovery
(18,19) and equal long-term outcomes (4,5,20) for colorectal cancer patients. Current
studies comparing laparoscopy and open surgery in elderly
colorectal cancer patients are limited and retrospective, making
the short- and long-term outcomes unclear.
To the best of our knowledge, there are 3
meta-analyses comparing the short-term outcomes following
laparoscopic and open colorectal resections in elderly populations.
Grailey et al pooled 11 studies and concluded the reduction
in length of hospital stay, intraoperative blood loss, incidence of
postoperative pneumonia, time to return of normal bowel function,
incidence of postoperative cardiac complications, and wound
infections in the elderly population (≥70) receiving laparoscopic
colorectal resection (6). Li et
al pooled 10 studies and concluded that laparoscopy can reduce
the length of hospital stay, intraoperative blood loss, time to
return of normal bowel function, incidence of postoperative
pneumonia, wound infection and postoperative ileus in the elderly
population (≥80) receiving laparoscopic colorectal resection
(7). Xie et al performed a
similar meta-analysis including 7 studies in octogenarian patients
and concluded that the laparoscopic approach was associated with a
lower rate of mortality, and prolonged ileus, quicker bowel
function return, and shorter length of hospital stay (8). It is noteworthy that Xie et al
found a lower rate of mortality in the laparoscopic group (P=0.03)
(8), which is different from that
identified by Grailey (P=0.82) (6)
and Li et al (P=0.05) (7) in
their respective meta-analyses. Thus, laparoscopic colorectal
resection is associated with improved short-term outcomes, and the
evidence was reinforced by several recent cohort studies (12,15,21).
However, the abovementioned meta-analyses did not report long-term
outcomes.
We collected the previous comparative studies and
performed a meta-analysis in long-term outcomes for elderly
colorectal cancer patients. Our meta-analysis showed that, compared
with open surgery, the laparoscopic surgery had a higher 3-year
survival rate and an equivalent 5-year survival in elderly
colorectal cancer patients. Koh et al performed a matched
case-control study in octogenarian patients, and concluded that
patients who have undergone laparoscopic surgery were associated
with a better 1-year survival rate (94.4 vs. 75.0%, P=0.09)
compared with those who underwent open surgery (22). Of note is that the deaths in the open
group were due to causes unrelated to surgery, the author explained
the longer lasting effect of open surgery on physiological reserve
may play an important role (22). In
general population, no statistical significance was found in
long-term outcomes between laparoscopic and open surgery (4,5,20,23).
Jiang et al pooled 12 studies and found disease-free
survival (OR=1.80, P=0.18) and overall survival (OR=1.44, P=0.33)
were similar between laparoscopic and open surgery for low rectal
cancer (24). Feinberg et al
performed a similar meta-analysis for pT4 colon cancer patients and
concluded there was no significant difference in overall survival
(HR, 1.28; 95% CI, 0.94–1.72), and disease-free survival (HR, 1.20;
95% CI, 0.90–1.61) between laparoscopic and open surgery (25). Thus, the issue raised is why
long-term outcomes are different between the general and elderly
populations. One reason may be that the poorer function reserve and
more serious comorbidities after larger trauma of open surgery may
impair the anti-tumor ability of immunologic function, which may
cause earlier tumor recurrence and cancer-related death. By
contrast, elderly colorectal cancer patients with advanced disease
(11,26,27),
larger tumor size (15,27,28) and
emergency events (10) are often
advised to undergo open surgery, which may lead to the open surgery
group having inferior outcomes.
There are limitations to our meta-analysis that
should be considered. First, all our eligible studies are non-RCTs,
which may have selection bias. Second, obvious heterogeneity has
been found between studies on the 3- and 5-year survival, which may
be explained by confounding factors such as tumor location,
adjuvant chemotherapy, level of hospital stay and surgeon. Third,
only 8 studies were included in our meta-analysis; thus a larger
cohort is required to confirm the results. Fourth, the age was
limited to individuals aged ≥65 in 2 studies (10,13), ≥70
in 2 studies (9,16), ≥75 in 1 study (14), and ≥80 in 3 studies (11,12,15).
Varying age may play an important role on reliability. Fourth, the
majority of the eligible studies did not show a 3- and 5-year
survival rate directly, and this had to be estimated by survival
curve, which may lead to errors. Finally, the difference of tumor
location between eligible studies may also influence the
reliability [colorectal cancer in 4 studies (11–13,15),
colon cancer in 2 studies (10,14), and
rectal cancer in 2 studies (9,16)].
However, we performed funnel plot and Begg's test to evaluate the
publication bias of the included studies and no obvious publication
bias was found, which demonstrated our meta-analysis was
reliable.
Improved long-term outcomes have been found for the
laparoscopic surgery group for elderly colorectal cancer patients
in our meta-analysis. However, most of the previous studies are
non-RCTs exhibiting selection bias. Consequently, large sample and
multicenter RCTs are needed to identify the optimal operation style
for elderly colorectal cancer patients.
Acknowledgements
We would like to thank M. Yan and W.M. Yan for their
assistance on our meta-analysis.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
RR
|
risk ratio
|
|
R
|
rectal cancer
|
|
C
|
colon cancer
|
|
CRC
|
colorectal cancer
|
|
CS
|
cohort study
|
|
lap
|
laparoscopy
|
|
Open
|
open surgery
|
|
NM
|
not mentioned
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winther Braendegaard S, Baatrup G,
Pfeiffer P and Qvortrup C: Academy of Geriatric Cancer Research
(AgeCare): Trends in colorectal cancer in the elderly in Denmark,
1980–2012. Acta Oncol. 55 Suppl 1:S29–S39. 2016. View Article : Google Scholar
|
|
3
|
Hermans E, van Schaik PM, Prins HA, Ernst
MF, Dautzenberg PJ and Bosscha K: Outcome of colonic surgery in
elderly patients with colon cancer. J Oncol. 2010:8659082010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bayar R, Mzoughi Z, Djebbi A, Halek G and
Khalfallah MT: Laparoscopic colectomy versus colectomy performed
via laparotomy in the treatment of non-metastatic colic
adenocarcinomas. Pan Afr Med J. 25:1652016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA,
van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA,
Andersson J, Angenete E, et al: A randomized trial of laparoscopic
versus open surgery for rectal cancer. N Engl J Med. 372:1324–1332.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grailey K, Markar SR, Karthikesalingam A,
Aboud R, Ziprin P and Faiz O: Laparoscopic versus open colorectal
resection in the elderly population. Surg Endosc. 27:19–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang S, Gao S, Yang C, Yang W and
Guo S: Laparoscopic colorectal resection versus open colorectal
resection in octogenarians: A systematic review and meta-analysis
of safety and efficacy. Tech Coloproctol. 20:153–162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie M, Qin H, Luo Q, He X, Lan P and Lian
L: laparoscopic colorectal resection in octogenarian patients: Is
it Safe? A systematic review and meta-analysis. Medicine
(Baltimore). 94:e17652015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altuntas YE, Gezen C, Vural S, Okkabaz N,
Kement M and Oncel M: Laparoscopy for sigmoid colon and rectal
cancers in septuagenarians: A retrospective, comparative study.
Tech Coloproctol. 16:213–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cummings LC, Delaney CP and Cooper GS:
Laparoscopic versus open colectomy for colon cancer in an older
population: A cohort study. World J Surg Oncol. 10:312012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinoi T, Kawaguchi Y, Hattori M, Okajima
M, Ohdan H, Yamamoto S, Hasegawa H, Horie H, Murata K, Yamaguchi S,
et al: Laparoscopic versus open surgery for colorectal cancer in
elderly patients: A multicenter matched case-control study. Ann
Surg Oncol. 22:2040–2050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moon SY, Kim S, Lee SY, Han EC, Kang SB,
Jeong SY, Park KJ and Oh JH: SEoul COlorectal Group (SECOG):
Laparoscopic surgery for patients with colorectal cancer produces
better short-term outcomes with similar survival outcomes in
elderly patients compared to open surgery. Cancer Med. 5:1047–1054.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robinson CN, Balentine CJ, Marshall CL,
Wilks JA, Anaya D, Artinyan A, Berger DH and Albo D: Minimally
invasive surgery improves short-term outcomes in elderly colorectal
cancer patients. J Surg Res. 166:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
She WH, Poon JT, Fan JK, Lo OS and Law WL:
Outcome of laparoscopic colectomy for cancer in elderly patients.
Surg Endosc. 27:308–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shigeta K, Baba H, Yamafuji K, Asami A,
Takeshima K, Nagasaki K, Okamoto N, Murata T, Arai S, Kubochi K and
Kitagawa Y: Effects of laparoscopic surgery on the patterns of
death in elderly colorectal cancer patients: Competing risk
analysis compared with open surgery. Surg Today. 46:422–429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng WG, Zhou ZX, Hou HR, Liang JW, Zhou
HT, Wang Z, Zhang XM and Hu JJ: Outcome of laparoscopic versus open
resection for rectal cancer in elderly patients. J Surg Res.
193:613–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christensen K, Doblhammer G, Rau R and
Vaupel JW: Ageing populations: The challenges ahead. Lancet.
374:1196–1208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Pas MH, Haglind E, Cuesta MA,
Fürst A, Lacy AM, Hop WC and Bonjer HJ: COlorectal cancer
Laparoscopic or Open Resection II (COLOR II) Study Group:
Laparoscopic versus open surgery for rectal cancer (COLOR II):
Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol.
14:210–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto S, Inomata M, Katayama H,
Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y and
Kitano S: Japan Clinical Oncology Group Colorectal Cancer Study
Group: Short-term surgical outcomes from a randomized controlled
trial to evaluate laparoscopic and open D3 dissection for stage
II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404.
Ann Surg. 260:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong SY, Park JW, Nam BH, Kim S, Kang SB,
Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, et al: Open versus
laparoscopic surgery for mid-rectal or low-rectal cancer after
neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of
an open-label, non-inferiority, randomised controlled trial. Lancet
Oncol. 15:767–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niitsu H, Hinoi T, Kawaguchi Y, Ohdan H,
Hasegawa H, Suzuka I, Fukunaga Y, Yamaguchi T, Endo S, Tagami S, et
al: Laparoscopic surgery for colorectal cancer is safe and has
survival outcomes similar to those of open surgery in elderly
patients with a poor performance status: Subanalysis of a large
multicenter case-control study in Japan. J Gastroenterol. 51:43–54.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koh FH, Wong J, Tan JK, Tan KK, Cheong WK
and Lieske B: Laparoscopic colorectal surgery is safe and benefits
octogenarian patients with malignant disease: A matched
case-control study comparing laparoscopic and open colorectal
surgery. Int J Colorectal Dis. 30:963–968. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim DH, Kim IY, Kim BR and Kim YW: Factors
affecting the selection of minimally invasive surgery for stage 0/I
colorectal cancer. Int J Surg. 16:44–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang JB, Jiang K, Wang JJ, Dai Y, Xie FB
and Li XM: Short-term and long-term outcomes regarding laparoscopic
versus open surgery for low rectal cancer: A systematic review and
meta-analysis. Surg Laparosc Endosc Percutan Tech. 25:286–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feinberg AE, Chesney TR, Acuna SA, Sammour
T and Quereshy FA: Oncologic outcomes following laparoscopic versus
open resection of pT4 colon cancer: A systematic review and
meta-analysis. Dis Colon Rectum. 60:116–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyasaka Y, Mochidome N, Kobayashi K, Ryu
S, Akashi Y and Miyoshi A: Efficacy of laparoscopic resection in
elderly patients with colorectal cancer. Surg Today. 44:1834–1840.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura T, Sato T, Miura H, Ikeda A,
Tsutsui A, Naito M, Ogura N and Watanabe M: Feasibility and
outcomes of surgical therapy in very elderly patients with
colorectal cancer. Surg Laparosc Endosc Percutan Tech. 24:85–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tominaga T, Takeshita H, Arai J, Takagi K,
Kunizaki M, To K, Abo T, Hidaka S, Nanashima A, Nagayasu T and
Sawai T: Short-term outcomes of laparoscopic surgery for colorectal
cancer in oldest-old patients. Dig Surg. 32:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|