Introduction
Urothelial carcinoma (UC) is one of the most common
urological malignancies worldwide. Approximately 30% of UC patients
initially present with muscle invasion and metastasis (1). In addition, despite the performance of
radical surgery as a local therapy for patients with muscle
invasion, more than one-third of these patients ultimately develop
metastatic disease (2).
Cisplatin-based systemic chemotherapy is the gold
standard approach for patients with advanced or metastatic UC.
Combined chemotherapy with methotrexate, vinblastine, doxorubicin
and cisplatin (MVAC), which was developed in 1985, is an effective
and frequently used modality for these life-threatening diseases
(3–7). Recently, combined chemotherapy with
gemcitabine and cisplatin (GC) has become another standard
treatment for advanced UC, since GC therapy showed equivalent
efficacy and less toxicity in comparison to MVAC in a randomized
phase 3 trial (8). However, no
standard second-line chemotherapy regimens have been established
for cases in which a first-line cisplatin-based chemotherapy (such
as MVAC or GC) fails. The administration of vinflunine in
combination with the best supportive care (BSC) as a second-line
chemotherapy after the failure of cisplatin-based regimens was
associated with a 2.5-month increase in survival in comparison to
patients who received BSC alone in a phase 3 trial (9). However, vinflunine has only been
approved in Europe.
Various single agents and combinations of agents
have been reported as second-line chemotherapy regimens. Among
these, combined chemotherapy with paclitaxel and carboplatin (TC)
has been shown to have promising therapeutic activity against
cisplatin-refractory UC (10–12).
However, few reports have assessed the tolerability and efficacy of
TC therapy as a second-line regimen for UC showing resistance to GC
as a first-line chemotherapy regimen. Our institution has used MVAC
as a second-line chemotherapy regimen for patients with advanced
urothelial carcinoma after the failure of first-line GC since July
2009 and has used TC as second-line chemotherapy since April 2014.
In this study, we retrospectively assessed the prognostic factors
for overall survival (OS) in patients who received second-line
treatment that included BSC and investigated the tolerability and
efficacy of TC therapy.
Patients and methods
The data of 52 patients who received BSC or
second-line chemotherapy with MVAC or TC after the failure of
first-line chemotherapy with GC at our institution between June
2009 and November 2016 were retrospectively evaluated. UC was
histopathologically diagnosed and disease progression during
first-line chemotherapy with GC was radiologically confirmed.
Twenty-eight patients selected BSC and 24 received second-line
chemotherapy (MVAC, n=8; TC, n=16). Patients who received
second-line chemotherapy were required to have an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of ≤2 and
an adequate organ function, (defined by granulocyte count
≥1,500/mm3, platelet count ≥100,000/mm3,
serum total bilirubin <1.5 mg/dl, serum transaminase activity
<3 times that of normal and serum creatinine <2 times the
normal level).
In the MVAC regimen, methotrexate (30
mg/m2) was administered intravenously on days 1, 15 and
22; vinblastine (3.0 mg/m2) was administered
intravenously on days 2, 15 and 22; and doxorubicin (30
mg/m2) and cisplatin (70 mg/m2) were
administered intravenously on day 2. The cycle was basically
repeated every 28 days. In the TC regimen, paclitaxel (175
mg/m2) and carboplatin (area under the curve: 5) were
administered by intravenous infusion on day 1. The cycle was
repeated every 21 days. The two second-line regimens were repeated
until disease progression or unacceptable adverse events occurred.
Tumor measurements were generally performed by computed tomography
before and after every 2–3 cycles of the second-line chemotherapy.
Decisions regarding adverse events were made based on the Common
Terminology Criteria for Adverse Events, version 4.0 (13). The tumor response was evaluated as
the best response according to the Response Evaluation Criteria In
Solid Tumors, version 1.1 (14).
All of the patients provided their written informed
consent to participate in this study, and the study protocol was
approved by the Ethics Committee of the Kyushu Cancer Center
(Fukuoka, Japan).
Statistical analysis
The statistical analyses were carried out using the
JMP® Pro, version 12.2.0 software package (SAS
Institute, Inc., Cary, NC, USA). OS was calculated from the day
that BSC was selected or the day on which chemotherapy was started
until the date of the last follow-up examination or death from any
cause. The OS were evaluated using the Kaplan-Meier method, and the
log-rank test (Bonferroni correction procedure) was used to
determine differences between the second-line treatment groups. The
significance of associations between the clinical parameters and OS
was assessed using the Cox proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The clinical characteristics of the 52 (male, n=40;
female, n=12; median age, 70 years; range, 50–86 years) patients
are listed in Table I. All the
patients received GC as the first-line chemotherapy for urothelial
carcinoma, and selected BSC or second-line chemotherapy after the
failure of GC chemotherapy. Twenty-four patients had bladder UC, 22
patients had upper urinary tract UC and 6 patients had both types
of UC. Forty patients (76.9%) had visceral metastasis. With regard
to the second-line treatments, 28 patients (53.8%) selected BSC and
24 patients (46.2%) received second-line chemotherapy (MVAC, n=8;
TC, n=16).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic
(n=52) | No. of patients |
|---|
| Gender |
|
| Male | 40 |
|
Female | 12 |
| Age, years |
|
| Median
(range) | 70 (50–86) |
| ECOG PS |
|
| 0 | 10 |
| 1 | 21 |
| ≥2 | 21 |
| Anemia (male
<13.5, female <11.0) |
|
|
Yes | 45 |
| No | 7 |
| CRP |
|
|
<0.3 | 8 |
|
≥0.3 | 44 |
| Albumin |
|
|
≥4.0 | 12 |
|
<4.0 | 40 |
| NLR |
|
| Median
(range) | 3.0 (0.8–14.9) |
| Primary tumor
site |
|
|
Bladder | 24 |
| Upper
urinary tract | 22 |
| Bladder
+ upper urinary tract | 6 |
| Surgical treatment
for the primary tumor |
|
|
Cystectomy | 16 |
|
Cystectomy +
nephroureterectomy | 2 |
|
Nephroureterectomy | 15 |
|
Transurethral resection | 11 |
| Visceral
metastasis |
|
|
Negative | 12 |
|
Positive | 40 |
| Second-line
therapy |
|
|
BSC | 28 |
| TC
chemotherapy | 16 |
| MVAC
chemotherapy | 8 |
OS according to the second-line
treatments
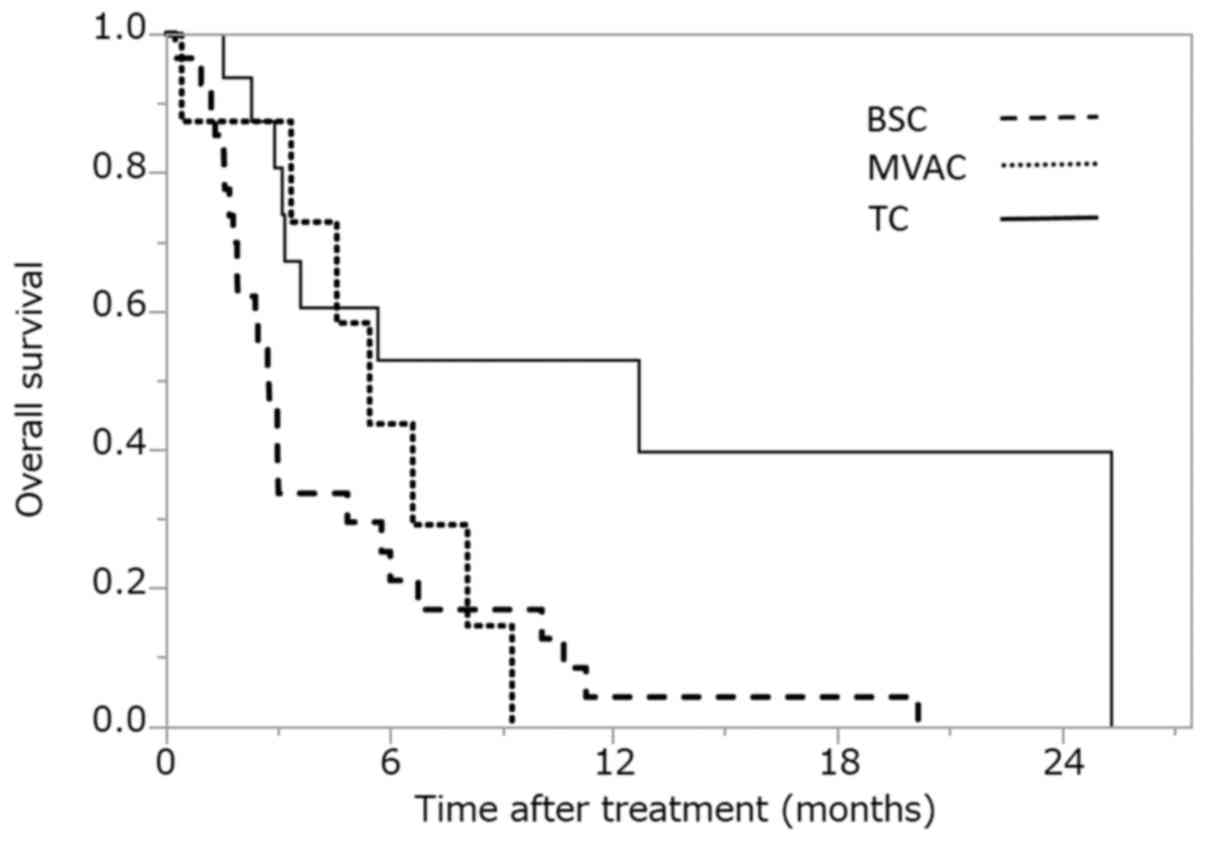
The OS according to the second-line treatment is
shown in Fig. 1. The median OS for
BSC was 2.8 months [95% confidence interval (CI), 1.8–4.9 months;
the median OS for MVAC was 5.4 months (95% CI, 0.4–8.1 months], and
the median OS for TC was 12.7 months (95% CI, 3.1–25.4 months). The
difference between BSC and MVAC (according to the log-rank test)
was not statistically significant (P=0.596). However, the
difference between BSC and TC was statistically significant after
Bonferroni correction (P=0.002).
Univariate and multivariate analyses of the
associations between various factors and the OS after the failure
of GC chemotherapy. To identify the prognostic factors associated
with OS after the failure of GC chemotherapy, we performed
univariate and multivariate analyses using the Cox proportional
hazards model (Table II).
Univariate analyses for various factors revealed that prior
nephrectomy, ECOG-PS, the neutrophil/lymphocyte ratio (NLR),
albumin, C-reactive protein (CRP), visceral metastasis and
second-line treatment were prognostic variables. The multivariate
analyses revealed that anemia (male <13.5; female <11.0) (HR,
7.047, 95% CI=1.553–35.636, P=0.011), the presence of visceral
metastasis (HR 4.174, 95% CI=1.506–13.429, P=0.005) and second-line
treatment (TC: HR 0.296, 95% CI=0.124–0.636, P=0.003) were
independent prognostic factors.
 | Table II.The univariate and multivariate
analyses of the factors associated with overall survival in
patients receiving second-line treatment. |
Table II.
The univariate and multivariate
analyses of the factors associated with overall survival in
patients receiving second-line treatment.
| Variable | Univariate HR (95%
CI) | P-value | Multivariate HR
(95% CI) | P-value |
|---|
| Age |
|
|
|
|
|
<70 | 1 |
|
|
|
|
≥70 | 0.824
(0.429–1.556) | 0.552 |
|
|
| Sex |
|
|
|
|
|
Male | 1 |
|
|
|
|
Female | 0.942
(0.421–1.903) | 0.875 |
|
|
| Histology |
|
|
|
|
| Pure
UC | 1 |
|
|
|
| Mixed
UC | 0.613
(0.282–1.243) | 0.180 |
|
|
| ECOG PS |
|
|
|
|
| 0 | 1 |
|
|
|
| 1 | 4.146
(1.529–14.462) | 0.004 |
|
|
| ≥2 | 5.946
(2.158–21.007) | <0.001 |
|
|
| Anemia (male
<13.5, female <11.0) |
|
|
|
|
|
Negative | 1 |
| 1 |
|
|
Positive | 2.248
(0.953–6.604) | 0.066 | 7.047
(1.553–35.636) | 0.011 |
| NLR |
|
|
|
|
|
<3.0 | 1 |
|
|
|
|
≥3.0 | 2.010
(1.055–3.903) | 0.034 |
|
|
| Albumin |
|
|
|
|
|
≥4.0 | 1 |
|
|
|
|
<4.0 | 2.963
(1.386–7.111) | 0.004 |
|
|
| CRP |
|
|
|
|
|
<0.3 | 1 |
|
|
|
|
≥0.3 | 3.323
(1.319–11.169) | 0.008 |
|
|
| Best response to GC
therapy |
|
|
|
|
| PD | 1 |
|
|
|
| SD | 0.712
(0.319–1.579) | 0.401 |
|
|
|
CR+PR | 0.785
(0.369–1.686) | 0.528 |
|
|
| Visceral
metastases |
|
|
|
|
|
Negative | 1 |
| 1 |
|
|
Positive | 2.289
(1.062–5.699) | 0.034 | 4.174
(1.506–13.429) | 0.005 |
| Second-line
treatmnet |
|
|
|
|
|
BSC | 1 |
| 1 |
|
|
MVAC | 0.734
(0.289–1.639) | 0.467 | 0.337
(0.093–1.181) | 0.089 |
| TC | 0.296
(0.124–0.636) | 0.001 | 0.202
(0.065–0.588) | 0.003 |
The response analysis and the
toxicities in patients who received TC as a second-line
chemotherapy regimen
The objective tumor responses are shown in Table III. Among the 16 patients who
received TC as a second-line chemotherapy regimen, a complete
response (CR) was confirmed in 1 patient (6.2%), while 2 patients
(12.5%) showed a partial response (PR), with an overall response
rate of 18.7%. The disease control rate (defined by the achievement
of CR, PR or stable disease [SD]), was 56.2%.
 | Table III.The analysis of the responses of
patients who received TC chemotherapy. |
Table III.
The analysis of the responses of
patients who received TC chemotherapy.
| Response | No. of
patients | Response rate
(%) |
|---|
| CR | 1 |
6.2 |
| PR | 2 | 12.5 |
| SD | 6 | 37.5 |
| PD | 7 | 43.8 |
| Overall response
rate (CR + PR) | 3 | 18.7 |
| Disease contorl
rate (CR + PR + SD) | 9 | 56.2 |
Table IV shows the
toxicities associated with TC chemotherapy. Myelosuppression was
the most common toxicity. Grade ≥3 neutropenia occurred in 10
patients (62.5%), while febrile neutropenia was only observed in 1
patient (6.3%); no patients showed severe infection.
 | Table IV.Toxicities in patients treated with
TC chemotherapy. |
Table IV.
Toxicities in patients treated with
TC chemotherapy.
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ≥Grade 3 (%) |
|---|
| Neutropenia | 0 | 1 | 5 | 5 | 62.5 |
| Anemia | 1 | 2 | 2 | 0 | 12.5 |
|
Thrombocytopenia | 0 | 1 | 2 | 0 | 12.5 |
| Febrile
neutropenia | – | – | 1 | 0 | 6.3 |
| Neuropathy | 3 | 1 | 1 | 0 | 6.3 |
| Muscle pain | 0 | 2 | – | – | – |
| Nausea,
vomiting | 1 | 0 | 0 | 0 | 0 |
| Anorexia | 5 | 3 | 1 | 0 | 6.3 |
| Malaise | 6 | 4 | – | – | 0 |
| Alopecia | 3 | 7 | – | – | – |
| Increased
creatinine | 7 | 0 | 0 | 0 | 0 |
| Liver
dysfunction | 0 | 0 | 0 | 0 | 0 |
Grade 3 anemia developed in 2 patients (12.5%) and
grade 3 thrombocytopenia developed in 2 patients (12.5%). With
regard to non-hematological toxicities, grade 3 neuropathy and
grade 3 anorexia developed in 1 patient each. All other toxicities
were less than grade 3. There were no treatment-related deaths
among the 16 patients.
Discussion
Cisplatin-based systemic chemotherapy is the gold
standard approach for the treatment of advanced or metastatic UC.
Recently, GC chemotherapy has become the standard first-line
treatment for advanced and metastatic UC because GC chemotherapy
showed equivalent efficacy and lower toxicity in comparison to MVAC
chemotherapy (8,15). However, long-term follow-up has
revealed that the rates of overall survival and progression-free
survival are poor, particularly in patients with metastatic UC
(7,15). In addition, the data were
insufficient to recommend TC as standard second-line chemotherapy
after the failure of cisplatin-based combination chemotherapy
(particularly GC). Thus, we retrospectively assessed the outcomes
and toxicities of patients with metastatic or advanced UC after the
failure of GC chemotherapy who selected to receive MVAC or TC as
second-line chemotherapy in comparison to those who received the
BSC.
Various single agents and combinations of agents
have been reported as second-line chemotherapy regimens (16–23).
Even in the NCCN guidelines, the second-line chemotherapy data are
highly variable and unclear in this setting; thus, no standard
therapy exists. The NCCN bladder cancer panel members highly
recommend the performance of a clinical trial (24). In our institution, MVAC regimen was
first selected as a second-line chemotherapy regimen after the
failure of GC chemotherapy. The reason for this was that MVAC was
the standard first-line chemotherapy before the GC regimen was
introduced. Thus, we were used to controlling the side effects.
However, this study also showed that the outcomes of second-line OS
were not necessarily satisfactory (Fig.
1). Thus, we employed the TC regimen (paclitaxel in combination
with carboplatin) as second-line chemotherapy from April 2014.
Paclitaxel is an antimitotic spindle drug that
promotes microtubular aggregation and interferes with certain cell
functions, including cell mitosis, transport and motility.
Single-agent paclitaxel was shown to have an overall response rate
of 42% in previously untreated patients with UC (25), and 70% when administered in
combination with cisplatin (26). On
the other hand, platinum-based agents have been frequently included
in salvage chemotherapy, which is provided even after the failure
of a platinum-based regimen, and the activity of this agent against
platinum-resistant disease has been reported (10–12,27).
However, patients with UC often have an impaired renal function due
to advanced age, prior platinum-containing chemotherapy, prior
nephrectomy and/or disease-related hydronephrosis. Carboplatin is a
less nephrotoxic and emetogenic platinum compound than cisplatin
(28); thus, carboplatin is
considered to be a favorable agent for second-line regimens.
We first assessed the OS according to the
second-line treatment (Fig. 1). All
the patients received GC as first-line chemotherapy for urothelial
carcinoma and selected BSC or second-line chemotherapy after the
failure of GC chemotherapy. The second-line treatments included BSC
(n=28; 53.8%) and second-line chemotherapy [n=24; 46.2% (MVAC, n=8;
TC, n=16)] (Table I). The median OS
of the patients who received BSC, MVAC and TC was 2.8, 5.4 and 12.7
months, respectively. The difference between BSC and MVAC was not
statistically significant (log-rank test, P=0.596). However, the
difference between BSC and TC was statistically significant after
Bonferroni correction (P=0.002). Although this study was
retrospective in nature and did not formally evaluate the quality
of life (QOL), the median OS of patients who received TC was longer
than that of the patients who received BSC or MVAC. Thus, from the
viewpoint of OS, it is possible that TC should be recommended as a
second-line chemotherapy regimen, rather than MVAC, when patients
indicate a desire to receive second-line chemotherapy, not BSC.
Subsequently, we performed univariate and
multivariate analyses using the Cox proportional hazards model to
investigate the relationship between the OS and the clinical
parameters after the failure of GC chemotherapy (Table II). Univariate analyses for various
factors revealed that prior nephrectomy, ECOG-PS, NLR, albumin,
CRP, visceral metastases and second-line treatment were prognostic
variables. Furthermore, the multivariate analyses revealed that
anemia (HR 7.047, 95% CI=1.553–35.636, P=0.011), the presence of
visceral metastasis (HR 4.174, 95% CI=1.506–13.429, P=0.005) and
second-line treatment (TC regimen: HR 0.296, 95% CI=0.124–0.636,
P=0.003) were independent prognostic factors. Several studies have
examined the various prognostic factors of patients. Buti et
al identified nine studies (29–37) that
aimed at evaluate the prognostic factors of 1,273 patients in a
second-line treatment setting. In most studies, PS, Hb, and
visceral metastasis were identified as the main independent
prognostic factors for OS (38).
These descriptions are consistent with our observations in the
present study. However, PS was not a significant factor in this
study. One reason was that second-line chemotherapy was
administered (especially TC) even to patients with a relatively
poor PS, because this regimen was well tolerated and could be
safely used (Table IV). The other
reason was that this study included patients who chose BSC, despite
having a relatively good PS, due to the fact that second-line
chemotherapy represented a palliative-rather than
curative-treatment. In addition, second-line chemotherapy with TC
but not MVAC, was an independent prognostic factor in this study
(TC: HR 0.296, 95% CI=0.124–0.636, P=0.003).
Thus, we assessed the outcome and toxicities of TC.
This study demonstrated that TC was associated with an 18.7%
overall response rate and a 56.2% disease control rate (Table III). The toxicities that occurred
in association with TC are shown in Table IV. Myelosuppression was the most
common grade ≥3 toxicity. Grade ≥3 neutropenia occurred in 10
patients (62.5%) and was easily managed with G-CSF. Febrile
neutropenia was only observed in 1 patient (6.3%); however, there
were no cases of severe infection. Although grade 3 anemia occurred
in 2 patients (12.5%) and grade 3 thrombocytopenia occurred in 2
patients (12.5%), transfusion was not required in any of these
cases. However, with regard to non-hematological toxicity, grade 3
neuropathy and grade 3 anorexia occurred in 1 patient each. The
other toxicities were less than grade 3 and no cases of
treatment-related death occurred among the 16 patients. These
findings suggest that the TC could be safely administered, even
after intensive treatment with GC as a first-line regimen. There
are some articles which have previously reported the efficacy and
tolerability of TC as second-line chemotherapy for advanced UC
resistant to first-line cisplatin-based chemotherapy in Japanese
patients (11,39). However, these studies did not compare
the outcomes of TC with the outcomes of other treatments, including
MVAC and BSC.
Recently, immune checkpoint inhibitors such as
anti-programmed cell death 1 and anti-programmed cell death-ligand
1 (PD-L1) antibodies have been reported to have durable effects in
various cancers (40,41). Thomas et al reported that
anti-PD-L1 antibody treatment was effective for patients with
metastatic bladder cancer, and reported that the ORR was 25% and
that severe adverse events were rare (42). In the future, studies should be
performed to compare anti-PD-L1 antibody therapy and chemotherapy
as second-line treatment for metastatic UC.
The present study is associated with some
limitations. First, the data related to the efficacy and
tolerability of TC as second-line chemotherapy were evaluated
retrospectively, and not in a randomized trial. Second, the study
population was relatively small. Further studies will be needed to
confirm our data in a larger study population. Although our
analysis relied on a small sample study population, TC achieved a
56.2% disease control rate with a tolerable toxicity profile as
second-line chemotherapy in patients with advanced or metastatic UC
who have previously received GC as first-line chemotherapy.
Although this study did not formally evaluate the QOL, the median
OS of the patients who received TC was longer than that of the
patients who received BSC or MVAC (12.7 vs. 2.8 months or 5.4
months, respectively). Additionally, TC chemotherapy was itself a
prognostic factor in the multivariate analysis. Taken together the
results suggest that at present, when there is no standard
second-line treatment for patients with advanced or metastatic UC
after failure of GC chemotherapy, TC chemotherapy can be a
preferred option for second-line chemotherapy.
References
|
1
|
Vaidya A, Soloway MS, Hawke C, Tiguert R
and Civantos F: De novo muscle invasive bladder cancer: Is there a
change in trend? J Urol. 165:47–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Ahmed T, Weiselberg LR, Geller N, Hollander PS, Herr HW, Sogani
PC, et al: Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loehrer PJ Sr, Einhorn LH, Elson PJ,
Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R,
Sarosdy MF, Lowe BA, et al: A randomized comparison of cisplatin
alone or in combination with methotrexate, vinblastine and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 10:1066–1073. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Geller N, Herr HW, Morse MJ, Sogani PC, Vaughan ED, Bander N,
et al: Methotrexate, vinblastine, doxorubicin, and cisplatin for
advanced transitional cell carcinoma of the urothelium. Efficacy
and patterns of response and relapse. Cancer. 64:2448–2458. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Herr HW, Morse MJ, Sogani PC, Vaughan ED Jr, Bander N,
Weiselberg LR, et al: M-VAC (methotrexate, vinblastine, doxorubicin
and cisplatin) for advanced transitional cell carcinoma of the
urothelium. J Urol. 139:461–469. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saxman SB, Propert KJ, Einhorn LH,
Crawford ED, Tannock I, Raghavan D, Loehrer PJ Sr and Trump D:
Long-term follow-up of a phase III intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 15:2564–2569. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaishampayan UN, Faulkner JR, Small EJ,
Redman BG, Keiser WL, Petrylak DP and Crawford ED: Phase II trial
of carboplatin and paclitaxel in cisplatin-pretreated advanced
transitional cell carcinoma: A Southwest Oncology Group study.
Cancer. 104:1627–1632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soga N, Onishi T, Arima K and Sugimura Y:
Paclitaxel Carboplatin chemotherapy as a second-line chemotherapy
for advanced platinum resistant urothelial cancer in Japanese
cases. Int J Urol. 14:828–832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kouno T, Ando M, Yonemori K, Matsumoto K,
Shimizu C, Katsumata N, Komiyama M, Okajima E, Matsuoka N, Fujimoto
H and Fujiwara Y: Weekly paclitaxel and carboplatin against
advanced transitional cell cancer after failure of a platinum-based
regimen. Eur Urol. 52:1115–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lorusso V, Pollera CF, Antimi M, Luporini
G, Gridelli C, Frassineti GL, Oliva C, Pacini M and De Lena M: A
phase II study of gemcitabine in patients with transitional cell
carcinoma of the urinary tract previously treated with platinum.
Italian Co-operative Group on Bladder Cancer. Eur J Cancer.
34:1208–1212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCaffrey JA, Hilton S, Mazumdar M, Sadan
S, Kelly WK, Scher HI and Bajorin DF: Phase II trial of docetaxel
in patients with advanced or metastatic transitional-cell
carcinoma. J Clin Oncol. 15:1853–1857. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papamichael D, Gallagher CJ, Oliver RT,
Johnson PW and Waxman J: Phase II study of paclitaxel in pretreated
patients with locally advanced/metastatic cancer of the bladder and
ureter. Br J Cancer. 75:606–607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vaughn DJ, Broome CM, Hussain M, Gutheil
JC and Markowitz AB: Phase II trial of weekly paclitaxel in
patients with previously treated advanced urothelial cancer. J Clin
Oncol. 20:937–940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn
DJ, Arning M, Curiel RE, Obasaju CK, Wang Y, Nicol SJ and Kaufman
DS: Phase II study of pemetrexed for second-line treatment of
transitional cell cancer of the urothelium. J Clin Oncol.
24:3451–3457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galsky MD, Mironov S, Iasonos A,
Scattergood J, Boyle MG and Bajorin DF: Phase II trial of
pemetrexed as second-line therapy in patients with metastatic
urothelial carcinoma. Invest New Drugs. 25:265–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Witte RS, Elson P, Bono B, Knop R,
Richardson RR, Dreicer R and Loehrer PJ Sr: Eastern Cooperative
Oncology Group phase II trial of ifosfamide in the treatment of
previously treated advanced urothelial carcinoma. J Clin Oncol.
15:589–593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han KS, Joung JY, Kim TS, Jeong IG, Seo
HK, Chung J and Lee KH: Methotrexate, vinblastine, doxorubicin and
cisplatin combination regimen as salvage chemotherapy for patients
with advanced or metastatic transitional cell carcinoma after
failure of gemcitabine and cisplatin chemotherapy. Br J Cancer.
98:86–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Comprehensive Cancer Network:
Guidelines on bladder cancer. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf/December
1–2016
|
|
25
|
Roth BJ, Dreicer R, Einhorn LH, Neuberg D,
Johnson DH, Smith JL, Hudes GR, Schultz SM and Loehrer PJ:
Significant activity of paclitaxel in advanced transitional-cell
carcinoma of the urothelium: A phase II trial of the Eastern
Cooperative Oncology Group. J Clin Oncol. 12:2264–2270. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burch PA, Richardson RL, Cha SS, Sargent
DJ, Pitot HC IV, Kaur JS and Camoriano JK: Phase II study of
paclitaxel and cisplatin for advanced urothelial cancer. J Urol.
164:1538–1542. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pagliaro LC, Millikan RE, Tu SM, Williams
D, Daliani D, Papandreou CN and Logothetis CJ: Cisplatin,
gemcitabine and ifosfamide as weekly therapy: A feasibility and
phase II study of salvage treatment for advanced transitional-cell
carcinoma. J Clin Oncol. 20:2965–2970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esteban-Fernández D, Verdaguer JM,
Ramírez-Camacho R, Palacios MA and Gómez-Gómez MM: Accumulation,
fractionation and analysis of platinum in toxicologically affected
tissues after cisplatin, oxaliplatin and carboplatin
administration. J Anal Toxicol. 32:140–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albers P, Park SI, Niegisch G, Fechner G,
Steiner U, Lehmann J, Heimbach D, Heidenreich A, Fimmers R and
Siener R: AUO Bladder Cancer Group: Randomized phase III trial of
2nd line gemcitabine and paclitaxel chemotherapy in patients with
advanced bladder cancer: Short-term versus prolonged treatment
[German Association of Urological Oncology (AUO) trial AB 20/99].
Ann Oncol. 22:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pond GR, Bellmunt J, Fougeray R, Choueiri
TK, Qu AQ, Niegisch G, Albers P, Di Lorenzo G, Salhi Y, Galsky MD,
et al: Impact of response to prior chemotherapy in patients with
advanced urothelial carcinoma receiving second-line therapy:
Implications for trial design. Clin Genitourin Cancer. 11:495–500.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bellmunt J, Choueiri TK, Fougeray R,
Schutz FA, Salhi Y, Winquist E, Culine S, von der Maase H, Vaughn
DJ and Rosenberg JE: Prognostic factors in patients with advanced
transitional cell carcinoma of the urothelial tract experiencing
treatment failure with platinum-containing regimens. J Clin Oncol.
28:1850–1855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niegisch G, Fimmers R, Siener R, Park SI
and Albers P: German Association of Urological Oncology Bladder
Cancer Group: Prognostic factors in second-line treatment of
urothelial cancers with gemcitabine and paclitaxel (German
Association of Urological Oncology trial AB20/99). Eur Urol.
60:1087–1096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikeda M, Matsumoto K, Tabata K, Minamida
S, Fujita T, Satoh T, Iwamura M and Baba S: Combination of
gemcitabine and paclitaxel is a favorable option for patients with
advanced or metastatic urothelial carcinoma previously treated with
cisplatin-based chemotherapy. Jpn J Clin Oncol. 41:1214–1220. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JL, Ahn JH, Park SH, Lim HY, Kwon JH,
Ahn S, Song C, Hong JH, Kim CS and Ahn H: Phase II study of a
cremophor-free, polymeric micelle formulation of paclitaxel for
patients with advanced urothelial cancer previously treated with
gemcitabine and platinum. Invest New Drugs. 30:1984–1990. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krajewski KM, Fougeray R, Bellmunt J, Pons
F, Schutz FA, Rosenberg JE, Salhi Y and Choueiri TK: Optimisation
of the size variation threshold for imaging evaluation of response
in patients with platinum-refractory advanced transitional cell
carcinoma of the urothelium treated with vinflunine. Eur J Cancer.
48:1495–1502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choueiri TK, Ross RW, Jacobus S,
Vaishampayan U, Yu EY, Quinn DI, Hahn NM, Hutson TE, Sonpavde G,
Morrissey SC, et al: Double-blind, randomized trial of docetaxel
plus vandetanib versus docetaxel plus placebo in
platinum-pretreated metastatic urothelial cancer. J Clin Oncol.
30:507–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ko YJ, Canil CM, Mukherjee SD, Winquist E,
Elser C, Eisen A, Reaume MN, Zhang L and Sridhar SS: Nanoparticle
albumin-bound paclitaxel for second-line treatment of metastatic
urothelial carcinoma: A single group, multicentre, phase 2 study.
Lancet Oncol. 14:769–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buti S, Ciccarese C, Zanoni D, Santoni M,
Modena A, Maines F, Gilli A, Bria E, Brunelli M, Rimanti A, et al:
Prognostic and predictive factors in patients treated with
chemotherapy for advanced urothelial cancer: Where do we stand?
Future Oncol. 11:107–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Terakawa T, Miyake H, Yokoyama N, Miyazaki
A, Tanaka H, Inoue T and Fujisawa M: Clinical outcome of paclitaxel
and carboplatin as second-line chemotherapy for advanced urothelial
carcinoma resistant to first-line therapy with gemcitabine and
cisplatin. Urol Int. 92:180–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weinstock M and McDermott D: Targeting
PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma.
Ther Adv Urol. 7:365–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|