Introduction
Cervical conization is the recommended treatment for
cervical intraepithelial neoplasia (CIN) grade 3 (1). Based on the available evidence, there
is no optimal surveillance strategy following treatment for CIN
(2). For early detection of
recurrence, long-term follow-up after cervical conization has been
recommend by the American College of Obstetricians and
Gynecologists (ACOG) guidelines (3).
In Japan, the national screening guidelines for
women under the National Health Insurance system state that
cervical conventional cytology using Pap smears is a standard
screening test for cervical cancer. Human papillomavirus (HPV) DNA
testing has not been recommended for population-based screening due
to the scarcity of scientific evidence. However, when conventional
cervical cytology shows atypical squamous cells of undetermined
significance (ASC-US), repeat cervical cytology after 6 and 12
months, immediate colposcopy, or HPV DNA triage have been
recommended by the National Cancer Comprehensive Network (NCCN)
guidelines (4).
Although ASC-US comprise a wide variety of cervical
cells, including benign and malignant cells, the presence of ASC-US
has been considered as a low-risk abnormal cervical cytological
characteristic (5). However, a
substantial proportion of cases displaying ASC-US have underlying
high-grade CIN (2 or 3) and, thus, are at an increased risk of
developing cervical cancer (6).
Based on these facts, it appears reasonable to consider women with
ASC-US following treatment for CIN to be at a relatively increased
risk of developing cervical cancer compared with women with ASC-US
after no treatment. As regards risk stratification for women
following treatment for CIN, an appropriate triage method used to
identify women with ASC-US who have or will develop a cervical
cancer precursor is crucial. The clinical significance of ASC-US
following cervical conization for CIN, particularly for CIN 3,
which has a high risk of recurrence, has not been fully elucidated.
The aims of the present study were to evaluate the clinical
significance of ASC-US following cervical conization for CIN 3 and
to suggest an appropriate triage method.
Patients and methods
Study population
This was a retrospective cohort study. In order to
identify cases with cytological abnormalities following conization,
the medical records of patients who received conization as a
conservative treatment for CIN 2–3 were reviewed. A total of 142
cases with CIN 3 that had been diagnosed using the conization
specimens between February 2005 and May 2015 in our hospital were
ultimately considered as eligible for review.
Conization procedure
Conization was performed using
yttrium-aluminum-garnet (YAG) laser or ultrasonic scalpel under
spinal anesthesia. Prior to resection, the squamous columnar
junction (SCJ) was examined using the Schiller test.
The YAG laser procedure was performed as follows:
The cervix was sutured, pulling the line to the outside of the SCJ.
Towing the line, cervical excision was performed with the YAG laser
at 12 W. The resection stump was coagulated with the laser.
The ultrasonic scalpel procedure was as follows: The
cervix was sutured, pulling the line to the outside of the SCJ.
Cervical excision was performed using output level 3 of the
Harmonic Scalpel. The use of equipment was determined by the
attending physician.
Surveillance after treatment for CIN
3
A conventional Pap smear was performed after
conization at a time left to the discretion of each physician. The
physicians conducted the follow-up based on the guidelines
determined by the Office of Gynecology in Japan and the NCCN
guidelines (4).
Identification of abnormal cytology
and recurrence after treatment
Abnormal cytology was defined as worse than ASC-US.
ASC-US was determined by one cytoscreener and one cytopathologist
based on the Bethesda guidelines (7). Recurrence was defined as a diagnosis
worse than CIN 2 in any pathological specimen at any timepoint
during the follow-up period. The pathological specimens were
independently reviewed by two gynecological pathologists.
HPV testing
Some ASC-US patients underwent high-risk HPV DNA
testing (Hybrid Capture test using SurePath (BD Biosciences,
Sparks, MD, USA) as the collection method). The high-risk HPV DNA
test detects 13 different HPV types (16, 18, 31, 33, 35, 39, 45,
51, 52, 56, 58, 59, 68) (8).
High-risk HPV DNA testing in patients with ASC-US was left to the
discretion of the physicians.
Statistical analysis
Data were analyzed using the SPSS 21.0 software (IBM
Corp., Armonk, NY, USA). Based on the postoperative cytology
results, patients with abnormal cytology after conization were
divided into an ASC-US group and a worse than low-grade squamous
intraepithelial lesion (LSIL) group. The data are presented as
means ± standard deviation (SD) for quantitative variables and
frequencies (%) for qualitative variables. Student's t-test was
used to compare means or medians, and the Chi-squared test or
Fisher's exact test were used, as appropriate, to compare the
frequency distributions of categorical variables. Pearson's
χ2 test was used to analyze categorical variables.
Kaplan-Meier survival curves with log-rank tests, with patient
status at the time of the last follow-up visit, were used to
compare the cumulative recurrence-free rates among the normal,
ASC-US, and worse than LSIL groups. A P-value of <0.05 was
considered to indicate statistically significant differences.
Results
Patient characteristics
The mean age of the 142 patients was 36.1 years. The
mean age was not significantly different between the normal and the
abnormal cytology groups. The mean follow-up period after
conization was 41.8 months.
Cytological abnormalities after conization were
observed in 27 patients (19%), whereas the remaining 115 patients
had normal cytological findings. There were no significant
differences in age, surgical instrument, postoperative visit
frequency, duration, or intervals between the abnormal and the
normal cytology groups (Table
I).
 | Table I.Patient characteristics (n=142). |
Table I.
Patient characteristics (n=142).
|
| Cytological findings
after treatment |
|---|
|
|
|
|---|
| Characteristics | Normal | Abnormal |
|---|
| Number of
patients | 115 | 27 |
| Mean age, years | 36.2 | 35.5 |
| Conization procedure,
n (%) |
|
|
|
Ultrasonic scalpel | 38 (33.0) | 12 (44.4) |
| YAG
laser | 77 (67.0) | 15 (55.6) |
| Margins status of the
excised specimens, n |
|
|
|
Negative | 66 | 13 |
|
Positive | 32 | 12 |
| Not
assessable | 17 | 2 |
| Postoperative
follow-up visits |
|
|
| Mean
total number of visits (median) | 7.1 (6) | 7.5 (6) |
| Mean
duration, months (median) | 42.0 (33) | 41.1 (31) |
| Mean
interval, months | 5.6 | 5.2 |
Identification of abnormal cytology
after treatment
Of all the participants in this study, 19 (13.3%)
had ASC-US, and 8 (5.6%) had a diagnosis worse than LSIL. There was
no significant difference in the mean age between the two groups
(35.3 vs. 35.9 years, respectively). The rate of cytological
abnormalities did not differ significantly among the negative
margin, the positive margin, and the non-assessable margin groups
(χ2=0.104). However, in the abnormal cytology group,
there was a significant positive association between the rate of
using YAG laser conization and the positive margin status of the
excised specimen group (χ2=0.036; Table II).
 | Table II.Association of abnormal cytology and
conization procedure with margin status. |
Table II.
Association of abnormal cytology and
conization procedure with margin status.
|
|
| Margin status of the
excised specimens, n |
|
|---|
|
|
|
|
|
|---|
| Cytology after
treatment | Conization
procedure | Negative | Positive | Unclear | Total | P-value (Pearson's
χ2 test) |
|---|
| Normal | YAG laser | 42 | 25 | 10 | 77 | 0.267 |
|
| Ultrasonic
scalpel | 24 | 7 | 7 | 38 |
|
|
| Total | 66 | 32 | 17 | 115 |
|
| Abnormal | YAG laser | 4 | 9 | 1 | 15 | 0.036 |
|
| Ultrasonic
scalpel | 9 | 3 | 1 | 12 |
|
|
| Total | 13 | 12 | 2 | 27 |
|
| Total | YAG laser | 46 | 34 | 11 | 92 | 0.104 |
|
| Ultrasonic
scalpel | 33 | 10 | 8 | 50 |
|
|
| Total | 79 | 44 | 19 | 142 |
|
ASC-US group
In this group, there were negative margins in 11
patients, positive margins in 7 patients, and a non-assessable
margin in 1 patient. There were 3 different approaches to
management based on the Japanese and NCCN guidelines. High-risk HPV
tests were performed in 11 cases (including 7 negative-margin
cases, 3 positive-margin cases and case with a non-assessable
margin); the high-risk HPV test was positive in 6 cases (including
2 negative-margin cases using the ultrasonic scalpel, 1
negative-margin case using the YAG laser, 2 positive-margin cases
using the YAG laser, and 1 non-assessable margin case using the YAG
laser). Colposcopy with cervical biopsy was performed in 4 cases; 2
cases of CIN 1 and 2 cases of CIN 2 were detected (1
positive-margin case using the YAG laser and 1 negative-margin case
using the ultrasonic scalpel). One patient underwent re-excision,
and the result was negative for dysplasia. A total of 5 patients
were negative for high-risk HPV (including 4 negative-margin
patients and 1 positive-margin patient). Of those 5 patients, 4
were followed up by repeat cervical cytology, and all the
cytological results were negative. In one case, hysterectomy was
performed at the patient's request, and the result was negative for
dysplasia.
The high-risk HPV test was not performed in 8 cases
(including 4 negative-margin and 4 positive-margin cases). Of the 8
cases, 5 (3 negative-margin and 2 positive-margin) were followed up
by repeat cervical cytology, and all the cytological results were
negative. Immediate colposcopy with cervical biopsy was performed
in 1 patient (with a positive margin), and no dysplasia was
detected. Two patients (1 negative-margin and 1 positive-margin)
underwent immediate colposcopy followed by hysterectomy at their
request; 1 of the patients had CIN 3, and the other patient had CIN
1 (Table III).
 | Table III.Course of ASC-US cases. |
Table III.
Course of ASC-US cases.
| Case | Age (years) | Conization
procedure | Margin status of
the excised specimens | Time to first
identification of abnormal cytology after treatment (months) | Management after
first identification of abnormal | High-risk HPV DNA
test | Follow-up after
first management | Final
evaluation | Follow-up time
(months) |
|---|
| 1 | 27 | Ultrasonic
scalpel | Negative | 14 | Repeat cervical
cytology |
| Repeat cervical
cytology | NILM | 35 |
| 2 | 30 | Ultrasonic
scalpel | Negative | 6 | Repeat cervical
cytology |
| Repeat cervical
cytology | NILM | 22 |
| 3 | 30 | Ultrasonic
scalpel | Negative | 8 | Repeat cervical
cytology |
| Repeat cervical
cytology | NILM | 18 |
| 4 | 36 | Ultrasonic
scalpel | Negative | 2 | High-risk HPV DNA
test | Negative | Repeat cervical
cytology | NILM | 22 |
| 5 | 35 | Ultrasonic
scalpel | Negative | 1 | High-risk HPV DNA
test | Negative | Hysterectomy | No dyplasia | 11 |
| 6 | 35 | Ultrasonic
scalpel | Negative | 12 | High-risk HPV DNA
test | Negative | Repeat cervical
cytology | NILM | 22 |
| 7 | 33 | Ultrasonic
scalpel | Negative | 8 | High-risk HPV DNA
test | Negative | Repeat cervical
cytology | NILM | 18 |
| 8 | 31 | Ultrasonic
scalpel | Negative | 11 | High-risk HPV DNA
test | Positive | Colposcopy with
cervical biopsy | CIN 2 followed by
ASC-US | 27 |
| 9 | 37 | Ultrasonic
scalpel | Negative | 41 | High-risk HPV DNA
test | Positive | Colposcopy with
cervical biopsy | No dysplasia
followed by ASC-US | 51 |
| 10 | 29 | YAG laser | Negative | 2 | High-risk HPV DNA
test | Positive | Re-excision | No dyplasia
followed by NILM | 44 |
| 11 | 41 | YAG laser | Negative | 84 | Immediate
colposcopy |
| Hysterectomy | CIN 3 | 103 |
| 12 | 28 | Ultrasonic
scalpel | Positive | 13 | Repeat cervical
cytology |
| Repeat cervical
cytology | NILM | 25 |
| 13 | 41 | Ultrasonic
scalpel | Positive | 5 | Repeat cervical
cytology |
| Repeat cervical
cytology | NILM followed by
pregnancy | 15 |
| 14 | 33 | YAG laser | Positive | 76 | High-risk HPV DNA
test | Negative | Repeat cervical
cytology | NILM | 86 |
| 15 | 34 | YAG laser | Positive | 32 | High-risk HPV DNA
test | Positive | Colposcopy with
cervical biopsy | CIN 1 followed by
HSIL | 49 |
| 16 | 42 | YAG laser | Positive | 101 | High-risk HPV DNA
test | Positive | Colposcopy with
cervical biopsy | CIN 2 followed by
NILM | 121 |
| 17 | 35 | YAG laser | Positive | 12 | Immediate
colposcopy |
| Colposcopy with
cervical biopsy | No dysplasia
followed by NILM | 32 |
| 18 | 62 | YAG laser | Positive | 9 | Immediate
colposcopy |
| Hysterectomy | CIN 1 | 31 |
| 19 | 32 | YAG laser | Not assessable | 43 | High-risk HPV DNA
test | Positive | Colposcopy with
cervical biopsy | CIN 1 followed by
NILM | 46 |
Worse than LSIL group
In this group, there were 2 negative-margin
patients, 5 positive-margin patients, and 1 non-assessable margin
patient. There were 4 cases of LSIL (3 positive-margin and 1
non-assessable margin), 3 cases of high-grade squamous
intraepithelial lesion (HSIL) (2 negative-margin and 1
positive-margin), and 1 case of atypical squamous cells, which
cannot exclude high-grade squamous intraepithelial lesion (ASC-H)
(a positive-margin case) (Table
IV).
 | Table IV.Course of worse than LSIL cases. |
Table IV.
Course of worse than LSIL cases.
| Case | Age (years) | Conization
procedure | Margin status of
the excised specimens | Time to first
identification of abnormal cytology after treatment (months) | Cytology
findings | Management after
first identification of abnormal cytology | Follow-up after
first management | Final
evaluation | Follow-up time
(months) |
|---|
| 1 | 34 | YAG laser | Negative | 1 | HSIL | Colposcopy with
cervical biopsy | Re-excision (CIN
3) | CIN 3 followed by
HPV, negative ASC-US | 49 |
| 2 | 45 | YAG laser | Negative | 4 | HSIL | Unknown |
|
| 4 |
| 3 | 50 | YAG laser | Positive | 1 | ASCH | Colposcopy with
cervical biopsy | Hysterectomy | CIN 3 | 31 |
| 4 | 26 | Ultrasonic
scalpel | Positive | 47 | LSIL | Colposcopy with
cervical biopsy | Re-excision (CIN
3) | CIN 3 followed by
NILM | 69 |
| 5 | 28 | YAG laser | Positive | 12 | LSIL | Colposcopy findings
followed by repeat cytology | Negative | NILM | 61 |
| 6 | 32 | YAG laser | Positive | 8 | LSIL | Colposcopy with
cervical biopsy | Re-excision (CIN
3) | CIN 3 followed by
NILM | 44 |
| 7 | 35 | YAG laser | Not assessable | 18 | LSIL | Colposcopy findings
followed by repeat cytology | Negative | NILM | 30 |
| 8 | 37 | YAG laser | Positive | 6 | HSIL | Colposcopy with
cervical biopsy | Re-excision (CIN
3) | CIN 3 followed by
NILM | 30 |
Of the 8 patients, 7 underwent immediate colposcopy.
Two patients with LSIL (both with non-assessable margin) had
negative findings on colposcopy and were then followed up with
repeat and cervical cytological examination, which have been normal
thus far. Colposcopy with cervical biopsy was performed after 4
re-excisions. All the patients exhibited CIN 3 (including 1 HSIL
with negative margins, 2 LSIL with positive margins, and 1 HSIL
with positive margins). In the single remaining case, hysterectomy
was performed at the patient's request, with ASC-H including a
positive margin, and CIN 3 was detected in this case. One patient
was lost to follow-up for unknown reasons (Table IV).
Identification of recurrent
disease
Based on colposcopy with cervical biopsy,
re-excision, and hysterectomy after detecting abnormal cytology,
CIN 2 and CIN 3 were diagnosed in 8 of the 142 cases. The
recurrence rate of CIN 2 and CIN 3 was 5.6% of all cases and 29.6%
(8/27) in the abnormal cytology cases. The recurrence rate was
15.7% (3/19) in the ASC-US group and 71.4% (5/7) in the worse than
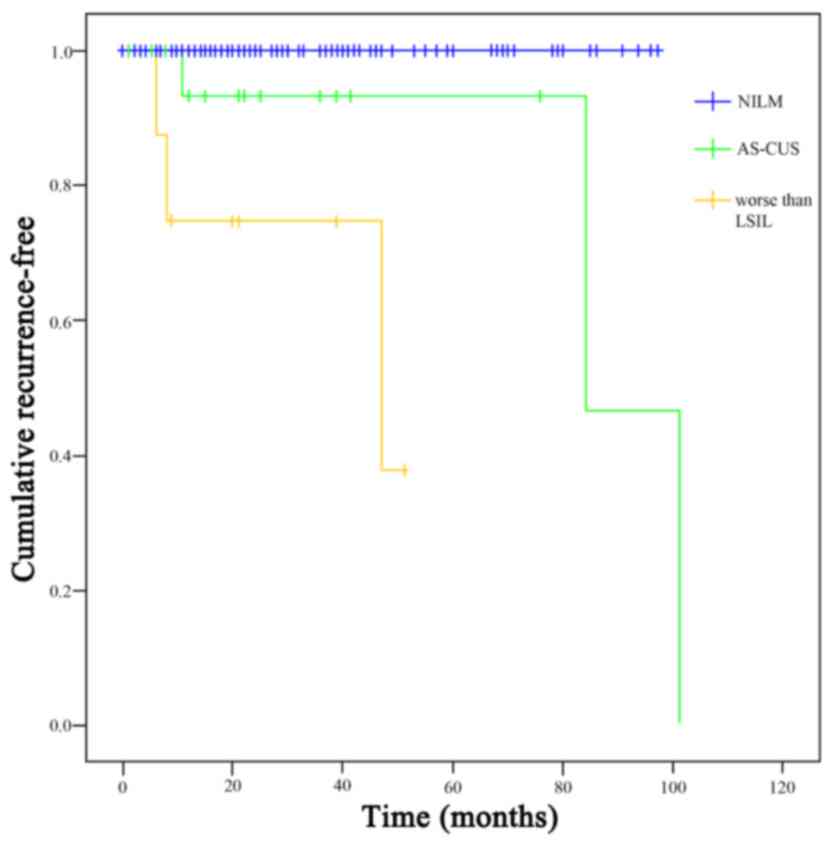
LSIL group. The cumulative recurrence-free rate was significantly
better for the ASC-US group compared with that in the worse than
LSIL group (log-rank test P<0.05; Fig. 1). All cases of worse than LSIL that
underwent histopathological examination were diagnosed with CIN
3.
Postoperative identification of
abnormal cytology and recurrence time
There was no significant difference in the time to
first identification of abnormal cytology after treatment between
the worse than LSIL and the ASC-US groups (12.12±15.2 vs.
24.73±30.6, respectively; P=0.054; Student's t-test). There was no
significant difference in the time to first identification of
abnormal cytology after treatment by margin status of the excised
specimens (positive, 16±23.5; negative, 28±3.24; non-assessable,
17.4±15.02 months; P=0586; Kruskal-Wallis test). However, there was
a significant difference in the time to first identification of
abnormal cytology after treatment between the recurrence and the no
recurrence groups (32.37±40.18 vs. 16.21±19.19, respectively;
P=0.003; Student's t-test).
Discussion
ASC-US was the most common abnormal cytological
finding after treatment for CIN 3. Among all participants, 19%
displayed cytological abnormalities after treatment in this study;
the rate of ASC-US was 13.3%, and that of worse than LSIL 5.6%. The
recurrence rate was 15.7% (3/19) in the ASC-US group and 71.4%
(5/7) in the worse than LSIL group.
The Bethesda System (2001 revision) was adopted in
1988 (7). ASC-US accounts for 90% of
ASC cases in American standard facilities, and ASC-H accounts for
10%. With respect to the frequency of ASC, it has been suggested
that good management requires that the ASC:SIL ratio be maintained
at <1.5, and the frequency of ASC be maintained at <5% of all
cervical screenings (7). With
respect to the accuracy of cancer screenings in our institute, the
rate of negative cytology results in conventional cancer screenings
(95%) was similar to the rate of normal cytology results using
conventional cytology (96%) in the Canadian Cervical Cancer
Screening Trial (9).
In the population-based screening phase, it has been
reported that ASC-US were found in <1% of the cases in a single
population-based study (10).
Solomon et al reported that <4% of U.S. women were given
an equivocal cervical cytological diagnosis (ASC-US) annually
(11). Based on these facts, the
results obtained in the present study revealed a rate of ASC-US
that appears to be quite high (13.3%) compared with these previous
reports. ASC-US comprises a wide variety of cervical cells,
including benign and malignant cells, and it appears reasonable to
suggest that a proportion of women who receive treatment for CIN
with ASC-US have postoperative inflammation resulting in altered
cell morphology.
In addition, it has been reported that, on
conventional cervical cytology alone, ASC-US accounts for 6.9% of
CIN 2, 2.6% of CIN 3, and 0.18% of cervical cancer cases (12). In the present study, the recurrence
rate was 15.7% (3/19) in the ASC-US group. CIN 2 and CIN 3 were the
final pathological diagnoses in 2/19 (10.5%) and 1/19 (5.2%)
patients, respectively. The 2 CIN 2 cases (1 with a positive margin
using the YAG laser, and the other with a negative margin using an
ultrasonic scalpel), were diagnosed with high-risk HPV. The
remaining CIN 3 case (negative margin using a YAG laser and
unknown, high-risk HPV status) was detected by immediate
colposcopy.
On the basis of these facts, it appears reasonable
to predict that women with ASC-US who have undergone treatment for
CIN would be at a greater risk of developing cervical cancer
compared with women with ASC-US in population-based screenings. As
regards the risk stratification of women following treatment for
CIN, it is crucial to determine an appropriate triage method able
to identify women with ASC-US that have or will develop a cervical
cancer precursor.
The risk of developing recurrent high-grade CIN (CIN
2 or CIN 3) and cervical cancer with a positive margin after
treatment is high. The margin-positive recurrence rate after
conization is 9–16% (13), whereas
the margin-negative recurrence rate has been reported to be 2–4%
(14,15). Melnikow et al noted that
recurrence was defined by initial CIN grade and treatment type
(5). A positive margin after
treatment is one of the most important risk factors for recurrence
(12), but evaluation of margins
after conization may be difficult; therefore, in the present study,
margin status was not assessable in some of the cases. Although
there was no significant difference in recurrence of CIN 3 between
the YAG laser and the ultrasonic scalpel, the use of the ultrasonic
scalpel was more frequent in the abnormal group. During conization
with a YAG laser, the margins are often cauterized; this may have
resulted in a low incidence of abnormal cytological findings. In
our hospital, a coin-shaped resection is performed for nulliparous
women, often resulting in unclear or positive margins.
However, even women with clear excision margins are
at risk for disease recurrence (16). The risk of developing invasive cancer
after treatment for high-grade CIN is five times higher compared
with that in the general population (17), which justifies closer surveillance of
such patients with annual cytology and colposcopy follow-up for 10
years after treatment (18).
Therefore, for women treated for CIN 3, it has been recommended
that they have cytological follow-up at least 6 and 12 months after
treatment, and annual cytology for the next 9 years, before
resuming screening at the routine interval (19).
There was a significant difference in the time to
first identification of abnormal cytology after treatment between
the recurrence and the no recurrence groups (32.37±40.18 vs.
16.21±19.19, respectively; P=0.003; Student's t-test). However, the
cumulative recurrence-free rate was a significantly better in the
ASC-US group compared with that in the worse than LSIL group
(log-rank test P<0.05).
Cytological abnormalities during the early
postoperative period are due to persistent lesions or postoperative
inflammation resulting in altered cell morphology, whereas those in
the late postoperative period are due to the generation of new
dysplasia. According to the ACOG guidelines and a Cochrane Database
systemic review (2,3), CIN recurrence was often observed in the
first 24 months; however, in the present study, it appeared that
follow-up for 48 months after surgery is necessary. Even CIN 3
recurrence was observed 5 years after surgery. Although follow-up
for >48 months is necessary, the appropriate intervals and
methods have not been fully elucidated (2).
For the first 4 years after surgery, close
observation is considered necessary, but the number of patient
follow-up visits decreased over time. In our hospital, ~50% of
cases in were followed-up for 48 months. When no cytological
abnormality was identified, the follow-up was often interrupted. As
only 50% of cases returned, the proportion of recurrent cases was
higher. Even if the HPV test is negative, follow-up for 20 years
after conization is recommended in the ACOG guidelines (2,3).
Continuous observation of all cases for 48 months is difficult. In
order to prevent abnormal cytology or CIN 3 recurrence, margin
ablation is important, particularly in coin resection cases. Margin
ablation is important in the prevention of recurrence after
conization, and it is possible to reduce the follow-up visits after
surgery in such cases (20,21).
Katki et al reported that, among ASC-US
patients who tested positive for high-risk HPV, CIN 2 was diagnosed
in 18%, CIN 3 in 6.8%, and cervical cancer in 0.41% of the cases
(12). However, in patients who
tested negative for high-risk HPV, CIN 2 was diagnosed in 1.1%, and
CIN 3 in 0.43% of the patients in previous studies (12,22).
High-risk HPV tests are used for risk stratification and they may
be a reasonable alternative to post-treatment Pap smear cytology
based on sensitivity and specificity. However, these sensitivity
and specificity values are likely not applicable in a
post-treatment surveillance setting, in which the prevalence of
high-risk HPV is significantly higher compared with the screening
phase (5,23). In the post-treatment setting, it is
important to distinguish between a newly detected HPV genotype or
recurrent detection of a lesion-associated HPV genotype, as it has
been reported that most HPV infections are cleared 12 months after
surgery, whereas very few are cleared after this interval (24). Thus, the interpretation of the role
of high-risk HPV tests for post-treatment surveillance may be
difficult.
Abnormal cytology worse than LSIL suggests
recurrence of CIN 3. Cytology may predict the presence and grade of
dysplasia. Approximately one-fifth of patients who had abnormal
postoperative cytology develop recurrence. Worse than LSIL cases
are particularly likely to develop recurrence. Recurrence of CIN 3
was identified in 6 cases (4.2% of all patients); this recurrence
rate was somewhat higher compared with the reported margin-negative
cases (14,15). CIN 3 recurrent cases were observed
within 12 months and after 42 months following conization. For
early detection of recurrence, cervical smears should be performed
within at least 12 months after surgery.
Although several options for post-treatment
surveillance have been proposed, the current recommendations by the
ACOG and the American Society of Colposcopy and Cervical Pathology
suggest that, after treatment, women may require follow-up with a
combination of cytology, colposcopy and the high-risk HPV test
(2–4). A Cochrane Database Systematic Review
found no evidence from randomized controlled trials to update
decisions on the optimal surveillance strategy following treatment
for CIN (2). If surveillance
cytology shows ASC-US, immediate colposcopy is recommended based on
the results of the present study.
Approximately one-fifth of the conization cases had
CIN 3 as a postoperative cytological abnormality worse than ASC-US.
Approximately one-fifth of patients with abnormal cytological
findings after conization had recurrence. Worse than LSIL cases are
more likely to develop recurrence. Cytological abnormalities and
CIN 3 recurrence require close postoperative follow-up. As regards
the risk stratification of women following treatment for CIN, if
surveillance cytology shows ASC-US, immediate colposcopy is
recommended, along with long-term follow-up.
References
|
1
|
Massad LS, Einstein MH, Huh WK, Katki HA,
Kinney WK, Schiffman M, Solomon D, Wentzensen N and Lawson HW: 2012
ASCCP Consensus Guidelines Conference: 2012 updated consensus
guidelines for the management of abnormal cervical cancer screening
tests and cancer precursors. Obstet Gynecol. 121:829–846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Heijden E, Lopes AD, Bryant A,
Bekkers R and Galaal K: Follow-up strategies after treatment (large
loop excision of the transformation zone (LLETZ)) for cervical
intraepithelial neoplasia (CIN): Impact of human papillomavirus
(HPV) test. Cochrane Database Syst Rev. 1:CD0107572015.PubMed/NCBI
|
|
3
|
American College of Obstetricians and
Gynecologists: Practice Bulletin No. 140: Management of abnormal
cervical cancer screening test results and cervical cancer
precursors. Obstet Gynecol. 122:1338–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanley SJ, Fujita H, Tamakoshi A, Dong P
and Sakuragi N: Challenges in breast and cervical cancer control in
Japan. Lancet Oncol. 17:e3722016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melnikow J, Kulasingam S, Slee C, Helms
LJ, Kuppermann M, Birch S, McGahan CE, Coldman A, Chan BK and
Sawaya GF: Surveillance after treatment for cervical
intraepithelial neoplasia: Outcomes, costs, and cost-effectiveness.
Obstet Gynecol. 116:1158–1170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arbyn M, Roelens J, Simoens C, Buntinx F,
Paraskevaidis E, Martin-Hirsch PP and Prendiville WJ: Human
papillomavirus testing versus repeat cytology for triage of minor
cytological cervical lesions. Cochrane Database Syst Rev: CD008054.
2013. View Article : Google Scholar
|
|
7
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et
al: The 2001 Bethesda System: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cox JT, Castle PE, Behrens CM, Sharma A,
Wright TC Jr and Cuzick J: Athena HPV Study Group: Comparison of
cervical cancer screening strategies incorporating different
combinations of cytology, HPV testing, and genotyping for HPV
16/18: Results from the ATHENA HPV study. Am J Obstet Gynecol.
208:184.e1–184.e11. 2013. View Article : Google Scholar
|
|
9
|
Mayrand MH, Duarte-Franco E, Rodrigues I,
Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlée F and Franco EL:
Canadian Cervical Cancer Screening Trial Study Group: Human
papillomavirus DNA versus Papanicolaou screening tests for cervical
cancer. N Engl J Med. 357:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Alegría F, Poblete OQ, De Lorenzi DS
and Oyanedel JC: Clinical management of the first ASCUS report in
Chile. Prospective single-cohort study. Sao Paulo Med J.
133:480–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solomon D, Schiffman M and Tarone R: ALTS
Study group: Comparison of three management strategies for patients
with atypical squamous cells of undetermined significance: Baseline
results from a randomized trial. J Natl Cancer Inst. 93:293–299.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katki HA, Schiffman M, Castle PE,
Fetterman B, Poitras NE, Lorey T, Cheung LC, Raine-Bennett T, Gage
JC and Kinney WK: Five-year risk of recurrence after treatment of
CIN 2, CIN 3, or AIS: Performance of HPV and Pap cotesting in
posttreatment management. J Low Genit Tract Dis. 17 5 Suppl
1:S78–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morris M, Mitchell MF, Silva EG, Copeland
LJ and Gershenson DM: Cervical conization as definitive therapy for
early invasive squamous carcinoma of the cervix. Gynecol Oncol.
51:193–196. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vedel P, Jakobsen H, Kryger-Baggesen N,
Rank F and Bostofte E: Five-year follow up of patients with
cervical intra-epithelial neoplasia in the cone margins after
conization. Eur J Obstet Gynecol Reprod Biol. 50:71–76. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
White CD, Cooper WL and Williams RR:
Cervical intraepithelial neoplasia extending to the margins of
resection in conization of the cervix. J Reprod Med. 36:635–638.
1991.PubMed/NCBI
|
|
16
|
Paraskevaidis E, Kalantaridou SN, Kaponis
A, Chouliara S, Agnantis NJ, Dousias V, Zikopoulos K, Paschopoulos
M, Stamatopoulos P and Lolis DE: Surgical management of early stage
cervical cancer: Ten years experience from one Greek health region.
Eur J Gynaecol Oncol. 23:341–344. 2002.PubMed/NCBI
|
|
17
|
Brown JV, Peters WA and Corwin DJ:
Invasive carcinoma after cone biopsy for cervical intraepithelial
neoplasia. Gynecol Oncol. 40:25–28. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong NH, Lee NW, Kim HJ, Kim T and Lee
KW: High-risk human papillomavirus testing for monitoring patients
treated for high-grade cervical intraepithelial neoplasia. J Obstet
Gynaecol Res. 35:706–711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leguevaque P, Motton S, Decharme A,
Soulé-Tholy M, Escourrou G and Hoff J: Predictors of recurrence in
high-grade cervical lesions and a plan of management. Eur J Surg
Oncol. 36:1073–1079. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bar-Am A, Daniel Y, Ron IG, Niv J,
Kupferminc MJ, Bornstein J and Lessing JB: Combined colposcopy,
loop conization, and laser vaporization reduces recurrent abnormal
cytology and residual disease in cervical dysplasia. Gynecol Oncol.
78:47–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duesing N, Schwarz J, Choschzick M,
Jaenicke F, Gieseking F, Issa R, Mahner S and Woelber L: Assessment
of cervical intraepithelial neoplasia (CIN) with colposcopic biopsy
and efficacy of loop electrosurgical excision procedure (LEEP).
Arch Gynecol Obstet. 286:1549–1554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishimura M, Miyatake T, Nakashima A,
Miyoshi A, Mimura M, Nagamatsu M, Ogita K and Yokoi T: Clinical
significance of atypical squamous cells of undetermined
significance among patients undergoing cervical conization. Asian
Pac J Cancer Prev. 16:8145–8147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan BK, Melnikow J, Slee CA, Arellanes R
and Sawaya GF: Posttreatment human papillomavirus testing for
recurrent cervical intraepithelial neoplasia: A systematic review.
Am J Obstet Gynecol. 200(422): e1–e9. 2009.
|
|
24
|
Pirtea L, Grigoraş D, Matusz P, Pirtea M,
Moleriu L, Tudor A, Ilina R, Secoşan C, Horhat F and Mazilu O:
Human papilloma virus persistence after cone excision in women with
cervical high grade squamous intraepithelial lesion: A prospective
study. Can J Infect Dis Med Microbiol. 2016:30763802016.PubMed/NCBI
|