Introduction
The current management of musculoskeletal
malignancies is limb sparing, and depending on the specific tumor,
chemotherapy, surgery and radiotherapy are used in various
combinations (1). Surgery is the
mainstay of malignant bone tumor treatment with en bloc resection,
followed by implantation of autograft, allograft or prosthesis to
ensure skeletal continuity (1).
Reconstruction, precise fit and stability of the materials are the
main challenges to overcome. Different reconstruction methods have
different advantages and disadvantages. Aseptic loosening of
prosthesis and fracture of the allograft are common problems in
clinical practice (2,3).
In 1968, Spira and Lubin (4) first reported intra-operative
extracorporeal irradiation (ECI) and re-implantation of resected
bone as a useful method of limb salvage for malignant bone tumors.
However, this method does not have a widespread use according to
literature; it has only been used in some cases of osteosarcoma,
Ewing sarcoma or chondrosarcoma with minimal lytic destruction or
predominantly sclerotic changes (5).
The dose of radiation produces a dead autogenous bone graft for
re-implantation and reconstruction with correct dimensions.
Available results have demonstrated excellent oncological outcomes
in terms of local control and overall survival (5,6).
Previous reports have indicated that ECI and re-implantation of the
involved bone were more beneficial than other treatments, including
reconstruction using allograft or prosthesis (4–7). ECI and
re-implantation is an economic technique that provides an
anatomically size-matched graft for a lifelong biological
reconstruction and preservation of joint mobility, thus avoiding
the problems of revision due to prosthetic wear. Additionally, this
technique also removes the requirement for bone banks and other
problems associated with allografts, including graft rejection and
risk of viral transmission (4–7). ECI and
re-implantation of the involved bone was introduced to Southwest
Hospital, Third Military Medical University (Chongqing, China) in
2004. The present study reported the results and complications in
23 patients who had undergone this procedure.
Patients and methods
Patients
Between June 2005 and December 2014, ECI and
re-implantation of the involved bone was used in 39 patients in
Southwest Hospital, Third Military Medical University. The present
study retrospectively reviewed the radiographs, histology slides,
medical records (history and operative procedure) and follow-up
information for each patient. The present study and the treatment
protocols were approved by the Research Ethics Committee of
Southwest Hospital, Third Military Medical University. The
inclusion criteria were as follows: i) Malignant bone tumors
involving the limbs; ii) ECI and re-implantation of the involved
bone had been performed; and iii) patients had complete follow-up
information. Patients with aggressive benign bone tumors were
excluded from the present study.
The present study included 23 patients (12 males and
11 females) with an average age of 23.4 years (range, 9–56 years).
Patients were followed-up with a mean time of 77.6 months (range,
17–116 months), measured from the date of surgery. The involved
bone was the femur in 12 patients, tibia in 10 patients and the
humerus in 1 patient. The malignancies included 4 Ewing's sarcomas,
13 osteosarcomas, 1 chondrosarcomas, 2 malignant fibrous
histiocytoma and 3 metastatic tumors (Table I). Preoperatively, the stage and
extent of the tumor was evaluated using physical examination, plain
radiographs, computed tomography, magnetic resonance imaging and
bone scans.
 | Table I.Characteristics and results of the 23
patients. |
Table I.
Characteristics and results of the 23
patients.
|
|
|
|
|
|
|
|
|
|
| Oncologic
outcome |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Sex | Age, years | Diagnosis | Bone involved | Location | Resected segment
length, cm | Follow-up,
months | Complications | Re-operation | Relapse | Patient status | Time for graft union,
months | MSTS, % |
|---|
| 1 | M | 17 | Malignant fibrous
histiocytoma | Femur | Distal | 16 | 76 | None | None | None | NED | 8 | 90.0 |
| 2 | M | 13 | Ewing sarcoma | Tibia | Shaft | 10 | 89 | Part of the graft
resorption | None | None | NED | 13 | 93.3 |
| 3 | M | 9 | Osteosarcoma | Tibia | Proximal | 10 | 9 | None | Amputation | Local recurrence,
metastasis | STD | NA | 66.7 |
| 4 | M | 32 | Chondrosarcoma | Femur | Shaft | 17 | 24 | None | None | None | NED | 12 | 86.7 |
| 5 | F | 15 | Ewing sarcoma | Femur | Distal | 12 | 7 | None | None | None | STD | NA | 70.0 |
| 6 | F | 25 | Osteosarcoma | Femur | Distal | 16 | 74 | Non-union, Failure
of metalwork | Revision fixation +
graft planned | None | NED | 15 | 80.0 |
| 7 | M | 15 | Ewing sarcoma | Tibia | Shaft | 16 | 116 | None | None | None | NED | 12 | 93.3 |
| 8 | F | 13 | Osteosarcoma | Tibia | Proximal | 15 | 92 | 4 cm short | Limb lengthening,
resection of the metastatic lesion | Metastasis | AWD | 7 | 70.0 |
| 9 | F | 18 | Osteosarcoma | Tibia | Proximal | 15 | 87 | Peroneal nerve
palsy, part of the graft resorption | None | None | NED | 9 | 50.0 |
| 10 | F | 17 | Osteosarcoma | Tibia | Distal | 14 | 96 | Minor resorption of
the graft | None | None | NED | 11 | 86.7 |
| 11 | F | 19 | Osteosarcoma | Tibia | Proximal | 15 | 135 | Deep vein
thrombosis, part of the graft resorption | None | None | NED | 8 | 83.3 |
| 12 | M | 12 | Osteosarcoma | Tibia | Distal | 10 | 18 | None | Amputation | Local recurrence,
metastasis | STD | 10 | 63.3 |
| 13 | F | 56 | Metastatic tumor of
ovarian carcinoma | Humerus | Shaft | 11 | 43 | None | None | Metastasis | STD | 13 | 86.7 |
| 14 | F | 17 | Osteosarcoma | Femur | Distal | 18 | 79 | Delayed healing of
proximal osteotomy | Graft planned | None | NED | 14 | 86.7 |
| 15 | M | 16 | Osteosarcoma | Femur | Proximal | 14 | 66 | None | None | None | NED | 6 | 90.0 |
| 16 | M | 49 | Metastatic tumor of
pulmonary carcinoma | Femur | Shaft | 18 | 17 | None | None | None | NED | 11 | 83.3 |
| 17 | F | 38 | Malignant fibrous
histiocytoma | Femur | Distal | 16 | 41 | Non-union, stress
fracture | Revision fixation +
graft planned | None | NED | 16 | 80.0 |
| 18 | F | 47 | Osteosarcoma | Femur | Distal | 17 | 135 | None | None | None | NED | 6 | 93.3 |
| 19 | M | 16 | Ewing sarcoma | Femur | Proximal | 17 | 84 | None | None | None | NED | 7 | 86.7 |
| 20 | M | 15 | Osteosarcoma | Femur | Distal | 14 | 83 | Non-union, failure
of fixation fracture | Revision fixation +
graft planned | None | NED | 14 | 43.3 |
| 21 | F | 52 | Metastatic tumor of
thyroid carcinoma | Femur | Shaft | 19 | 38 | None | None | None | NED | 10 | 83.3 |
| 22 | M | 11 | Osteosarcoma | Tibia | Proximal | 10 | 23 | Wound
infection | Wound debridement,
amputation | Local recurrence,
metastasis | STD | 13 | 70.0 |
| 23 | M | 16 | Osteosarcoma | Tibia | Shaft | 13 | 64 | Wound infection,
partial skin edge necrosis | Wound debridement,
skin flap transfer | None | NED | 12 | 76.7 |
Chemotherapy
All cases of osteosarcomas and Ewing sarcomas were
treated with similar neoadjuvant chemotherapy protocols of three
cycles at three-week intervals with the drugs of cisplatin (Qilu
Pharmaceutical Co., Ltd., Haikou, China) and Adriamycin (Zhejiang
Haizheng Pharmaceutical Co., Ltd., Fuyang, China). In each cycle,
the patient was administered intravenously with Adriamycin (60
mg/m2) and cisplatin (120 mg/m2) for 2 days.
Following completion of the neoadjuvant chemotherapy, re-evaluation
of the stage and extent of the tumor was performed using the same
preoperative imaging studies. No radiotherapy was given prior to
surgery. In 1 patient, post-operative radiotherapy was given due to
marginal resection (patient 3).
Surgical procedure
Informed consent was provided by the patients and/or
their guardians, who were aware of the risks of the surgery and the
availability of other standard methods for surgical reconstruction.
Senior surgeon (Xu-quan Wang) performed all surgical procedures,
with the purpose of removing the tumor with satisfactory margins.
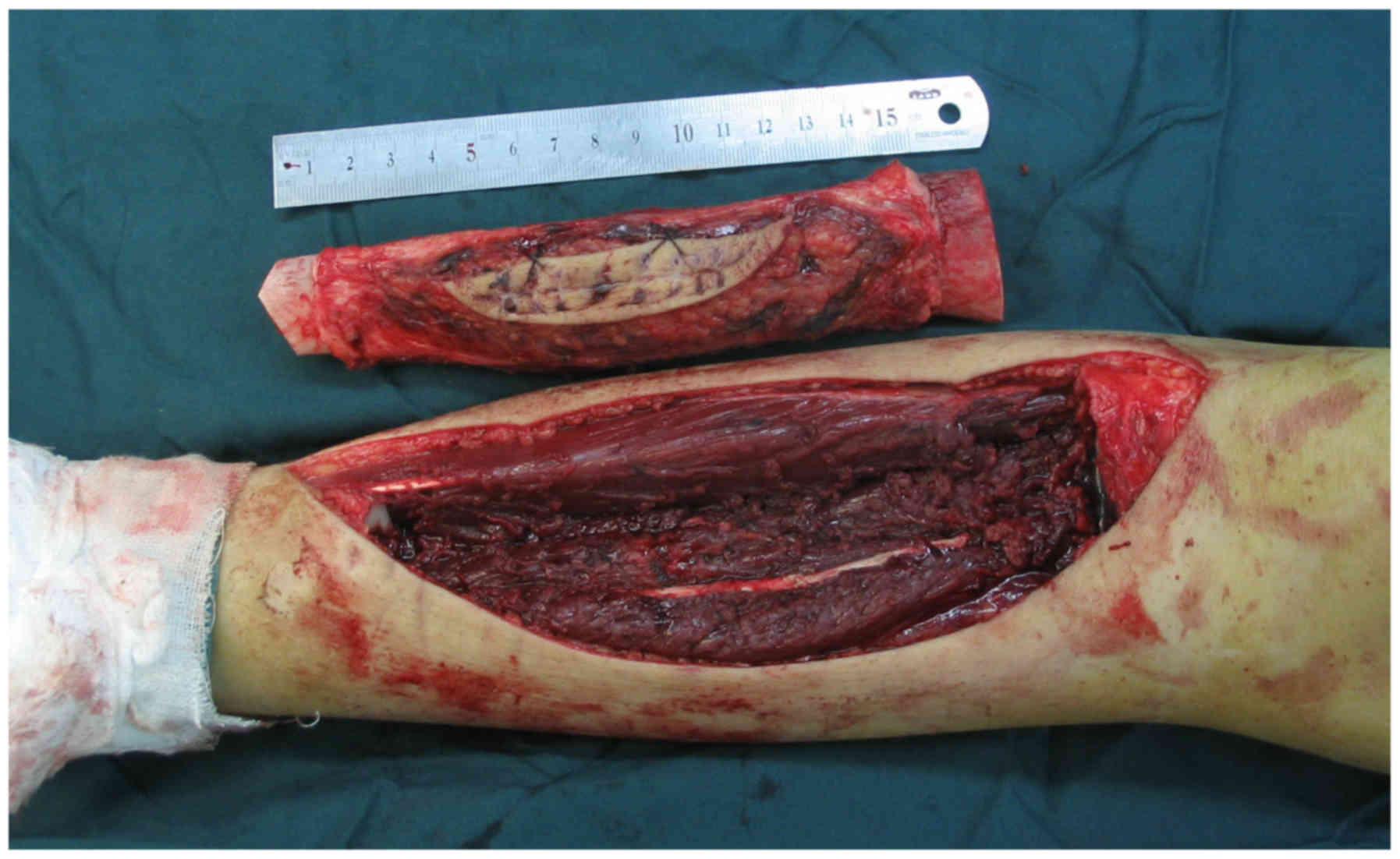
The surgical procedure included three main steps. The first step
was en bloc resection of the tumor and the involved bone, along
with soft tissues, through the MRI scan as far as possible
(Fig. 1). Following resection, the
second step involved a thorough debridement of all the tumor and
soft tissues from the resected bony segment, leaving only the
insertions of important ligaments and tendons. All tissue removed
from the tumor was sent for histopathological examination,
performed by a pathologist. Then the bone was placed into two
sterile plastic bags and delivered for radiotherapy. Bone segments
were irradiated with 4–6 MV photons from a linear accelerator,
having a single midplane dose of 50 Gy at a rate of 1.8–2.0 Gy per
min (6,7). For irradiation, each segment was placed
in the center of a cylindrical plastic canister and
water-equivalent material was used to fill the remaining space. The
mean time from collection of the bone to its return to the
operating room was 40 min. During ECI, the surgical site was
prepared for re-implantation, and biopsy was performed at all
osteotomy sites by the surgeon to assess the resection margins for
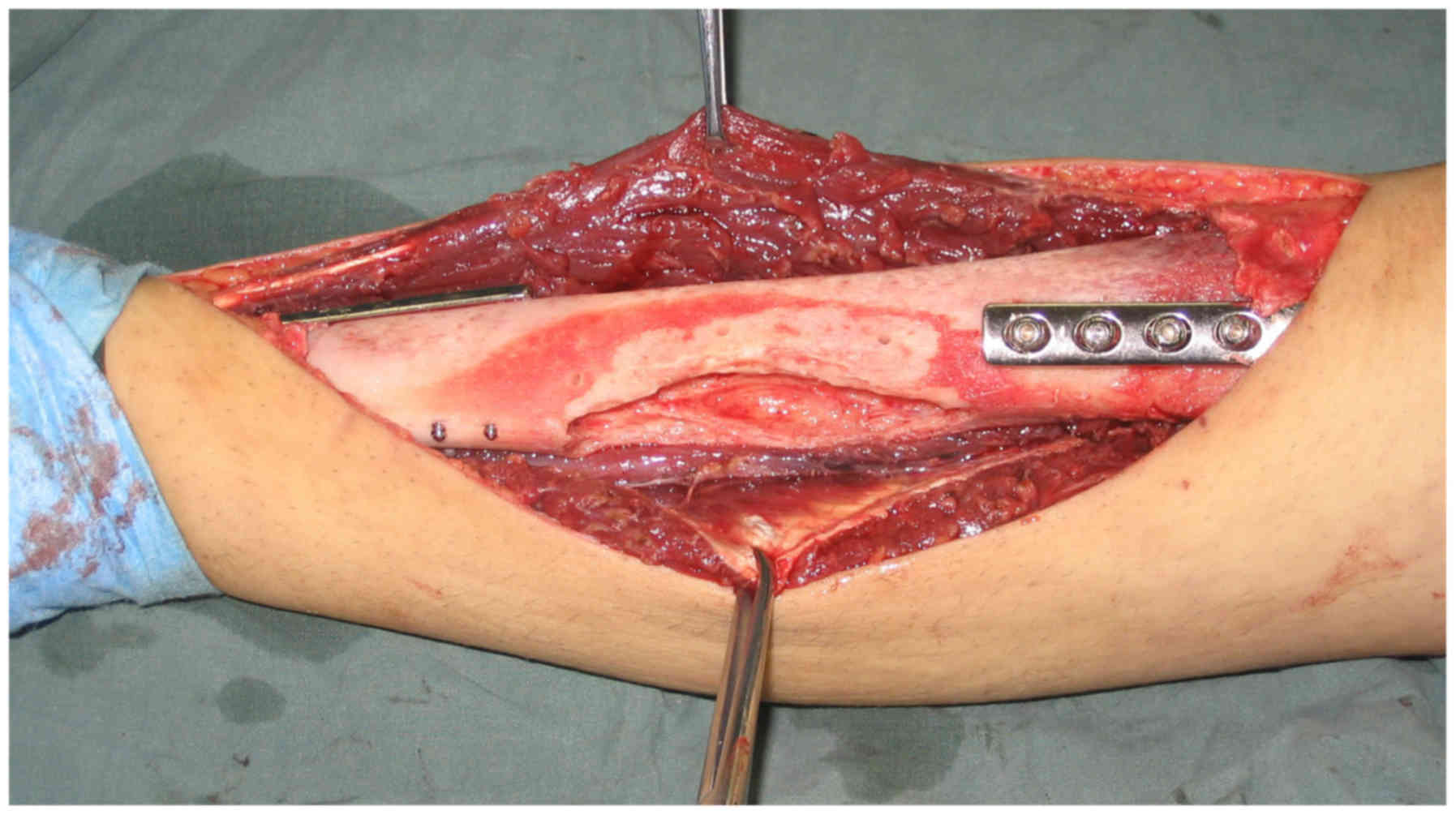
safety. The irradiated bone segments were then re-implanted in the
excision site using fixation methods, such as plates and
intramedullary nailing (Fig. 2).
When ECI and re-implantation was first introduced into our
hospital, some irradiated bone tissue was taken for pathological
examination to ensure that the inactivated bone had no live tumor
cells. In recent years, cancellous bone graft harvested from the
iliac crest is applied at the osteotomy site to promote bone
healing, which was performed in some cases in the present
study.
Each patient was managed with the standard
perioperative protocol, including intravenous antibiotics until all
drains and catheters were removed, prophylaxis against venous
thrombosis, physical therapy and pain control. Post-operatively,
patients were usually kept in bed for 5–7 days, and were then
allowed to walk without weight-bearing on crutches for ~3 months.
After this stage, partial weight-bearing was allowed, progressing
to full weight-bearing for another 3 months according to the plain
radiographs, albeit protected by one or two crutches until both
osteotomies had united. Some patients were immobilized in a brace
for the first 6–8 weeks to maintain knee stability and protect the
implants. The majority of patients received a course of
physiotherapy for 4–6 weeks following surgery.
Follow-up
Patients were followed-up at 3-month intervals
within 3 years of surgery, and at 6- or 12-month intervals 3 years
later, for local control, metastatic spread, function of the limb,
graft union and side effects. Meanwhile, the patients keep in touch
with us via email or telephone. Functional outcome was evaluated by
the Musculoskeletal Tumor Society (MSTS) system at the final
follow-up, which is based on six parameters, including walking
ability, pain, emotional acceptance, functional activities, the use
of external support and gait (8).
All patients with Ewing sarcomas and osteosarcomas received
postoperative chemotherapy.
Results
Oncologic outcome
The details and functional outcome of patients are
summarized in Table I, which
includes the notable incidence of complications and problems. At
the final follow-up, the living patients had a mean follow-up time
of 77.6 months (range, 17–116 months), measured from the date of
surgery. Of the 23 patients included in the present study, 17
(73.9%) demonstrated no evidence of disease, 5 (21.7%) succumbed to
the disease and 1 (4.3%) was alive with the disease at the final
follow-up. Local recurrence occurred in 3 patients (13.0%), with
the recurrence site in the bed outside of the irradiated graft, and
amputation surgery was performed. A total of 3 of the patients who
lost their lives with osteosarcoma (patients 3, 12 and 22) all
presented with a large soft tissue swelling and unsatisfactory
response to the neoadjuvant chemotherapy, and 1 had a metastatic
tumor of ovarian carcinoma of the humerus shaft (patient 13). Of
the 5 patients who lost their lives, 4 demonstrated disseminated
disease, while the remaining patient lost their life due to
coagulopathy and myocardial damage due to postoperative
chemotherapy (patient 5). Osteosarcoma of the proximal tibia was
observed in 1 patient (patient 8) who had pulmonary metastases 72
months after surgery. This was managed by surgical resection at
another hospital and chemotherapy. At the last follow-up, this
patient was still alive with the disease.
Orthopedic outcome
The marginal biopsies obtained intraoperatively were
adequate for assessing the resection margin, and histopathological
study revealed no viable tumor cells in the irradiated bone. The
mean value of the MSTS score was 78.8% (range, 50–93.3%). The
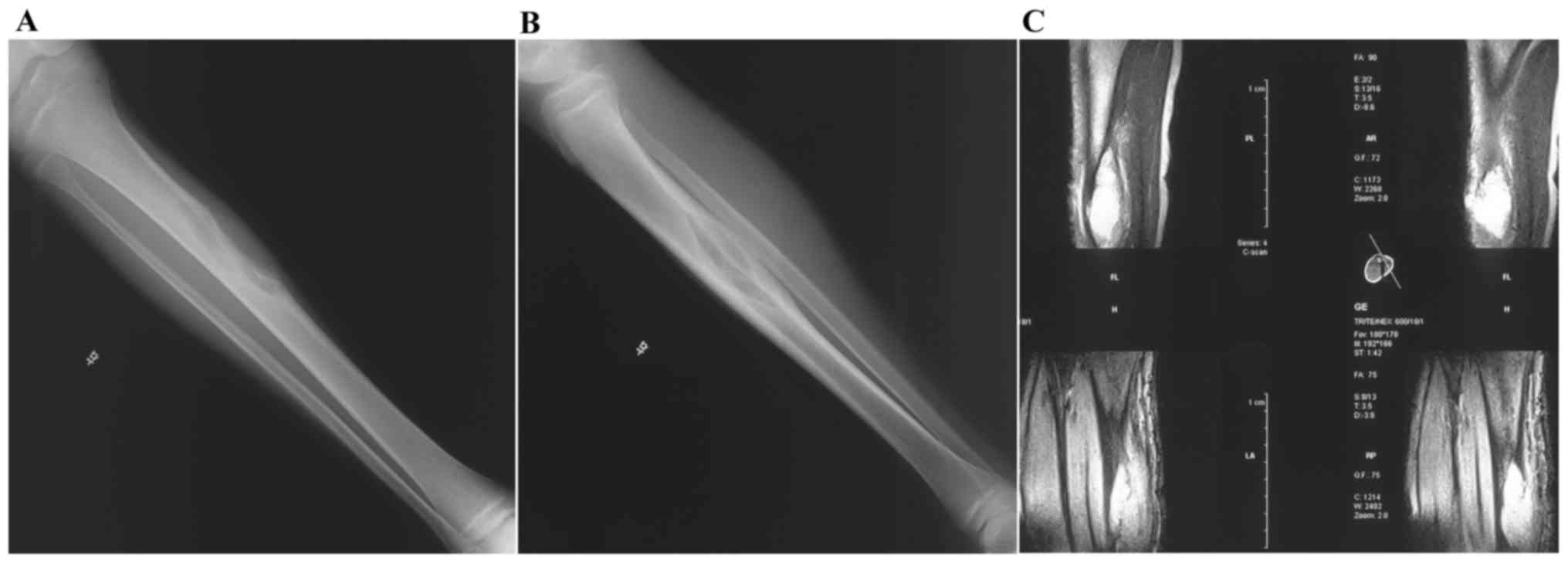
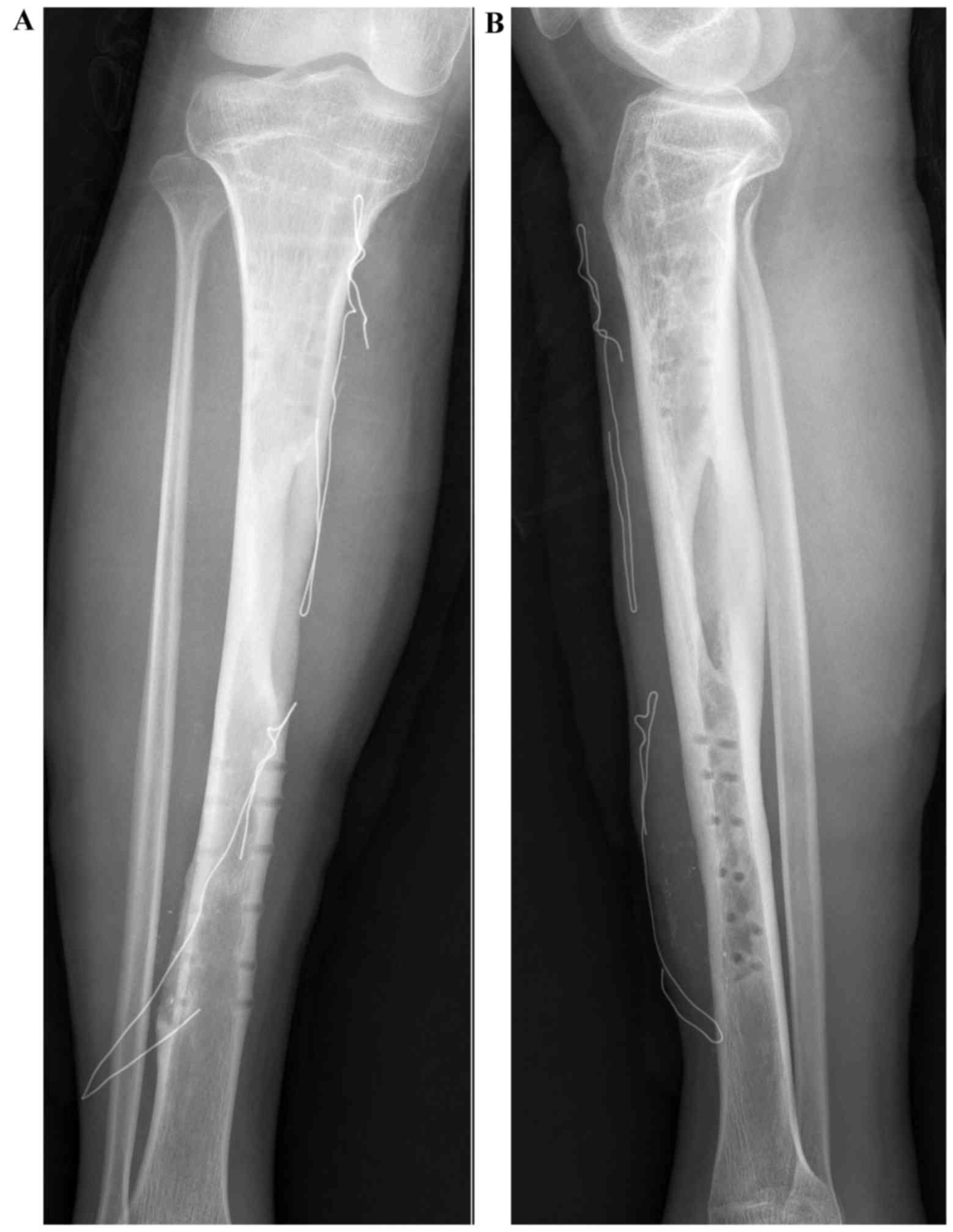
majority of patients demonstrated solid bony union (Figs. 3–5);
however, 3 patients (patients 6, 17 and 20) had non-union (13.0%)
and were revised by exchange of the fixation implant and additional
bone grafting, 2 patients (patients 3 and 5) had no union due to
amputation and mortality, respectively, and 1 patient (patient 14)
had a delayed union (4.3%) and underwent re-operation with
autogenous bone graft. Radiological union at the osteotomy site was
assessed according to literature, with graft union defined as
uninterrupted external bony borders between the graft and the
recipient bone except obscured or absent osteotomy lines at both
junctions (9). According to this
criterion, the host-irradiated bone junctions healed after a mean
time of 10.8 months (range, 6–16 months) in all but 2 patients who
lost their lives or required amputation before union. In the
present study, it was observed that bony union tended to occur more
rapidly in cases of the use of bone graft at the osteotomy site, or
secure reduction and fixation using plates or intramedullary
nailing.
Complications
Complications occurred in 11 patients (47.8%),
including early or late complications related to tumor resection
and irradiated bone reconstruction with implant. The main
complications were non-union, delayed union and failure of
fixation, and 3 of the 4 patients who experiences these
complications were revised by exchange of the fixation device and a
bone graft. All non-unions and delayed unions had united 3–5 months
after re-operation, whereas resorption of the irradiated bone was
observed (patients 2, 9, 10 and 11), particularly in the long
follow-up cases. A total of 2 patients sustained deep vein
thrombosis caused by stretching during the surgery and a long
bedridden time, and a neuropraxia of the peroneal nerve potentially
caused by stretching during the surgery, respectively. Both
patients recovered, and partial limb function was preserved
following conservative treatment for 6 months. Superficial skin
infection was observed in 2 patients, and 1 patient was treated
with intravenous antibiotics, debridement, frequent dressing and
immobilization without removal of the irradiated bone. In the other
patient, the skin necrosis was large and healed by debridement and
skin flap transfer.
Discussion
The incidence of malignant bone tumor is low and
usually occurs in the limbs; however, the morbidity and mortality
is high (10). Currently, limb
salvage is usually the first choice for treatment, which may result
in increased survival rates and disease-free periods that equal
those achieved with amputation, and may also offer an improved
psychological acceptance and an intact limb (11). Although it is generally believed that
limb-sparing surgery may lead to an increased risk of recurrence
and metastasis, there is no convincing evidence that en bloc
resection of the tumor leads to decreased survival time, and any
higher rate of recurrence and metastasis, even in the cases with
inadequate margins of resection or inappropriate adjuvant
chemotherapy and radiotherapy (12).
Along with the advancements in imaging and reconstruction methods,
chemotherapy or radiotherapy has made limb salvage an alternative
to amputation for limb malignancies.
The ideal reconstruction of the defect created
following en bloc resection of the bone tumor is controversial.
Tumor prostheses provide advantages, such as convenience, rapid
mobilization and weight bearing capabilities (13). However, tumor prostheses incur a high
financial cost and involve various complications, including
infection, aseptic loosening, breakage during the long-term
follow-up and a higher probability of revision surgery being
required within 10 years, as reported in some studies (13–15).
Allografts are a common reconstructive method utilized in some
western countries, whereas this technique is forbidden in some
Asian and African countries due to religious reasons (16). Although it is a biological
reconstructive method, it carries a high risk of transmission of
infectious diseases, fracture, tissue rejection, articular
cartilage degeneration and poor union rate (3). In addition, bone banking requires
substantial time, energy and money. Furthermore, as the concept of
bone donation is not widely accepted in some Asian countries,
allografting may be difficult to carry out.
Recycling of the resected bone segment is a
beneficial alternative method that is more economic than the
prosthetic devices, as well as durable and more psychological
accepted because of its biological nature, and may be achieved by
irradiation, freezing or heating (17–19).
Various methods of sterilization of tumor-bearing bone have been
described. Autoclaving and boiling lead to the complete destruction
of bone cells, but have the disadvantage of leading to considerable
decrease in the biological and biomechanical properties of the
graft compared to pasteurization and irradiation, causing severe
injury to the collagen matrix and bone proteins (17). Liquid nitrogen as an effective and
available cryogenic agent may be used for either tissue
preservation or destruction. A quick freeze and slow thaw lead to
tissue destruction, while a slow freeze and quick thaw allow for
tissue preservation (18). Two
studies have reported the use of liquid nitrogen to inactivate and
re-implant the resected tumor-bearing segment following en bloc
resection (20,21). Among them, a study by Abdel et
al (21) questioned the
effectiveness of reconstruction by re-implanting the tumor-bearing
segment after recycling in liquid nitrogen in a prospective
clinical study. A total of 10 patients with osteosarcoma of the
femur or tibia were treated using this technology. At the final
follow-up, all patients were alive and free from any signs of local
or systemic recurrence, with the graft united at both junctions
(21). Therefore, the authors
recommend this technique as an appropriate method of reconstruction
in properly selected patients. However, a larger series and a
longer follow-up are required to verify the long-term efficacy of
this technique. Currently, to the best of our knowledge,
re-implantation of the resected tumor-bearing segment following
recycling using freezing by liquid nitrogen has been described in a
limited number of studies.
For bone tumors with minimal lytic destruction or
predominantly sclerotic changes, ECI and re-implantation of the
involved bone has several potential advantages over other methods
of limb reconstruction (6,19). Firstly, the affected bone segment is
removed from the body and irradiated and therefore, avoidance of
radiation injury to the un-irradiated bone, muscles, joint and
other healthy tissues of the body is achieved. Secondly, it is an
economic technique that provides an anatomically size-matched graft
for a lifelong biological reconstruction and preservation of joint
mobility, thus avoiding the problems of revision due to prosthetic
wear. Thirdly, this technique also removes the requirement for bone
banks and some of the other problems associated with allografts,
such as graft rejection and risk of viral transmission (22). Fourth, in pediatric patients, this
technique may potentially avoid the growth discrepancy commonly
observed in prosthetic replacement by avoiding resection of the
normal growth plate and appositional bone growth from surrounding
healthy bone (23).
Currently, the optimal dose of radiation has not
been determined. Radiation doses of 50–300 Gy have been used in
some centers with no evidence of local recurrence from irradiated
bone segments (6,7,10,16,19,24,25).
In a histological study, Davidson et al (6) demonstrated the complete eradication of
tumor cells in grafts by a single radiation dose of 50 Gy, which is
equivalent to 250 Gy in the conventional fraction using the linear
quadratic model. They believed the radiation dose was immensely
higher than that of the standard fractionated external-beam
irradiation treatment for bone tumors, and there was no risk of
local recurrence or of radiation-induced malignancy. They also
believed that higher doses were unnecessary, take longer to
administer and may increase detrimental effects to bone. Other
studies have also conformed this point of view and believe this
dose should be sufficient to produce a tumor elimination rate of
100% (23,26). Studies by Sabo et al (27) and Currey et al (28) have demonstrated a radiation
dose-dependent reduction in strength and also suggested reduced
revascularization and osteoconductive properties, thereby
increasing the time to union and incorporation. Therefore, the
present study used a radiation dose of 50 Gy.
When compared with other methods of limb
preservation, such as megaprosthesis replacement and allograft
reconstruction, there was no evidence of increased rate of
recurrence with ECI in the present study or other similar studies
(5,16,19,24).
Furthermore, local recurrence in the irradiated segments was not
observed in the present study, and seldom local recurrence has
occurred in an ECI autografting over the last 20 years. In the
present study, 3 recurrent cases were observed; however, the local
recurrence occurred in the bed outside of the irradiated graft,
most likely attributable to inadequate resection. In agreement with
a study by Uyttendaele et al (29), the risk of local recurrence may be
minimized by careful preoperative planning and staging by imaging
techniques, intraoperative marginal biopsies, and the use of
appropriate chemotherapy or radiotherapy regimens.
Non-union (13.0%) and delayed union (4.3%) occurred
in 4 of the 23 patients in the present study. The rate of non-union
and delayed union corresponded with other reports of ECI
autografting of the limb (6,10,16,25,26), and
were lower than those observed with reconstructions using
allografts (22,30,31).
Union occurred faster at the metaphyseal than at the diaphyseal
junction in some cases; however, statistical tests were not
performed to determine whether these differences were significant.
This phenomenon was also observed in a study by Krieg et al
(32) in ECI autografting. The
authors of the present study are in agreement with Chen et
al (24) and believe that
perfect anatomical reduction, stable internal fixation and proper
muscle coverage are the most important factors in promoting healing
of the graft-host junction. In order to promote bone healing,
cancellous bone graft harvested from the iliac crest was applied at
the osteotomy site in some of the cases in the present study.
Theoretically, radiological remodeling of the large ECI graft is
limited, complete replacement with living bone does not occur and
the graft remains a framework of dead bone that requires the
long-term support of the metal implants. For these reason, some
studies recommend a vascularized fibular graft to supplement the
reconstruction and promote healing of the graft-host junction
(6,10,33). In
the present study, resorption of the irradiated bone was observed,
particularly in the long follow-up cases. Therefore, it may not be
appropriate to remove the implants following bone union in some of
our cases, which was strongly required by patients because of
religious reasons. Only longer follow-up with biopsies of the
irradiated autografts could properly answer this question.
One of the drawbacks of the use of ECI autografts is
the lack of material available for histopathological examination of
the effects of chemotherapy and the adequacy of the resection
margins. As the bone is re-implanted, the pathologist cannot
examine the whole material and only has access to soft tissues,
bone marrow and some small bone fragments. The surgeon must perform
biopsies from the margins of the bone to overcome the issue and to
define the status of the surgical margins.
In the present study, early or late complications,
such as infection, non-union, fracture and resorption of the
irradiated graft, occurred in 11 patients. The complication rate
(47.8%) and re-operation rate (39.1%) were high; however, the
functional results of patient survival were acceptable. To reduce
the complication rate, several techniques, such as irradiated
autograft prosthesis composites (24), use of a vascularized fibular graft to
supplement the reconstruction, vancomycin prophylaxis and adequate
cover of the irradiated bone, were proposed. Preoperative
treatment, surgical technique, postoperative support and follow-up
by a dedicated team are also major factors in achieving successful
outcomes.
In conclusion, when considering the present mid-term
results and overall experiences, ECI is a useful technique
following en bloc resection for limb salvage when there is
reasonable residual bone stock in appropriately selected patients.
The patient's own bone fits perfectly to the resection site, risk
of local recurrence due to tumor re-implantation is not possible
with current radiation doses and complications are usually
manageable. In order to confirm this, this technique should be used
as part of a multidisciplinary approach, using a larger series with
a longer follow-up.
References
|
1
|
Gitelis S, Bayne CO, Frank JM, Fillingham
YA and Kent PM: Surgery in malignant bone tumors. Curr Probl
Cancer. 37:192–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Unwin PS, Cannon SR, Grimer RJ, Kemp HB,
Sneath RS and Walker PS: Aseptic loosening in cemented custom-made
prosthetic replacements for bone tumours of the lower limb. J Bone
Joint Surg Br. 78:5–13. 1996.PubMed/NCBI
|
|
3
|
Matejovsky Z Jr, Matejovsky Z and Kofranek
I: Massive allografts in tumour surgery. Int Orthop. 30:478–483.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spira E and Lubin E: Extracorporeal
irradiation of bone tumors. A preliminary report. Isr J Med Sci.
4:1015–1019. 1968.PubMed/NCBI
|
|
5
|
Böhm P, Fritz J, Thiede S and Budach W:
Reimplantation of extracorporeal irradiated bone segments in
musculoskeletal tumor surgery: Clinical experience in eight
patients and review of the literature. Langenbecks Arch Surg.
387:355–365. 2003.PubMed/NCBI
|
|
6
|
Davidson AW, Hong A, McCarthy SW and
Stalley PD: En-bloc resection, extracorporeal irradiation, and
re-implantation in limb salvage for bony malignancies. J Bone Joint
Surg Br. 87:851–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong A, Stevens G, Stalley P, Pendlebury
S, Ahern V, Ralston A, Estoesta E and Barrett I: Extracorporeal
irradiation for malignant bone tumors. Int J Radiat Oncol Biol
Phys. 50:441–447. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 241–246.
1993.PubMed/NCBI
|
|
9
|
Hsu RW, Wood MB, Sim FH and Chao EY: Free
vascularised fibular grafting for reconstruction after tumour
resection. J Bone Joint Surg Br. 79:36–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muramatsu K, Ihara K, Hashimoto T, Seto S
and Taguchi T: Combined use of free vascularised bone graft and
extracorporeally-irradiated autograft for the reconstruction of
massive bone defects after resection of malignant tumour. J Plast
Reconstr Aesthet Surg. 60:1013–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sluga M, Windhager R, Lang S, Heinzl H,
Bielack S and Kotz R: Local and systemic control after ablative and
limb sparing surgery in patients with osteosarcoma. Clin Orthop
Relat Res. 120–127. 1999.PubMed/NCBI
|
|
12
|
Bacci G, Ferrari S, Bertoni F, Rimondini
S, Longhi A, Bacchini P, Forni C, Manfrini M, Donati D and Picci P:
Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated
with adjuvant chemotherapy: Analysis of 359 patients at the
Istituto Ortopedico Rizzoli. J Clin Oncol. 18:4–11. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heller L and Kronowitz SJ: Lower extremity
reconstruction. J Surg Oncol. 94:479–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai A, Muschler GF, Lane JM, Otis JC and
Healey JH: Prosthetic knee replacement after resection of a
malignant tumor of the distal part of the femur. Medium to
long-term results. J Bone Joint Surg Am. 80:636–647. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Natarajan MV, Annamalai K, Williams S,
Selvaraj R and Rajagopal TS: Limb salvage in distal tibial
osteosarcoma using a custom mega prosthesis. Int Orthop.
24:282–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Araki N, Myoui A, Kuratsu S, Hashimoto N,
Inoue T, Kudawara I, Ueda T, Yoshikawa H, Masaki N and Uchida A:
Intraoperative extracorporeal autogenous irradiated bone grafts in
tumor surgery. Clin Orthop Relat Res. 196–206. 1999.PubMed/NCBI
|
|
17
|
Singh VA, Nagalingam J, Saad M and Pailoor
J: Which is the best method of sterilization of tumour bone for
reimplantation? A biomechanical and histopathological study. Biomed
Eng Online. 9:482010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bickels J, Meller I, Shmookler BM and
Malawer MM: The role and biology of cryosurgery in the treatment of
bone tumors. A review. Acta Orthop Scand. 70:308–315. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puri A, Gulia A, Agarwal M, Jambhekar N
and Laskar S: Extracorporeal irradiated tumor bone: A
reconstruction option in diaphyseal Ewing's sarcomas. Indian J
Orthop. 44:390–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuchiya H, Wan SL, Sakayama K, Yamamoto
N, Nishida H and Tomita K: Reconstruction using an autograft
containing tumour treated by liquid nitrogen. J Bone Joint Surg Br.
87:218–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel Rahman M, Bassiony A and Shalaby H:
Reimplantation of the resected tumour-bearing segment after
recycling using liquid nitrogen for osteosarcoma. Int Orthop.
33:1365–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donati D, Capanna R, Campanacci D, Del Ben
M, Ercolani C, Masetti C, Taminiau A, Exner GU, Dubousset JF,
Paitout D, et al: The use of massive bone allografts for
intercalary reconstruction and arthrodeses after tumor resection. A
multicentric European study. Chir Organi Mov. 78:81–94. 1993.(In
English, Italian). PubMed/NCBI
|
|
23
|
Hong AM, Millington S, Ahern V, McCowage
G, Boyle R, Tattersall M, Haydu L and Stalley PD: Limb preservation
surgery with extracorporeal irradiation in the management of
malignant bone tumor: The oncological outcomes of 101 patients. Ann
Oncol. 24:2676–2680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WM, Chen TH, Huang CK, Chiang CC and
Lo WH: Treatment of malignant bone tumours by extracorporeally
irradiated autograft-prosthetic composite arthroplasty. J Bone
Joint Surg Br. 84:1156–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabo D, Bernd L, Buchner M, Treiber M,
Wannenmacher M, Ewerbeck V and Parsch D: Intraoperative
extracorporeal irradiation and replantation in local treatment of
primary malignant bone tumors. Orthopade. 32:1003–1012. 2003.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotb SZ and Mostafa MF: Recycling of
extracorporeally irradiated autograft for malignant bone tumors:
Long-term follow-up. Ann Plast Surg. 71:493–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sabo D, Brocai DR, Eble M, Wannenmacher M
and Ewerbeck V: Influence of extracorporeal irradiation on the
reintegration of autologous grafts of bone and joint. Study in a
canine model. J Bone Joint Surg Br. 82:276–282. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Currey JD, Foreman J, Laketić I, Mitchell
J, Pegg DE and Reilly GC: Effects of ionizing radiation on the
mechanical properties of human bone. J Orthop Res. 15:111–117.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uyttendaele D, De Schryver A, Claessens H,
Roels H, Berkvens P and Mondelaers W: Limb conservation in primary
bone tumours by resection, extracorporeal irradiation and
re-implantation. J Bone Joint Surg Br. 70:348–353. 1988.PubMed/NCBI
|
|
30
|
Donati D, Di Liddo M, Zavatta M, Manfrini
M, Bacci G, Picci P, Capanna R and Mercuri M: Massive bone
allograft reconstruction in high-grade osteosarcoma. Clin Orthop
Relat Res. 186–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cara JA, Laclériga A and Cañadell J:
Intercalary bone allografts. 23 tumor cases followed for 3 years.
Acta Orthop Scand. 65:42–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krieg AH, Davidson AW and Stalley PD:
Intercalary femoral reconstruction with extracorporeal irradiated
autogenous bone graft in limb-salvage surgery. J Bone Joint Surg
Br. 89:366–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muramatsu K, Fukano R, Ihara K, Iwanaga R
and Taguchi T: Reconstruction of the proximal humerus by combined
use of extracorporeally-irradiated osteochondral graft and free
vascularized fibula following resection of Ewing sarcoma. J Plast
Reconstr Aesthet Surg. 63:2177–2180. 2010. View Article : Google Scholar : PubMed/NCBI
|