Introduction
Infectious mononucleosis (IM) is an important
clinical entity that is associated with Epstein-Barr virus (EBV)
infection (1,2). This clinical manifestation was first
described in 1889, but the term IM was coined in 1920, when it was
discovered that a number of patients with glandular fever had
similar blood films (3). In 1968,
the then newly discovered EBV was identified as the cause of IM
(4). The currently estimated
incidence of IM is at ~500 cases per 100,000 persons annually. IM
diagnosis is often established with the classical clinical triad of
pharyngitis, fever and lymphadenopathy. Serological testing for the
identification of EBV antibodies is required for a definitive
diagnosis (1,2). The treatment of patients with IM is
mainly supportive. Corticosteroids are considered as the standard
treatment for severe complications associated with IM (1,2).
Uric acid (UA) is a purine degradation metabolite. A
high serum level of UA is considered harmful. Hyperuricemia is
considered to be closely associated with a number of metabolic
disorders (5–7). For example, it was previously
demonstrated that UA and metabolic syndrome were closely
associated, and young women with hyperuricemia were at the highest
risk of developing metabolic syndrome (5). Our recent study investigated
subclinical thyroid dysfunction and hyperuricemia. It was
demonstrated that, in subjects with hyperuricemia, mild
hypothyroidism was a risk factor for men, while not for women
(6). UA is also an important
endogenous antioxidant, as well as a natural scavenger of
peroxynitrate. Abnormalities in the serum levels of UA have been
observed in several diseases. For example, a low UA level has been
detected in stroke (8–10), multiple sclerosis (MS) (11,12),
infections of the central nervous system (CNS) (13,14) and
leprosy reaction episodes (15). As
regards IM, the number of previous related studies is limited and
the results are conflicting. A total of three early articles
(16–18) with small number of recruited subjects
and one previous case report (19)
were retrieved. Dylewski et al (16) investigated 35 cases with IM after a
case report, and reported that 7 men and 2 women had UA levels
above the laboratory's upper limit of normal. Cowdrey (17) reported UA elevation during the first
10 days of the disease course in 21 patients. However, Sugita et
al (19) described a case of a
27-month-old boy with persistent EBV infection and CNS
manifestations, who had lymphadenopathy and low UA levels.
Therefore, the aim of the present study was to
analyze the associations between UA and IM in a comprehensive
manner, in order to determine whether low UA is a significant risk
factor for IM, and whether there is a sex difference.
Patients and methods
Patients
The present study was conducted under collaboration
between the Departments of Infectious Diseases, Nuclear Medicine
and Health Management of Tianjin Medical University General
Hospital (Tianjin, China). Between December 2014 and December 2015,
a total of 95 patients (47 men and 48 women) with a confirmed
diagnosis of IM were recruited. All the patients were admitted to
the Department of Infectious Diseases of our hospital.
Controls
Between June 2015 and September 2015, 95 healthy
subjects (47 men and 48 women) were enrolled in the normal control
cohort from the Department of Health Management of our hospital.
The control subjects visited our institution to receive a routine
annual health checkup.
Ethics
The Institutional Review Board of Tianjin Medical
University General Hospital approved the ethical and methodological
aspects of the study protocol and all the participants provided
written informed consent. All the methods were performed in
accordance with the relevant ethical regulations.
Parameter measurements
For patients with IM, blood tests and anthropometric
measurements were performed upon admission to the Department of
Infectious Diseases. For the healthy controls, blood tests and
anthropometric measurements were performed upon visiting our
institution.
Physical examination included body height (BH) and
body weight (BW) measurement. Body mass index (BMI) was calculated
as BW divided by BH squared (kg/m2). Fasting blood tests
were performed following venipuncture, and serological parameters
were measured.
White blood cell (WBC) count, red blood cell (RBC)
count, hemoglobin (Hb) level and platelet (PLT) count were measured
using a hemocytometer (Sysmex Corporation, Kobe, Japan). Alanine
aminotransferase (ALT), aspartate aminotransferase (AST), total
bilirubin (TB), blood urea nitrogen (BUN), creatinine (Cr) and UA
were enzymatically determined by an auto-analyzer (model 7170;
Hitachi, Tokyo, Japan).
Antibodies (IgM and IgG) against specific EBV
antigens were measured by the enzyme-linked immunosorbent assay
method using a commercial kit (Euroimmun; Medizinische
Labordiagnostika AG, Lübeck, Germany).
Diagnostic criteria
The diagnosis of IM was generally based on the
clinical presentation, the presence of atypical lymphocytes on a
peripheral blood smear, and a positive heterophile antibody test.
Serological testing for the identification of antibodies against
specific EBV antigens was required in order to establish a
definitive diagnosis (1,2). Hyperuricemia was defined as UA >420
µmol/l in men and >360 µmol/l in women (5).
Statistical analysis
All data are presented as mean ± standard deviation;
men and women were separately analyzed. Differences in indices
between the two groups of patients were measured by the independent
samples t-test. The Chi-squared test was used to compare
differences in prevalence. Pearson's bivariate correlation was used
to assess the correlation between UA and other variables. Receiver
operating characteristic (ROC) curves were drawn and diagnostic
efficacies were then determined. After the optimal cut-off UA value
was selected, the sensitivity, specificity, diagnostic accuracy,
positive predictive value and negative predictive value for
differential diagnosis were assessed. By stratifying data with UA
quartiles, odds ratio (OR) for IM with 95% confidence interval (CI)
was calculated by binary logistic regression models. SPSS version
17.0 (SPSS Inc., Chicago, IL, USA) was used to conduct statistical
analyses and significance was set at P<0.05.
Results
Characteristics of the
participants
The measured variables were separately compared in
men and women (Tables I and II). In men, WBC count, ALT and AST were
significantly higher in patients with IM compared with control
subjects, whereas RBC count, Hb and UA levels were significantly
lower in patients with IM compared with control subjects. In women,
ALT and AST were significantly higher in IM patients, whereas RBC
count, Hb, TB, BUN, Cr and UA levels were significantly lower in IM
patients compared with controls.
 | Table I.Parameter characteristics in men. |
Table I.
Parameter characteristics in men.
| Parameters | Patients with
IM | Controls | P-value |
|---|
| Number of
subjects | 47 | 47 |
|
| Age (years) | 37.40±16.83 | 37.68±16.83 | −0.080 |
| BMI
(kg/m2) | 23.58±3.02 | 24.74±3.50 | −1.725 |
| WBC
(×109/l) | 7.17±3.81 | 5.93±1.49 | 2.085a |
| RBC
(×1012/l) | 4.46±0.51 | 5.19±0.38 | −7.870b |
| Hb (g/l) | 133.19±14.59 | 155.15±10.21 | −8.453b |
| PLT
(×109/l) | 208.47±80.43 | 214.81±46.92 | −0.467 |
| ALT (U/l) | 86.72±108.67 | 27.57±29.00 | 3.605b |
| AST (U/l) | 55.53±76.95 | 24.45±30.67 | 2.573a |
| TB (µmol/l) | 12.24±11.86 | 14.12±6.62 | −0.952 |
| BUN (mmol/l) | 4.10±1.74 | 4.56±1.22 | −1.496 |
| Cr (µmol/l) | 76.30±28.92 | 83.34±12.75 | −1.528 |
| UA (µmol/l) | 278.98±96.58 | 373.00±72.49 | −5.338b |
 | Table II.Parameter characteristics in
women. |
Table II.
Parameter characteristics in
women.
| Parameters | Patients with
IM | Controls | P-value |
|---|
| Number of
subjects | 48 | 48 |
|
| Age (years) | 41.27±17.54 | 41.19±17.35 | 0.023 |
| BMI
(kg/m2) | 23.52±3.81 | 22.60±2.94 | 1.314 |
| WBC
(×109/l) | 6.29±3.62 | 5.17±1.54 | 1.975 |
| RBC
(×1012/l) | 3.93±0.43 | 4.44±0.27 | −6.954a |
| Hb (g/l) | 113.54±13.34 | 130.73±8.49 | −7.530a |
| PLT
(×109/l) | 235.65±90.10 | 226.15±50.15 | 0.638 |
| ALT (U/l) | 61.35±103.51 | 14.19±6.84 | 3.150a |
| AST (U/l) | 45.60±58.11 | 16.68±6.08 | 3.393a |
| TB (µmol/l) | 7.63±5.42 | 11.03±6.01 | −2.913a |
| BUN (mmol/l) | 2.97±1.05 | 4.15±1.15 | −5.242a |
| Cr (µmol/l) | 52.13±9.46 | 60.77±9.59 | −4.446a |
| UA (µmol/l) | 195.27±61.25 | 272.75±56.41 | −6.446a |
UA differences between sexess
Comparison of UA levels between sexes in IM patients
revealed significantly higher levels in men (t=5.056, P<0.01).
Similarly, comparison of UA levels between sexes in control
subjects also revealed significantly higher levels in men (t=7.531,
P<0.01). There was a lower incidence of hyperuricemia in men
with IM, but the difference was not statistically significant.
However, no women with IM had hyperuricemia, which was
statistically significantly different from the control group
(Table III).
 | Table III.Comparison of hyperuricemia incidence
between sexes. |
Table III.
Comparison of hyperuricemia incidence
between sexes.
|
| Incidence (case
number count) in different sexes |
|---|
|
|
|
|---|
|
| Men | Women |
|---|
|
|
|
|
|---|
| Incidence
comparisons | IM | Control | IM | Control |
|---|
| Normal UA | 89.36% (42) | 78.72% (37) | 100.00% (48) | 87.50% (42) |
|
Hyperuricemiaa | 10.64% (5) | 21.28% (10) | 0.00% (0) | 12.50% (6) |
| Chi-squared
valueb | 1.983 |
| 6.400c |
|
Correlations between key
variables
Correlation coefficients between UA and other
variables were calculated to determine whether there were any
significant associations (Table
IV). Statistically significant positive correlations were found
between UA and BMI, RBC count, Hb, TB, BUN and Cr in men. In women,
UA was statistically significantly positively correlated with RBC
count, Hb, BUN and Cr.
 | Table IV.Pearson's bivariate correlations
between UA and other variables in the two sexes. |
Table IV.
Pearson's bivariate correlations
between UA and other variables in the two sexes.
|
| Correlation
coefficients |
|---|
|
|
|
|---|
| Parameters | Men | Women |
|---|
| Age | −0.069 | −0.069 |
| BMI | 0.492b | 0.195 |
| WBC | 0.112 | 0.127 |
| RBC | 0.419b | 0.474b |
| Hb | 0.388b | 0.445b |
| PLT | 0.118 | 0.065 |
| ALT | 0.060 | −0.166 |
| AST | −0.015 | −0.132 |
| TB | 0.225a | 0.196 |
| BUN | 0.247a | 0.506b |
| Cr | 0.452b | 0.496b |
Diagnostic and predictive values of UA
for IM
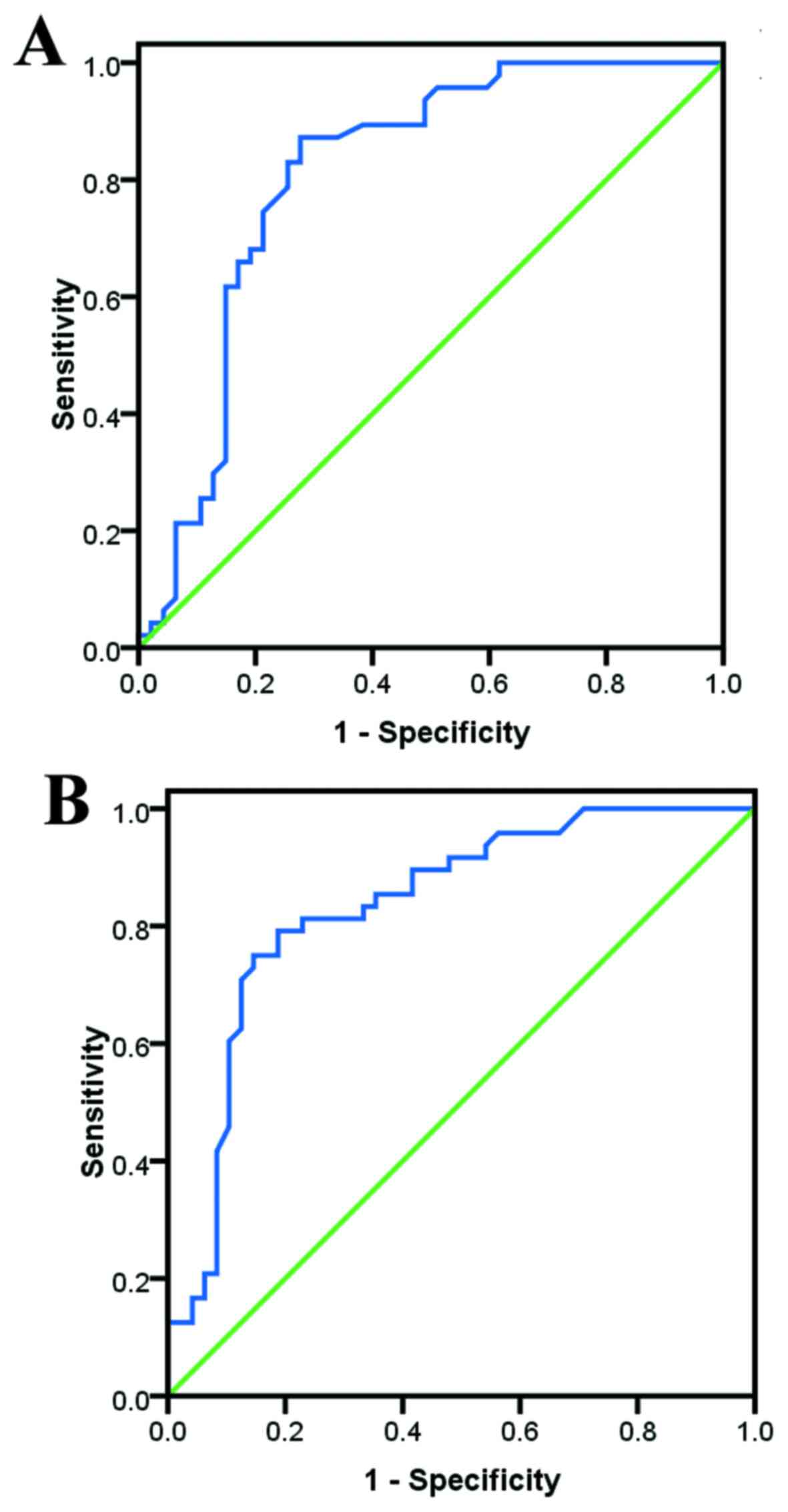
Based on the ROC analysis, UA demonstrated good
diagnostic and predictive values for IM (Fig. 1). The cut-off values were calculated
as 326.00 and 243.50 µmol/l in men and women, respectively, with
area under the curve values of 0.809 and 0.835, respectively (both
P<0.01). The sensitivity, specificity, diagnostic accuracy,
positive predictive value and negative predictive value were found
to be 74.500, 78.700, 76.596, 75.510 and 77.778%, respectively, for
men, while the respective values for women were 75.000, 85.400,
80.208, 77.358 and 83.721%.
Risk of IM in different UA
quartiles
Binary logistic regression models were used to
calculate the risk of IM in the two sexes (Table V). Crude OR calculation was performed
with UA in the highest quartile as reference, and significant risk
was demonstrated for IM in quartile 1 and 2 for both sexes.
Adjusted OR calculation included age and BMI as covariates. A
significantly enhanced risk for IM was displayed in quartile 1 and
2 for both sexes. Of note, women with low serum UA appeared to be
more susceptible to IM. The crude ORs in quartile 1 were 24.000
(95% CI: 4.381–131.472) and 52.500 (95% CI: 8.640–319.028) for men
and women, and the adjusted ORs were 31.437 (95% CI: 4.680–211.181)
and 301.746 (95% CI: 25.160–3618.861), respectively (all
P<0.01).
 | Table V.Risk of IM according to UA quartiles
in the two sexes. |
Table V.
Risk of IM according to UA quartiles
in the two sexes.
|
| Men | Women |
|---|
|
|
|
|
|---|
| UA quartiles | UA values | Crude OR
(CI)c | Adjusted OR
(CI)d | UA values | Crude OR
(CI)c | Adjusted OR
(CI)d |
|---|
| Quartile 1 | <255.75 | 24.000
(4.381–131.472)b | 31.437
(4.680–211.181)b | <184.00 | 52.500
(8.640–319.028)b | 301.746
(25.160–3618.861)b |
| Quartile 2 |
255.75≤UA<324.00 | 3.810
(1.132–12.816)a | 4.447
(1.172–16.874)a |
184.00≤UA<236.50 | 10.625
(2.718–41.534)b | 34.806
(5.825–207.958)b |
| Quartile 3 |
324.00≤UA<384.25 | 0.457
(0.113–1.841) | 0.510
(0.120–2.171) |
236.50≤UA<279.50 | 1.667
(0.404–6.870) | 3.941
(0.762–20.382) |
| Quartile 4 | ≥384.25 (µmol/l,
reference) |
|
| ≥279.50 (µmol/l,
reference) |
|
|
Discussion
The aim of the present study was to investigate
whether UA has diagnostic and predictive value for IM, prompted by
the fact that a low UA level was found to be associated with
pathological conditions such as stroke (8–10), MS
(11,12,20) and
CNS infections (13,14). Our research group previously
investigated UA, but the focus was the association of hyperuricemia
with various metabolic disorders (5–7). The
fact that low UA levels have important clinical implications has
become intriguing; therefore, collaborative efforts were focused on
investigating the association between UA and IM. It was
demonstrated that UA was significantly lower in patients with IM
compared with healthy controls. Low UA level was found to have
adequate diagnostic and predictive power for IM. Subjects with low
UA levels, indicating low antioxidant reserve, were significantly
more likely to develop IM, and these effects were more pronounced
in women.
IM commonly affects patients who have had a primary
EBV infection during childhood or adolescence. As the overall
socioeconomic and sanitary conditions have improved, EBV infection
in early childhood has become less common (1), with no obvious annual cycles or
seasonal changes in incidence, and no apparent predisposition of
either sex (1). IM usually runs a
self-limiting course. The majority of IM patients recover without
sequelae and return to normal activities ~2 months after the onset.
As numerous individuals are EBV-positive, special precautions
against transmission are not necessary. However, severe
complications (including upper airway obstruction, hemolytic
anemia, thrombocytopenia, hepatitis, myocarditis, splenic rupture,
neurological and hematological complications) may occur, and
fulminant infection is also possible. Clinical experience suggests
that corticosteroids are helpful in the management of these
complications, although randomized trials evaluating their efficacy
are limited (1,2). No specific guidelines are currently
available for the treatment of IM, and no serum factor for
predicting IM in either sexes has been identified (1,2). The
findings of the present study indicate that UA levels may be such a
predictor.
There are established theories as to why normal
level of UA is important. Humans cannot efficiently catabolize UA
to a more soluble compound (allantoin), due to lack of urate
oxidase function. This hepatic enzyme is inactivated during early
primate evolution due to two independent nonsense mutations
(21). As a result, humans naturally
have higher levels of UA compared with most non-primates. This
genetic modification actually confers an evolutionary advantage.
Under conditions of increased oxidative stress, UA may be oxidized
into allantoin and other metabolites via non-enzymatic oxidation
and through exposure to pro-oxidant molecules (22). UA is the most abundant natural
antioxidant in humans and it accounts for two-thirds of the
antioxidant capacity of the plasma (23). However, too high a level of UA is
also detrimental, as it exerts a pro-oxidant effect. In the
clinical setting, higher levels of UA have been associated with
gout (24,25), and associations between hyperuricemia
and an increased risk of various metabolic disorders have also been
described (5,6). In fact, a U-shaped association between
extremely low or high UA levels and worse outcome has been
described in stroke (10,26). However, it appears that, under
conditions of increased oxidative stress, as occurs in acute
ischemic stroke, the balance between anti- and pro-oxidant
properties shifts to promote neuroprotection (27–30).
In ischemic stroke, highly reactive oxidant
molecules are the major force driving the ischemic cascade
(31). The brain develops enzymatic
and non-enzymatic endogenous antioxidant defenses. UA, being a
non-enzymatic molecule, is a powerful antioxidant at physiological
concentrations. It was observed that a gradual depletion of UA
occurred during the acute phase of stroke (32). Moreover, decreases in UA after stroke
onset have been correlated with increased severity and poor
long-term outcome (33). Based on
promising pre-clinical evidence (32,34),
more clinical trials of exogenous administration of UA for stroke
are currently performed (8). The
mechanisms underlying the role of UA in MS have also been
extensively investigated. It has been observed that MS and gout are
mutually exclusive (35). It is now
generally accepted that the lower serum UA level in MS patients may
be due to the intrinsically reduced antioxidant capacity, as well
as the increased consumption of UA in MS (11,12). The
mechanisms of CNS injury during infection are complex. It has been
indicated that oxidative stress and antioxidant imbalance play a
central role in the pathophysiology of meningitis (36–38).
Recently, Liu et al (13)
reported that the serum levels of UA in patients with various types
of CNS infections were significantly lower compared with those in
normal subjects. However, after effective therapy, the UA levels
increased significantly compared with prior to treatment, and were
almost restored to normal in some patients.
The design of the present study framework focused on
EBV infection causing IM. In fact, it is known that increased
oxidative stress plays a fundamental role in the pathogenesis of
several types of infections, causing extensive cellular and tissue
damage. Previous studies have demonstrated that this mechanism
exists in various pathogens, including influenza virus (39), hepatitis virus (40), respiratory viruses (41), human immunodeficiency virus (42), Staphylococcus aureus (43), Helicobacter pylori (44), spirochetal bacteria (45) and mycoplasma (46), among others. It would be reasonable
to deduce that infection due to EBV may also cause oxidative
stress, leading to obvious depletion of antioxidants, such as UA.
In addition, three early clinical studies demonstrated a transitory
UA increase during acute onset of IM, which was explained by the
increase in de novo purine biosynthesis necessary to
accommodate the stepped-up nucleic acid production in IM (16–18). In
fact, IM patients visiting our hospital (a tertiary hospital in
Tianjin Municipality with a population of ~20 million) were often
cases with more severe complications, with an IM disease duration
of >10-14 days. In such patients, oxidative stress and depletion
of the antioxidants may well overwhelm the de novo purine
biosynthesis of UA. Therefore, this may be considered as the
mechanism underlying the findings of the present study.
However, the reason for the obvious female
predisposition to IM under conditions of low UA levels remains
unclear. It is a common phenomenon that men have a significantly
higher level of serum UA compared with women, and the rate of
increase in UA levels is also significantly higher in men (5). The present study also confirmed this
finding (Table III). A higher
level of UA may promote a stronger antioxidant protection in men.
Thus, women may be more vulnerable to oxidative stress-related UA
depletion, which was also demonstrated by our findings. As a
result, a decreased UA level may be more predictive of IM in women
(Fig. 1, Table V).
There were certain limitations to the present study.
First, the cross-sectional nature of the investigation meant that
no causality could be determined from the results. A prospective
study should be planned in the future. Second, a limited number of
IM patients and controls were included. More participants should be
recruited in order to limit the case number-related inherent
drawback. Third, due to study budget limitations, measurements such
as reactive oxygen species and activities of antioxidants were not
performed, which should be included in future investigations.
Finally, administration of UA as an adjuvant therapy should be
investigated in the future to validate the findings of the present
study.
To the best of our knowledge, this is the first
study to demonstrate the inverse association between UA and IM,
suggesting a progressive decrease of antioxidant reserve in IM.
Moreover, low UA level is predictive for IM, particularly in
women.
Acknowledgements
The present study was supported by the National Key
Clinical Specialty Project, awarded to the Departments of Nuclear
Medicine and Radiology; the Tianjin Medical University General
Hospital New Century Excellent Talent Program, Young and
Middle-aged Innovative Talent Training Program from Tianjin
Education Committee, and Talent Fostering Program (the 131 Project)
from the Tianjin Education Committee, Tianjin Human Resources and
Social Security Bureau, awarded to Zhaowei Meng; the China National
Natural Science Foundation (grant no. 81571709), Key Project of
Tianjin Science and Technology Committee Foundation (grant no.
16JCZDJC34300), awarded to Zhaowei Meng; and the Tianjin Science
and Technology Committee Foundation (grant nos. 11ZCGYSY05700,
12ZCZDSY20400 and 13ZCZDSY20200) awarded to Qing Zhang, Qiyu Jia
and Kun Song.
References
|
1
|
Luzuriaga K and Sullivan JL: Infectious
mononucleosis. N Engl J Med. 362:1993–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vouloumanou EK, Rafailidis PI and Falagas
ME: Current diagnosis and management of infectious mononucleosis.
Curr Opin Hematol. 19:14–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lennon P, O'Neill JP, Fenton JE and
O'Dwyer T: Challenging the use of the lymphocyte to white cell
count ratio in the diagnosis of infectious mononucleosis by
analysis of a large cohort of monospot test results. Clin
Otolaryngol. 35:397–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henle G, Henle W and Diehl V: Relation of
Burkitt's tumor-associated herpes-ytpe virus to infectious
mononucleosis. Proc Natl Acad Sci USA. 59:pp. 94–101. 1968,
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Lou S, Meng Z and Ren X: Gender
and age impacts on the correlations between hyperuricemia and
metabolic syndrome in Chinese. Clin Rheumatol. 30:777–787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Meng Z, Zhang Q, Liu L, Song K,
Tan J, Li X, Jia Q, Zhang G and He Y: Gender impact on the
correlations between subclinical thyroid dysfunction and
hyperuricemia in Chinese. Clin Rheumatol. 35:143–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Lou S, Xu K, Meng Z, Zhang Q and
Song K: Relationship between lifestyle choices and hyperuricemia in
Chinese men and women. Clin Rheumatol. 32:233–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llull L, Amaro S and Chamorro Á:
Administration of uric acid in the emergency treatment of acute
ischemic stroke. Curr Neurol Neurosci Rep. 16:42016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Lin Y, Liu Y, Chen Y, Wang B, Li
C, Yan S, Wang Y and Zhao W: Serum uric acid levels and outcomes
after acute ischemic stroke. Mol Neurobiol. 53:1753–1759. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Huang ZC, Lu TS, You SJ, Cao YJ
and Liu CF: Prognostic significance of uric acid levels in ischemic
stroke patients. Neurotox Res. 29:10–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Shen Y, Xiao K, Tang Y, Cen L and
Wei J: Serum uric acid levels in patients with multiple sclerosis:
A meta-analysis. Neurol Res. 34:163–171. 2012.PubMed/NCBI
|
|
12
|
Moccia M, Lanzillo R, Costabile T, Russo
C, Carotenuto A, Sasso G, Postiglione E, De Luca Picione C, Vastola
M, Maniscalco GT, et al: Uric acid in relapsing-remitting multiple
sclerosis: A 2-year longitudinal study. J Neurol. 262:961–967.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Li M, Wang X, Yi H, Xu L, Zhong XF
and Peng FH: Serum uric acid levels in patients with infections of
central nervous system. Acta Neurol Belg. 116:303–308. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collazos J, Blanco MS, Guerra E, Mayo J
and Martínez E: Sequential evaluation of serum urate concentrations
in AIDS patients with infections of the central nervous system.
Clin Chem Lab Med. 38:1293–1296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morato-Conceicao YT, Alves-Junior ER,
Arruda TA, Lopes JC and Fontes CJ: Serum uric acid levels during
leprosy reaction episodes. PeerJ. 4:e17992016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dylewski JS and Gerson M: Hyperuricemia in
patients with infectious mononucleosis. Can Med Assoc J.
132:1169–1170. 1985.PubMed/NCBI
|
|
17
|
Cowdrey SC: Hyperuricemia in infectious
mononucleosis. JAMA. 196:319–321. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cowdrey SC: Hyperuricemia in infectious
mononucleosis: Further observations. J Am Coll Health Assoc.
18:382–383. 1970.PubMed/NCBI
|
|
19
|
Sugita K, Hagisawa S, Satoh Y, Eguchi M
and Furukawa T: Recurrent hepatosplenomegaly and peripheral blood
cytopenia, persistent epstein-barr virus infection and central
nervous system manifestation in a patient with lymphadenopathy and
low serum uric acid. Acta Paediatr Jpn. 40:362–366. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moccia M, Lanzillo R, Palladino R, Russo
C, Carotenuto A, Massarelli M, Vacca G, Vacchiano V, Nardone A,
Triassi M and Morra VB: Uric acid: A potential biomarker of
multiple sclerosis and of its disability. Clin Chem Lab Med.
53:753–759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu XW, Muzny DM, Lee CC and Caskey CT: Two
independent mutational events in the loss of urate oxidase during
hominoid evolution. J Mol Evol. 34:78–84. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santos CX, Anjos EI and Augusto O: Uric
acid oxidation by peroxynitrite: Multiple reactions, free radical
formation and amplification of lipid oxidation. Arch Biochem
Biophys. 372:285–294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becker BF: Towards the physiological
function of uric acid. Free Radic Biol Med. 14:615–631. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Messerli FH, Makani H and Halpern D: Gout.
N Engl J Med. 364:1876–1877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neogi T: Clinical practice. gout. N Engl J
Med. 364:443–452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanellis J and Kang DH: Uric acid as a
mediator of endothelial dysfunction, inflammation and vascular
disease. Semin Nephrol. 25:39–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sautin YY and Johnson RJ: Uric acid: The
oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids.
27:608–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Proctor PH: Uric acid: Neuroprotective or
neurotoxic? Stroke. 39:e882008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dawson J, Quinn T, Lees K and Walters M:
The continued yin and yang of uric acid. Stroke. 39:e92008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Proctor PH: Uric acid and neuroprotection.
Stroke. 39:e1262008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lipton P: Ischemic cell death in brain
neurons. Physiol Rev. 79:1431–1568. 1999.PubMed/NCBI
|
|
32
|
Amaro S, Soy D, Obach V, Cervera A, Planas
AM and Chamorro A: A pilot study of dual treatment with recombinant
tissue plasminogen activator and uric acid in acute ischemic
stroke. Stroke. 38:2173–2175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brouns R, Wauters A, Van De Vijver G, De
Surgeloose D, Sheorajpanday R and De Deyn PP: Decrease in uric acid
in acute ischemic stroke correlates with stroke severity, evolution
and outcome. Clin Chem Lab Med. 48:383–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Onetti Y, Dantas AP, Pérez B, Cugota R,
Chamorro A, Planas AM, Vila E and Jiménez-Altayó F: Middle cerebral
artery remodeling following transient brain ischemia is linked to
early postischemic hyperemia: A target of uric acid treatment. Am J
Physiol Heart Circ Physiol. 308:H862–H874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hooper DC, Spitsin S, Kean RB, Champion
JM, Dickson GM, Chaudhry I and Koprowski H: Uric acid, a natural
scavenger of peroxynitrite, in experimental allergic
encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA.
95:pp. 675–680. 1998, View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nudelman Y and Tunkel AR: Bacterial
meningitis: Epidemiology, pathogenesis and management update.
Drugs. 69:2577–2596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liechti FD, Grandgirard D and Leib SL:
Bacterial meningitis: Insights into pathogenesis and evaluation of
new treatment options: A perspective from experimental studies.
Future Microbiol. 10:1195–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aycicek A, Iscan A, Erel O, Akcali M and
Ocak AR: Oxidant and antioxidant parameters in the treatment of
meningitis. Pediatr Neurol. 37:117–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Checconi P, Salzano S, Bowler L, Mullen L,
Mengozzi M, Hanschmann EM, Lillig CH, Sgarbanti R, Panella S,
Nencioni L, et al: Redox proteomics of the inflammatory secretome
identifies a common set of redoxins and other glutathionylated
proteins released in inflammation, influenza virus infection and
oxidative stress. PLoS One. 10:e01270862015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zuwała-Jagiełło J, Warwas M and
Pazgan-Simon M: Ischemia-modified albumin (IMA) is increased in
patients with chronic hepatitis C infection and related to markers
of oxidative stress and inflammation. Acta Biochim Pol. 59:661–667.
2012.PubMed/NCBI
|
|
41
|
Komaravelli N and Casola A: Respiratory
Viral Infections and subversion of cellular antioxidant defenses. J
Pharmacogenomics Pharmacoproteomics. 5:10001412014.PubMed/NCBI
|
|
42
|
Ngondi JL, Oben J, Forkah DM, Etame LH and
Mbanya D: The effect of different combination therapies on
oxidative stress markers in HIV infected patients in Cameroon. AIDS
Res Ther. 3:192006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chakraborty SP, Das S, Chattopadhyay S,
Tripathy S, Dash SK, Pramanik P and Roy S: Staphylococcus
aureus infection induced redox signaling and DNA fragmentation
in T-lymphocytes: Possible ameliorative role of nanoconjugated
vancomycin. Toxicol Mech Methods. 22:193–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aslan M, Nazligul Y, Horoz M, Bolukbas C,
Bolukbas FF, Aksoy N, Celik H and Erel O: Serum prolidase activity
and oxidative status in Helicobacter pylori infection. Clin
Biochem. 40:37–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hébert-Schuster M, Borderie D, Grange PA,
Lemarechal H, Kavian-Tessler N, Batteux F and Dupin N: Oxidative
stress markers are increased since early stages of infection in
syphilitic patients. Arch Dermatol Res. 304:689–697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kariya C, Chu HW, Huang J, Leitner H,
Martin RJ and Day BJ: Mycoplasma pneumoniae infection and
environmental tobacco smoke inhibit lung glutathione adaptive
responses and increase oxidative stress. Infect Immun.
76:4455–4462. 2008. View Article : Google Scholar : PubMed/NCBI
|