Introduction
Lung cancer is the leading cause of cancer-related
death worldwide (1), and bone
metastases occur in 20–30% of patients with lung cancer (2). Bone metastases reduce the quality of
life (QOL) of cancer patients by causing pain or skeletal-related
events (SREs) such as pathological fractures, spinal cord
compression, and hypercalcemia. Nitrogen containing bisphosphonates
(N-BPs) are widely used to prevent SREs in cancer patients with
bone metastases (3,4). Preclinical studies have demonstrated
the in vitro anticancer activity of N-BPs in multiple
myeloma (5). In particular, the N-BP
zoledronate (ZOL) has demonstrated survival benefits in
premenopausal women with early-stage breast cancer and in
previously untreated patients with multiple myeloma (6,7).
However, the survival benefit of ZOL in patients with cancer,
including lung cancer, remains unclear.
N-BPs inhibit osteoclastic bone resorption by
blocking farnesyl-pyrophosphate-synthase, an enzyme in the
mevalonate pathway. The first administration of intravenous N-BPs
is occasionally associated with the development of fever, which is
due to the activation of γδ T cells, resulting in the release of
interferon-γ, interleukin-6, and tumor necrosis factor-α (8,9). γδ T
cells, a small subset of T lymphocytes, play an active role in
immunosurveillance against tumors as well as infections, as
components of innate immunity (10).
Therefore, we hypothesized that the development of
fever after the first administration of N-BPs would be associated
with the survival of patients with cancer. The aim of this
retrospective study was to estimate the prognostic value of fever
development after the first ZOL administration (ZOL-related fever)
in advanced or recurrent non-small cell lung cancer (NSCLC)
patients with bone metastases.
Patients and methods
Materials
We retrospectively reviewed the data of patients who
received a diagnosis of advanced or recurrent NSCLC between March
2009 and March 2011 at Tottori University Hospital in Japan and who
received ZOL therapy for bone metastases. This retrospective study
was approved by our institutional human research committee. The
onset rate of ZOL-related fever is known to peak within the first 2
days after ZOL infusion and is short lived (11). Therefore, ZOL-related fever was
defined as fever (>37.5°C) that occurred within 2 days after the
first ZOL administration and that did not persist for >2 days.
Fever treated with antibiotics was not regarded as ZOL-related
fever.
Methods and statistical analysis
The exclusion criteria included an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) score of
2–4 at diagnosis, and no data concerning fever after the treatment
with ZOL, even if records of ZOL administration were available. We
collected clinicopathological data such as age, sex, smoking
history, ECOG-PS, disease stage, histological type, epidermal
growth factor receptor (EGFR) mutation status, the time from
the first ZOL administration to first SRE, and overall survival
(OS) from the time of diagnosis of advanced disease or recurrence.
The characteristics of patients with ZOL-related fever (fever
group) were compared with those of patients without ZOL-related
fever (non-fever group) by using the Mann-Whitney test and Fisher's
exact test for numerical and categorized data, respectively. The OS
and the time to first SRE were assessed using the Kaplan-Meier
method and compared using the log-rank test. Multivariate testing
was performed using Cox regression analysis with adjustments for
factors that tended to be associated with OS (P<0.3), such as
smoking history, histological type, EGFR mutation status,
PS, and ZOL-related fever.
P<0.05 was considered statistically significant.
Statistical analyses were performed using PASW Statistics 19 (IBM
SPSS Statistics, Somers, NY, USA).
Results
A total of 62 patients with advanced or recurrent
NSCLC who were administered ZOL therapy were screened. Of these, 16
patients were excluded because of the lack of data concerning
ZOL-related fever, and 46 patients were included in this
retrospective study. The median follow-up time of patients was 15.7
months (range, 4.2–107.3 months). The patient characteristics for
all 46 patients are listed in Table
I. The age of patients ranged from 47 to 86 years (median age,
65 years), with 30 (63.2%) men. Of the 46 patients, 37 (80.4%) had
non-squamous cell carcinomas (non-Sq) (adenocarcinoma; n=36, large
cell carcinoma; n=1), and 6 had Sq. Thirty-seven (80.4%) patients
had stage IV disease, and 7 (15.2%) had stage IIIB disease. The
remaining 2 (4.3%) patients had recurrent disease after surgery.
EGFR mutation status was tested in 39 of the 46 patients and
was positive in 16 (34.8%) patients. Seven patients had unknown
EGFR mutation status, and all of these 7 patients were
diagnosed with Sq (n=6) or large cell carcinoma (n=1). All 16
patients harboring EGFR mutations received EGFR-tyrosine
kinase inhibitors at least once. Twenty-nine patients (80.5%) were
former or current smokers.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Total | Fever | Non-fever | P-value |
|---|
| No. of patients | 46 | 15 | 31 |
|
| Age in years, median
(range) | 65 (47–86) | 62 (47–78) | 66 (48–86) | 0.13 |
| Sex |
|
|
| 0.33 |
|
Female | 16 (34.8%) | 7 (46.7%) | 9 (29.0%) |
|
| Male | 30 (63.2%) | 8 (53.3%) | 22 (71.0%) |
|
| Disease stage |
|
|
| 0.69 |
| IIIB | 7 (15.2%) | 3 (20%) | 4 (12.9%) |
|
| IV | 37 (80.4%) | 11 (73.3%) | 26 (83.9%) |
|
|
Recurrent | 2 (4.3%) | 1 (6.7%) | 1 (3.2%) |
|
| Histological
type |
|
|
| 0.45 |
| Squamous
cell carcinoma | 9 (19.6%) | 4 (26.7%) | 5 (16.1%) |
|
|
Non-squamous cell
carcinoma | 37 (80.4%) | 11 (73.3%) | 26 (83.9%) |
|
| EGFR mutation
status |
|
|
| 0.74 |
|
Positive | 16 (34.8%) | 6 (40%) | 10 (32.2%) |
|
| Negative
or unknown | 30 (65.2%) | 9 (60%) | 21 (67.8%) |
|
| ECOG-PS score |
|
|
| 0.26 |
| 0 | 17 (37.0%) | 5 (33.3%) | 5 (16.1%) |
|
| 1 | 29 (63.0%) | 10 (66.7%) | 26 (83.9%) |
|
| Smoking history |
|
|
| 0.52 |
| Never
smoker | 10 (21.7%) | 7 (46.7%) | 10 (32.2%) |
|
| Former or
current smoker | 36 (78.3%) | 8 (53.3%) | 21 (67.8%) |
|
Patient characteristics including age, sex,
EGFR mutation status, ECOG-PS, and smoking history were
compared between the two groups (Table
I). Compared with patients in the non-fever group, the patients
in the fever group tended to be younger (median age, 62 vs. 66
years) and female (53.3 vs. 29.0%), with a higher prevalence of
ECOG-PS score 0 (33.3 vs. 16.1%), but these differences were not
significant.
Of the 46 patients, 15 (32.6%) experienced
ZOL-related fever after the first ZOL administration. To elucidate
whether ZOL-related fever was associated with clinical outcome, we
compared OS and time to the first SRE between the two groups. As
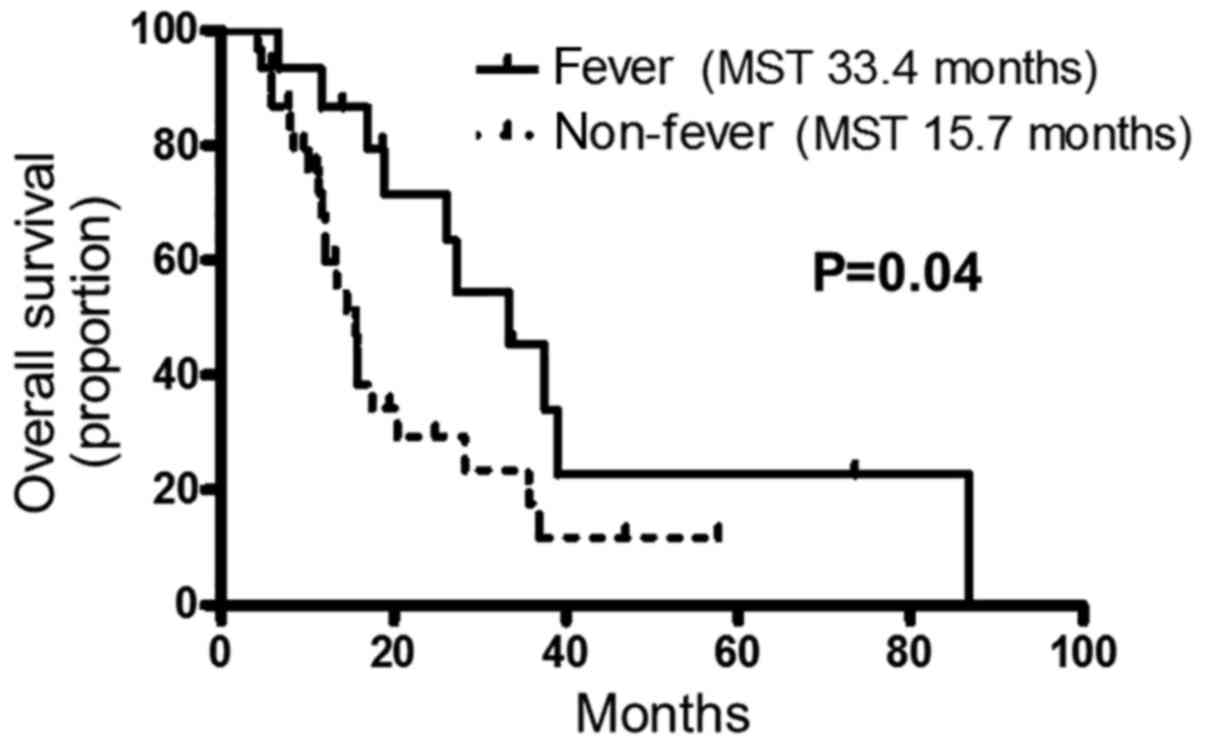
shown in Fig. 1, the OS of fever
group was significantly longer than that of the non-fever group
(median survival time: 33.4 vs. 15.7 months, P=0.04) with a hazard
ratio (HR) [95% confidence interval (CI)] of 0.46 (0.22–0.97). In
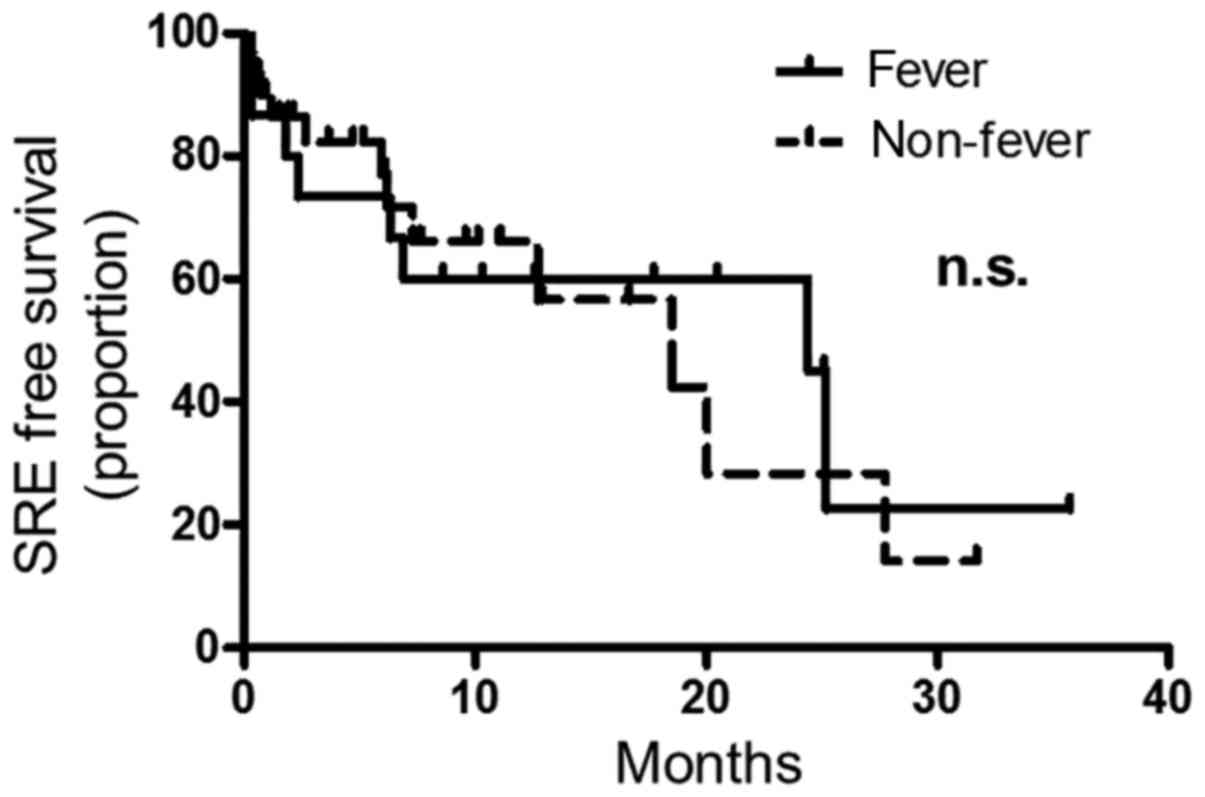
addition, the median time to first SRE was 24.4 months in the fever
group and 18.5 months in the non-fever group, with no significant
difference between the two groups [HR (95% CI) 1.3 (0.91–1.73),
P=0.88] (Fig. 2).
Positive EGFR mutation status [HR (95% CI)
0.41 (0.20–0.86), P=0.016], non-Sq histology [HR (95% CI) 0.24
(0.07–0.80), P=0.02], no smoking history [HR (95% CI) 0.37
(0.20–0.80), P=0.001], and female sex [HR (95% CI) 0.46
(0.22–0.95), P=0.036] were significantly associated with longer OS
(Table II). In addition,
multivariate analysis performed to test whether ZOL-related fever
was an independent prognostic factor revealed that the presence of
ZOL-related fever [HR (95% CI) 0.37 (0.16–0.83), P=0.02] and the
absence of smoking history [HR (95% CI) 0.13 (0.20–0.85), P=0.03]
were significant independent prognostic factors (Table II). The presence of EGFR
mutation tended to be associated with better OS [HR (95% CI) 0.44
(0.15–1.2), P=0.12], although this finding was not statistically
significant.
 | Table II.Univariate and multivariate analyses
of overall survival. |
Table II.
Univariate and multivariate analyses
of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (>70 vs. ≤70
years) | 1.4 | 0.62–3.2 | 0.42 | – | – | – |
| ECOG-PS score (0 vs.
1) | 0.88 | 0.4–2.1 | 0.79 | – | – | – |
| Sex (female vs.
male) | 0.46 | 0.22–0.95 | 0.04 | 0.27 | 0.04–1.64 | 0.16 |
| Histology (non-Sq
vs. Sq) | 0.24 | 0.07–0.80 | 0.02 | 0.44 | 0.15–1.2 | 0.12 |
| EGFR
mutation status (positive vs. negative or unknown) | 0.41 | 0.20–0.86 | 0.02 | 0.44 | 0.18–1.1 | 0.08 |
| Smoking history
(never smoker vs. former or current smoker) | 0.37 | 0.20–0.80 | <0.01 | 0.13 | 0.20–0.85 | 0.03 |
| The presence of
ZOL-related fever (yes vs. no) | 0.46 | 0.22–0.97 | 0.04 | 0.37 | 0.16–0.83 | 0.02 |
Discussion
In the present study, we compared the
characteristics of patients in the fever group with those of
patients in the non-fever group in order to identify predictors of
ZOL-related fever. Reid et al reported that young age and
female sex are predictors of the development of fever after ZOL
administration in patients with osteoporosis (11). In our study, although patients in the
fever group tended to be younger than those in the non-fever group,
with a greater percentage of women, these differences were not
significant. Further large-scale studies are necessary to confirm
whether such characteristics are predictors of ZOL-related fever in
patients with cancer.
We compared the OS between the fever group and
non-fever group in NSCLC patients treated with ZOL. Although there
were no significant differences in patient characteristics between
the two groups, the OS in the fever group was significantly longer
than that in the non-fever group. This indicates that ZOL-related
fever is an important prognostic indicator in advanced or recurrent
NSCLC patients with bone metastases. To the best of our knowledge,
this is the first report of an association between ZOL-related
fever and clinical outcome in patients with cancer. As the time to
the first SRE was not significantly different between the two
groups, the difference in OS between the two groups is likely not
attributable to the preventive effect of ZOL on SREs.
In addition to ZOL-related fever, a positive
EGFR mutation status, non-Sq histology, no smoking history,
and female sex were significantly associated with longer OS. These
characteristics are known prognostic factors in patients with
NSCLC, and are strongly associated with the presence of EGFR
mutation (12–14). Owing to the emergence of EGFR
tyrosine kinase inhibitors, the survival of NSCLC patients with
EGFR mutation is better than that of patients without
EGFR mutation (13). We
performed multivariate analyses to exclude confounding influences,
and the results revealed that ZOL-related fever is independently
associated with better OS in advanced NSCLC patients with bone
metastases.
Two hypotheses may explain the association between
ZOL-related fever and improved OS. First, patients in the fever
group may have superior immunocompetence than those in the
non-fever group, resulting in better prognosis, and ZOL
administration results in the development of fever depending on the
immunocompetence of the patient. In other words, ZOL-related fever
is a prognostic factor regardless of any beneficial clinical
effects of ZOL. Second, patients in the fever group alone may
benefit from favorable effects of ZOL other than SRE inhibition.
Thus, ZOL-related fever is a predictive factor for an undefined,
survival-promoting effect of ZOL.
ZOL-related fever is associated with the activation
of γδ T cells, which recognize non-peptide antigens in a major
histocompatibility complex-unrestricted manner (15). γδ T cells have demonstrated
cytotoxicity against a variety of cell lines in vitro,
including peripheral blood mononuclear cells from patients with
NSCLC (16). Immunotherapies
utilizing γδ T cells are currently under development and have been
shown to be safe and feasible (17–19).
Such therapies could offer invaluable alternatives for cancer
treatment in the future. Thus, it is possible that ZOL-related
fever is a predictive factor for an additional survival-improving
effect of ZOL, and that γδ T cells were activated to a greater
extent by ZOL in the fever group, resulting in the activation of
cancer immunity and improved OS. However, further study is
necessary to clarify the mechanism by which ZOL prolongs OS and to
confirm whether γδ T cells are activated after ZOL administration
in the fever group. In this regard, we are conducting a prospective
observational study to confirm the reproducibility of the
correlation between ZOL-related fever and OS and to clarify the
precise mechanism of ZOL-related fever development in patients with
advanced NSCLC.
In conclusion, the development of ZOL-related fever
was independently correlated with better OS in advanced or
recurrent NSCLC patients with bone metastases. ZOL-related fever is
a predictive factor for an undefined, survival-promoting effect of
ZOL.
Acknowledgements
We would like to thank Editage for English language
editing.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
ECOG-PS
|
Eastern Cooperative Oncology
Group-performance status
|
|
EGFR
|
epidermal growth factor receptor
|
|
HR
|
hazard ratio
|
|
N-BPs
|
nitrogen-containing
bisphosphonates
|
|
Sq
|
squamous cell carcinomas
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
QOL
|
quality of life
|
|
SRE
|
skeletal-related events
|
|
ZOL
|
zoledronate
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong M, Feng FY, Zhang Y, Xie GR, Wang YJ,
Liu JW, Song ST, Zhou QH, Ren J, Jiao SC, et al: Phase III clinical
study of zoledronic acid in the treatment of pain induced by bone
metastasis from solid tumor or multiple myeloma. Zhonghua Zhong Liu
Za Zhi. 30:215–220. 2008.(In Chinese). PubMed/NCBI
|
|
4
|
Rosen LS, Gordon D, Tchekmedyian S,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng
M, Urbanowitz G, et al: Zoledronic acid versus placebo in the
treatment of skeletal metastases in patients with lung cancer and
other solid tumors: A phase III, double-blind, randomized trial-the
Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J
Clin Oncol. 21:3150–3157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunzmann V, Bauer E, Feurle J, Weissinger
F, Tony H and Wilhelm M: Stimulation of gammadelta T cells by
aminobisphosphonates and induction of antiplasma cell activity in
multiple myeloma. Blood. 96:384–392. 2000.PubMed/NCBI
|
|
6
|
Gnant M, Mlineritsch B, Stoeger H,
Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M,
Hubalek M, Pristauz G, et al: Adjuvant endocrine therapy plus
zoledronic acid in premenopausal women with early-stage breast
cancer: 62-month follow-up from the ABCSG-12 randomised trial.
Lancet Oncol. 12:631–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Avilés A, Nambo MJ, Neri N, Castañeda C,
Cleto S and Huerta-Guzmán J: Antitumor effect of zoledronic acid in
previously untreated patients with multiple myeloma. Med Oncol.
24:227–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kunzmann V, Bauer E and Wilhelm M:
Gamma/delta T-cell stimulation by pamidronate. N Engl J Med.
340:737–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dicuonzo G, Vincenzi B, Santini D,
Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola
R and Tonini G: Fever after zoledronic acid administration is due
to increase in TNF-alpha and IL-6. J Interferon Cytokine Res.
23:649–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonneville M and Scotet E: Human
Vgamma9Vdelta2 T cells: Promising new leads for immunotherapy of
infections and tumors. Curr Opin Immunol. 18:539–546. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reid IR, Gamble GD, Mesenbrink P, Lakatos
P and Black DM: Characterization of and risk factors for the
acute-phase response after zoledronic acid. J Clin Endocrinol
Metab. 95:4380–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura H, Ando K, Shinmyo T, Morita K,
Mochizuki A, Kurimoto N and Tatsunami S: Female gender is an
independent prognostic factor in non-small-cell lung cancer: A
meta-analysis. Ann Thorac Cardiovasc Surg. 17:469–480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kris MG, Johnson BE, Berry LD, et al: NIH
Public Access. Select Target Drugs. 311:1998–2006. 2014.
|
|
14
|
Mitsudomi T, Kosaka T, Endoh H, Horio Y,
Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T and Yatabe Y:
Mutations of the epidermal growth factor receptor gene predict
prolonged survival after gefitinib treatment in patients with
non-small-cell lung cancer with postoperative recurrence. J Clin
Oncol. 23:2513–2520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Triebel F and Hercend T: Subpopulations of
human peripheral T gamma delta lymphocytes. Immunol Today.
10:186–188. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kondo M, Sakuta K, Noguchi A, Ariyoshi N,
Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, et al:
Zoledronate facilitates large-scale ex vivo expansion of functional
gammadelta T cells from cancer patients for use in adoptive
immunotherapy. Cytotherapy. 10:842–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakamoto M, Nakajima J, Murakawa T, Fukami
T, Yoshida Y, Murayama T, Takamoto S, Matsushita H and Kakimi K:
Adoptive immunotherapy for advanced non-small cell lung cancer
using zoledronate-expanded γδTcells: A phase I clinical study. J
Immunother. 34:202–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi H, Tanaka Y, Yagi J, Minato N
and Tanabe K: Phase I/II study of adoptive transfer of γδ T cells
in combination with zoledronic acid and IL-2 to patients with
advanced renal cell carcinoma. Cancer Immunol Immunother.
60:1075–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi H and Tanaka Y: γδ T cell
immunotherapy-A review. Pharmaceuticals (Basel). 8:40–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|