Introduction
In endometrial carcinoma, undifferentiated carcinoma
with grade 1 or 2 endometrioid adenocarcinoma is defined as
dedifferentiated endometrial carcinoma (1). Due to a relatively newly recognized
entity, there are quite a few cases with dedifferentiated
endometrial carcinoma reported worldwide (2). Of note, there is reportedly an
association between dedifferentiated endometrial carcinoma and
Lynch syndrome (1), an autosomal
dominant inherited cancer susceptibility syndrome caused by MMR
genes including MLH1, MSH2, MSH6, and
PMS (2,3).
In the present study, we reported three cases of
dedifferentiated endometrial carcinoma treated in our hospital with
their immunohistochemical expression of MMR proteins.
Case reports
Clinical characteristics of three
cases
Table I shows the
summary of clinical characteristics in three cases of
dedifferentiated endometrial carcinoma treated at our hospital in
2014 and 2015. The mean age at diagnosis was 54 years. All three
cases presented with atypical genital bleeding as chief complaints
and elevated tumor markers (CEA, CA19-9, CA125) were detected.
Patients 2 and 3 were null gravid and had familial histories of
colon cancer. As for past medical history, patient 1 had a history
of ulcerative colitis and patient 3 had a history of renal cell
carcinoma. Preoperative endometrial biopsies were performed in all
the patients and histological type was endometrioid adenocarcinoma
G1 in patient 1 and high-grade adenocarcinoma in patient 3. In
patient 2, we did not pick up sufficient materials. All three
patients underwent surgery based on the diagnosis of endometrial
carcinoma. In patients 1 and 3, we accomplished complete surgery
without any residual tumor. By contrast, we did not accomplish
complete surgery in patient 2 as there were many unresectable
tumors in the retroperitoneal cavity. Patient 1 was early stage,
and patients 2 and 3 were advanced stage. The treatment strategy
for adjuvant therapy was different in the patients because of
different degrees of renal dysfunction: It was mild in patient 1,
moderate in patient 2, and severe in patient 3. Patient 1 was alive
with no evidence of disease 2 years post-operation, but patients 2
and 3 succumbed to the disease at 5 months and 7 months
post-operation, respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Patient 1 | Patient 2 | Patient 3 |
|---|
| Age at diagnosis
(years) | 66 | 48 | 48 |
| Pregnancy
history | 3G2P | 0G0P | 0G0P |
| Family history | None | Father: Colon
cancer | Father: Colon
cancer |
| Past history | Ulcerative
colitis | None | Renal cell
carcinoma |
| Chief complaint | Atypical genital
bleeding | Atypical genital
bleeding | Atypical genital
bleeding |
| Preoperative
endometrial | Endometrioid | Insufficient
materiala | High-grade |
| biopsy | adenocarcinoma
G1 |
| adenocarcinoma |
| Operation | TAH + BSO + LNX
(pelvis-paraaorta) | TAH + BSO + OMTX +
Right hemicolectomy + Hartmann operation | TAH + BSO + OMTX +
LNX (pelvis) + LNS (paraaorta) |
| FIGO stage | IA | IVB | IIIA |
| TNM
classification | pT1aN0M0 | pT4bNXM0 | pT3aN0M0 |
| Carcinoma components
confirmed in hysterectomy specimen | Endometrioid G1:
55% | Endometrioid G1:
10% | Endometrioid G1:
40% |
|
| Undifferentiated:
45% | Undifferentiated:
90% | Undifferentiated:
60% |
| Residual tumor | None | >5 cm above
ureter | None |
| Adjuvant therapy | AP protocol | TC protocol | Radiation |
|
| Adriamycin: 60
mg/m2, Cisplatin: 50 mg/m2 | Paclitaxel:180
mg/m2, Carboplatin: AUC 6 | Pelvis |
|
| every 3 weeks, 6
cycles | every 4 weeks, 4
cycles | 50 Gy |
| Progression-free
time | 2 years | 5 months | 5 months |
| Recurrent or
metastases sites | None | Enlargement of pelvic
tumor | Lung, vaginal
stump |
| Outcome | Alive | Death 5 months after
operation | Death 7 months after
operation |
The patients provided permission to publish these
features of her case, and the identity of the patient has been
protected. Furthermore, ethics approval was obtained from the
Ethics Comittee of the Jikei University School of Medicine
[approval no. 14-132(4001)] and written informed consent was
obtained from the patient for publication of this case study and
the accompanying images.
Pathological findings
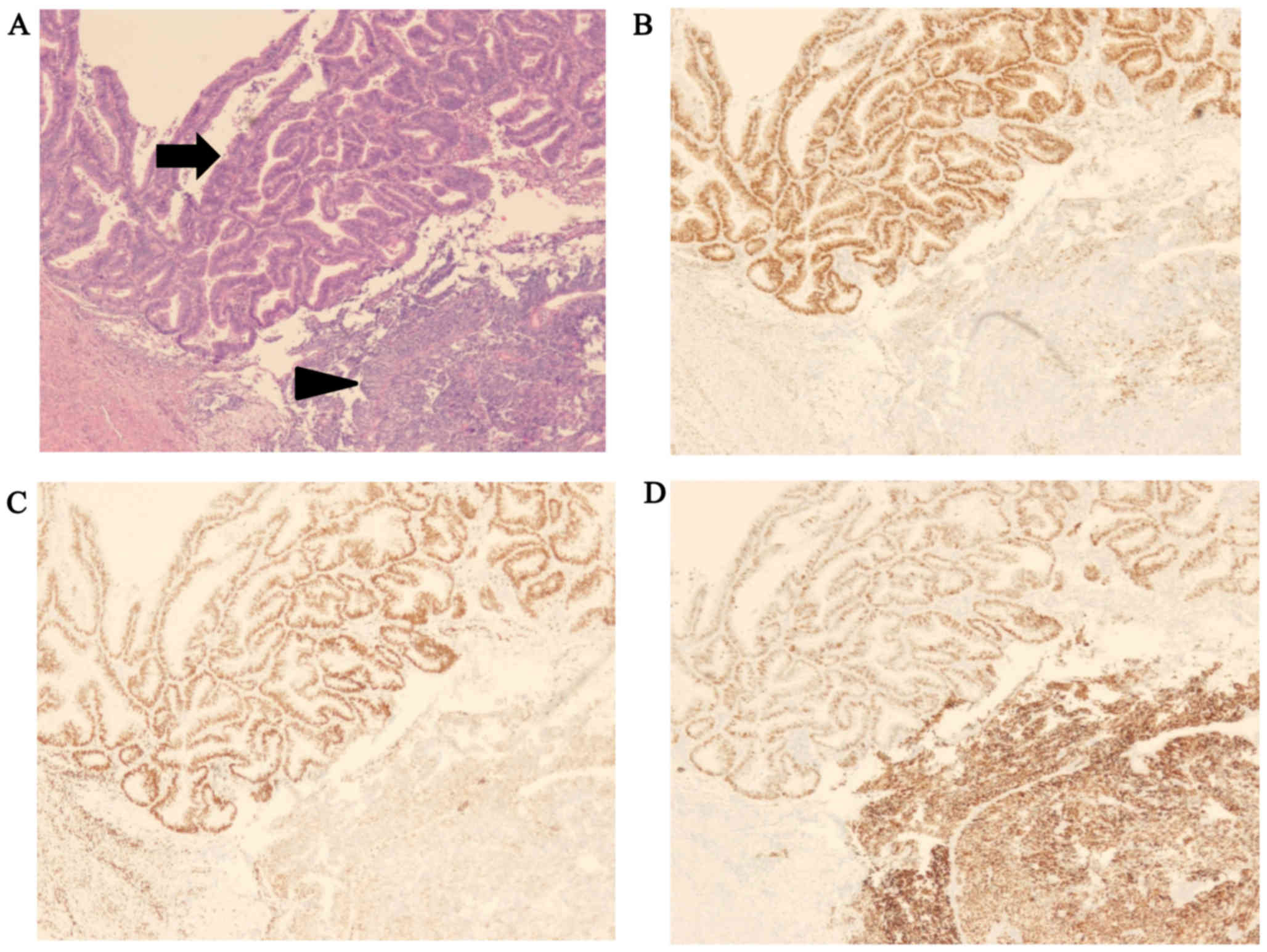
The histological examination performed in all three
cases revealed endometrial carcinoma containing low-grade
endometrioid adenocarcinoma and undifferentiated carcinoma, with
the abrupt transition of any two components showing a sharp border
(Fig. 1). The amount of
undifferentiated carcinoma components varied among the cases,
ranging from 45 to 90% (Table I).
Immunohistochemically, the expression for ER, PR, and p53 was
similar in all three cases of dedifferentiated carcinoma: ER and PR
were positive in the endometrioid adenocarcinoma component, and
negative for the undifferentiated carcinoma component, while p53
was overexpressed only in the undifferentiated carcinoma component
(Fig. 1).
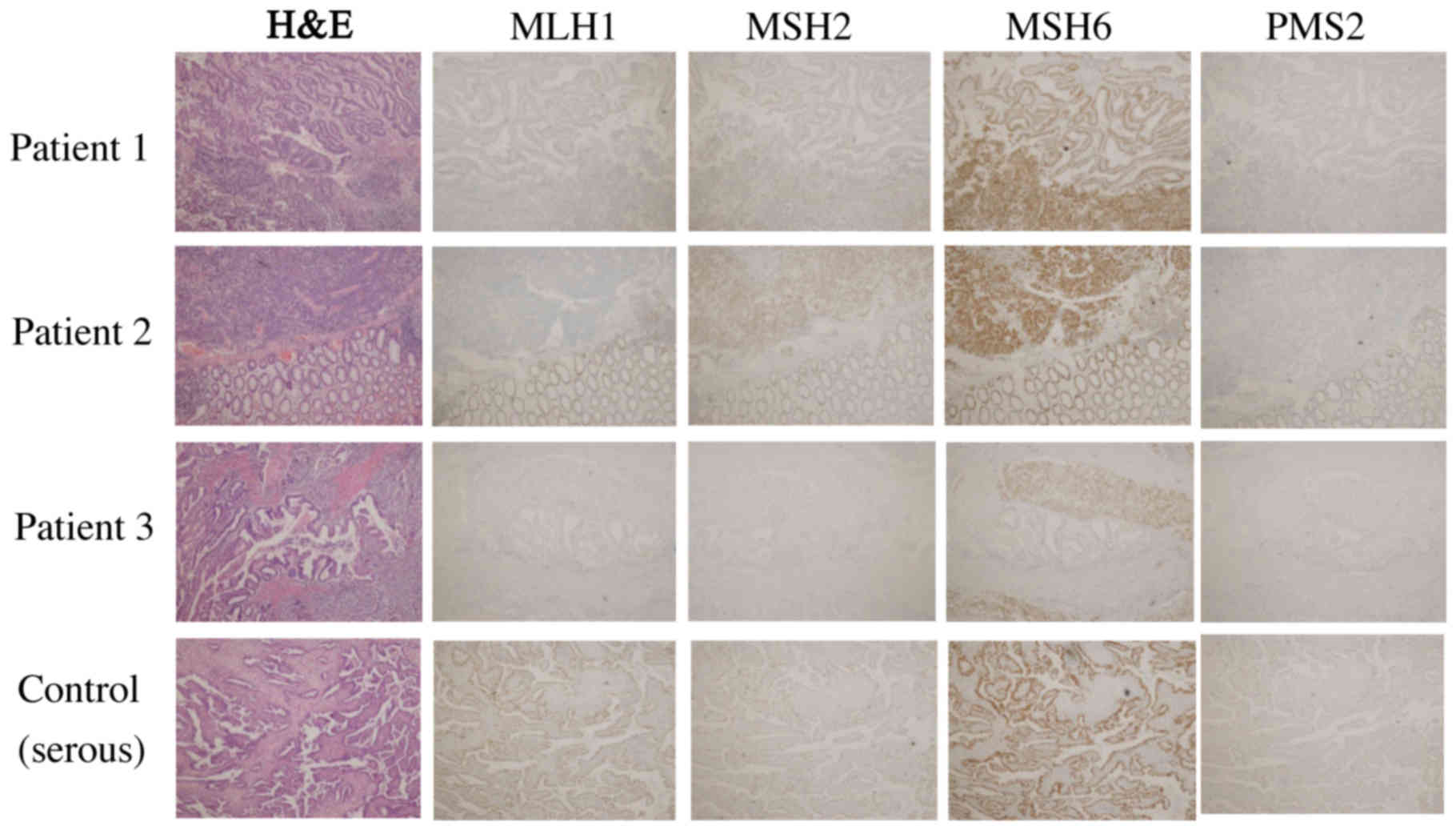
We also performed immunohistochemistry for four DNA
MMR proteins, i.e., MLH1, MSH2, MSH6, and PMS2, which served as
surrogate markers for Lynch syndrome, in three cases of
dedifferentiated carcinoma described above and the case of serous
carcinoma (control) (Fig. 2 and
Table II) (4). The undifferentiated carcinoma component
in three cases of dedifferentiated carcinoma showed loss of
MLH1/PMS2. These four DNA MMR proteins were retained in all the
serous carcinoma cases.
 | Table II.Immunohistochemical analyses of
dedifferentiated endometrial carcinoma cases. |
Table II.
Immunohistochemical analyses of
dedifferentiated endometrial carcinoma cases.
|
| MLH1 | MSH2 | MSH6 | PMS2 |
|---|
| Case 1 (DC) | Negative | Strongly
positive | Strongly
positive | Negative |
| Case 2 (DC) | Negative | Strongly
positive | Strongly
positive | Negative |
| Case 3 (DC) | Negative | EM: Negative | EM: Weakly
positive | Negative |
|
|
| UC: Strongly
positive | UC: Strongly
positive |
|
| Case 4 (Serous)
control case) | Strongly
positive | Strongly
positive | Strongly
positive | Strongly
positive |
Discussion
In 2006, Silva et al reported cases of
endometrial carcinoma in which low-grade endometrioid carcinoma was
combined with undifferentiated carcinoma, and designated them as
dedifferentiated endometrial carcinoma (5). The rate of each component was not
defined. It is reported that undifferentiated carcinoma comprises
9% of endometrial carcinoma (5). The
percentage of dedifferentiated endometrial carcinoma is thought to
be 40% of undifferentiated carcinoma (5). The peak age of dedifferentiated
endometrial carcinoma is 55 years, and the primary complaint is
post-menopausal atypical genital bleeding (1). The risk factor remains unclear but some
case reports have shown an association with Lynch syndrome
(1). According to Silva's report,
the frequency of stage I and II was 37.5% and stage III and IV was
62.5% (5). The clinical
characteristics of our cases are similar to previous reports.
The pathological characteristics of undifferentiated
carcinoma are as follows: Proliferation of small- to middle-size
cells without any differentiation; typically tumor cells are
positive for p53, EMA, CK18, and vimentin, negative for ER, PR, or
E-cadherin, and they may be negative for pan-cytokeratins (1). Undifferentiated carcinoma may arise
through transformation or dedifferentiation in well-differentiated
endometrioid adenocarcinoma (5).
According to the study by Wu et al, when dedifferentiated
endometrial carcinoma metastasizes, the majority of metastases are
comprised of the undifferentiated component. In the metastatic
lesions, ER and PR expression may be the tissue biomarkers to
distinguish the origin of the tumor (6). Hoang et al also reported that
the loss of PAX8 and ER expression may be a fundamental feature of
dedifferentiation (7). There is a
tendency for the well-differentiated endometrioid component to
exist mainly on the tumor surface and for the undifferentiated
component to exist in the deeper area (8). Due to this localization, it is possible
that the undifferentiated component cannot be identified by biopsy;
thus, an exact diagnosis and the appropriate operation are
difficult to determine. In the current report, there were no cases
of exact diagnosis using a biopsy specimen. According to Kanis
et al, the sensitivity of the preoperative endometrial
biopsy or curettage decreases with high-risk histology endometrial
cancer (9). It also has been
demonstrated that undifferentiated carcinoma component when
coexisting with endometrioid adenocarcinoma may be erroneously
recognized as solid component of endometrioid adenocarcinoma,
leading to misdiagnose the tumor as FIGO grade 2 or 3 endometrioid
adenocarcinoma (2). While the tumors
cells are discohesive with high-grade nuclear feature and grow in a
sheet-like manner in undifferentiated carcinoma, those of
endometrioid adenocarcinoma forming solid nests are cohesive and
show similar cytology to those forming glands. Previous findings
suggest the strategy to distinguish between undifferentiated
carcinoma and solid component of endometrioid adenocarcinoma. When
an undifferentiated carcinoma component is juxtaposed with
low-grade endometrioid adenocarcinoma, a sharp boundary is evident
between them, whereas a seamless transition from glandular
component to solid component is observed in high-grade endometrioid
adenocarcinoma (10). Ramalingan
et al reported that PAX8 may be an effective biomarker to
distinguish undifferentiated carcinoma (11).
The endometrioid component was ER (+) and PR (+),
and p53 (−). The undifferentiated component was ER (−) and PR (−),
and p53 (++) (Fig. 1). These
findings are characteristic of type 1 and type 2 cancer coexistence
(12). Furthermore, all the
components of undifferentiated carcinoma in dedifferentiated
carcinoma showed loss of MLH1/PMS2, whereas serous adenocarcinoma
was positive. Dedifferentiated carcinoma has been reported to be
associated with Lynch syndrome (1).
Lynch syndrome is an autosomal dominant inherited cancer
susceptibility syndrome caused by germline mutations in one of a
set of MMR genes (MLH1, MSH2, MSH6, and
PMS (2,3). Loss of expression is a predictive
marker for germline mutation. MLH1 dimerizes with PMS2 in
functional states, in order that MLH1 abnormality is accompanied by
the loss of PMS2. Garg et al reported that five of seven
dedifferentiated carcinomas were associated with abnormalities in
MLH1/PMS2 (13). However, loss of
MLH1 is caused by methylation of MLH1 as well as germline mutations
of MLH1. They did not perform genetic testing for cases with
abnormalities in MLH1/PMS2. In the study by Lu et al on
endometrial cancer at age younger than 50 years, only one of 13
cases with loss of MLH1 had germline mutation of MLH1 and the other
cases had methylation of MLH1 (14).
Personal and family history is very important for identifying
patients with high risk of Lynch syndrome (3). In the same study, they also reported
that women with a Lynch syndrome-associated cancer had a 43% chance
of germline mutation in MMR as compared to women without an
affected first-degree relative (14). Two of our three cases having family
history of colon cancer in a first-degree relative, were referred
for genetic counseling. According to the Berretta et al,
most of the patients diagnosed with dedifferentiated endometrial
carcinoma were deceased due to disease within one year, and the
appropriate treatment for dedifferentiated endometrial carcinoma
was not defined (15). In most
reports, operative therapy with adjuvant chemotherapy was
performed, but there is no evidence-based strategy, including
operative therapy, chemotherapy, and radiation therapy (15). In general, the prognosis of
dedifferentiated endometrial carcinoma is poor regardless of the
undifferentiated component percentage and the degree of
differentiation of endometrioid adenocarcinoma (4). The concept of the rare histological
type should be recognized when seeking a precise prognostic
analysis and the appropriate therapeutic strategy. In addition,
personal and family history and immunohistochemical analysis of MMR
protein for patients with dedifferentiated carcinoma of endometrium
should be considered to identify the risk of Lynch syndrome.
Acknowledgements
The present study has undergone English language
review by a native English speaker (Enago).
References
|
1
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumours of female reproductive
organs. Fourth Edition. World Health Organization; pp. 132–133.
2014
|
|
2
|
Shen Y, Wang Y, Shi Y, Liu J and Liu Y:
Clinicopathologic study of endometrial dedifferentiated
endometrioid adenocarcinoma: A case report. Int J Clin Exp Pathol.
5:77–82. 2012.PubMed/NCBI
|
|
3
|
Lynch HT and de la Chapelle A: Genetic
susceptibility to non-polyposis colorectal cancer. J Med Genet.
36:801–818. 1999.PubMed/NCBI
|
|
4
|
Kazu U, Kyosuke Y, Mitsuyoshi U, Yoshio I,
Misako S, Takashi N, Hiroyuki T, Aikou O, Misato S, et al:
Association of extracellular matrix metalloproteinase inducer in
endometrial carcinoma with patient outcomes and clinicopathogenesis
using monoclonal antibody 12C3. Oncol Rep. 17:731–735. 2006.
|
|
5
|
Silva EG, Deavers MT, Bodurka DC and
Malpica A: Association of low-grade endometrioid carcinoma of the
uterus and ovary with undifferentiated carcinoma: A new type of
dedifferentiated carcinoma? Int J Gynecol Pathol. 25:52–58. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu ES, Shih Le-M and Díaz-Montes TP:
Dedifferentiated endometrioid adenocarcinoma: An under-recognized
but aggressive tumor? Gynecol Oncol Rep. 5:25–27. 2013. View Article : Google Scholar
|
|
7
|
Hoang LN, Lee YS, Karnezis AN,
Tessier-Cloutier B, Almandani N, Coatham M, Gilks CB, Soslow RA,
Stewart CJ, Köbel M and Lee CH: Immunophenotypic features of
dedifferentiated endometrial carcinoma - insights from
BRG1/INI1-deficient tumours. Histopathology. 69:560–569. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tafe LJ, Garg K, Chew I, Tornos C and
Soslow RA: Endometrial and ovarian carcinomas with undifferentiated
components: Clinically aggressive and frequently underrecognized
neoplasms. Mod Pathol. 23:781–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanis MJ, Rahaman J, Moshier EL,
Zakashansky K, Chuang L and Kolev V: Detection and correlation of
pre-operative, frozen section, and final pathology in high-risk
endometrial cancer. Eur J Gynaecol Oncol. 37:338–341.
2016.PubMed/NCBI
|
|
10
|
Jiheun H, Eun YK, Sung ER, Soo YH and
Ahwon L: Dedifferentiated endometrioid carcinoma of the uterus:
report of four cases and review of literature. World J Surg Oncol.
15:172017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramalingam P, Masand RP, Euscher ED and
Malpica A: Undifferentiated carcinoma of the endometrium: an
expanded immunohistochemical analysis including PAX-8 and
basal-like carcinoma surrogate markers 35. Int J Gynecol Pathol.
410–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garg K, Leitao MM Jr, Kauff ND, Hansen J,
Kosarin K, Shia J and Soslow RA: Selection of endometrial
carcinomas for DNA mismatch repair protein immunohistochemistry
using patient age and tumor morphology enhances detection of
mismatch repair abnormalities. Am J Surg Pathol. 33:925–933. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu KH, Schorge JO, Rodabaugh KJ, Daniels
MS, Sun CC, Soliman PT, White KG, Luthra R, Gershenson DM and
Broaddus RR: Prospective determination of prevalence of lynch
syndrome in young women with endometrial cancer. J Clin Oncol.
25:5158–5164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berretta R, Patrelli TS, Faioli R, Mautone
D, Gizzo S, Mezzogiorno A, Giordano G and Modena AB:
Dedifferentiated endometrial cancer: An atypical case diagnosed
from cerebellar and adrenal metastasis: Case presentation and
review of literature. Int J Clin Exp Pathol. 6:1652–1657.
2013.PubMed/NCBI
|