Introduction
Choriocarcinoma is classified into two types:
gestational and non-gestational (1–3).
Gestational choriocarcinoma affects women at reproductive age and
is derived from pregnancies including hydatidiform mole,
miscarriage, ectopic pregnancy and term or pre-term deliveries
(4,5). Therefore, it theoretically contains
paternal genes. The incidence of gestational choriocarcinoma is
estimated in 1:40,000–50,000 pregnancies, and 1:40 hydatidiform
mole cases. Non-gestational choriocarcinoma is independent of
pregnancies and less sensitive to chemotherapy compared to
gestational choriocarcinoma (4–7).
Non-gestational choriocarcinoma is reported as a very rare tumor
(4,8). Choriocarcinoma in the female genital
tract is generally treated as the gestational type. However, when
the lesion is in the extragenital organs, differential diagnosis
will be difficult based on clinical data alone. Furthermore, since
there is little difference in morphological characterizations on
pathological examination between the two types of choriocarcinomas,
gene analysis is recommended to obtain definitive diagnosis in some
cases (9).
In general, a single DNA strand conformation
polymorphism analysis to evaluate short tandem repeat sites is
employed for gene analysis of choriocarcinoma using resected
lesions following surgery. However, the reliability of this method
may be reduced in cases of low amounts of tumor DNA and/or marked
contamination by maternal DNA. On the other hand, when gestational
carcinomas have originated from male conceptuses, we can prove the
presence of paternal genes by detecting Y-chromosome-specific
genes.
Based on this background, we here report a case of
extragenital choriocarcinoma in the kidney that was successfully
diagnosed as a gestational type by detection of the Sex-determining
region Y (SRY) gene in needle-biopsied fine tissue samples. The
informed consent of this report was obtained from the patient.
Case report
Five months after the second parturition, a
36-year-old woman visited a nearby hospital due to secondary
amenorrhea with nausea after obtaining a positive result on a
urinary pregnancy test. She had a past history of two normal
vaginal deliveries of male babies 4.5 years and six months ago,
respectively, and received artificial abortion one time. Although
the serum level of human chorionic gonadotropin (hCG) was elevated
at 51,800 mIU/ml, a gestational sac was not observed in the uterus
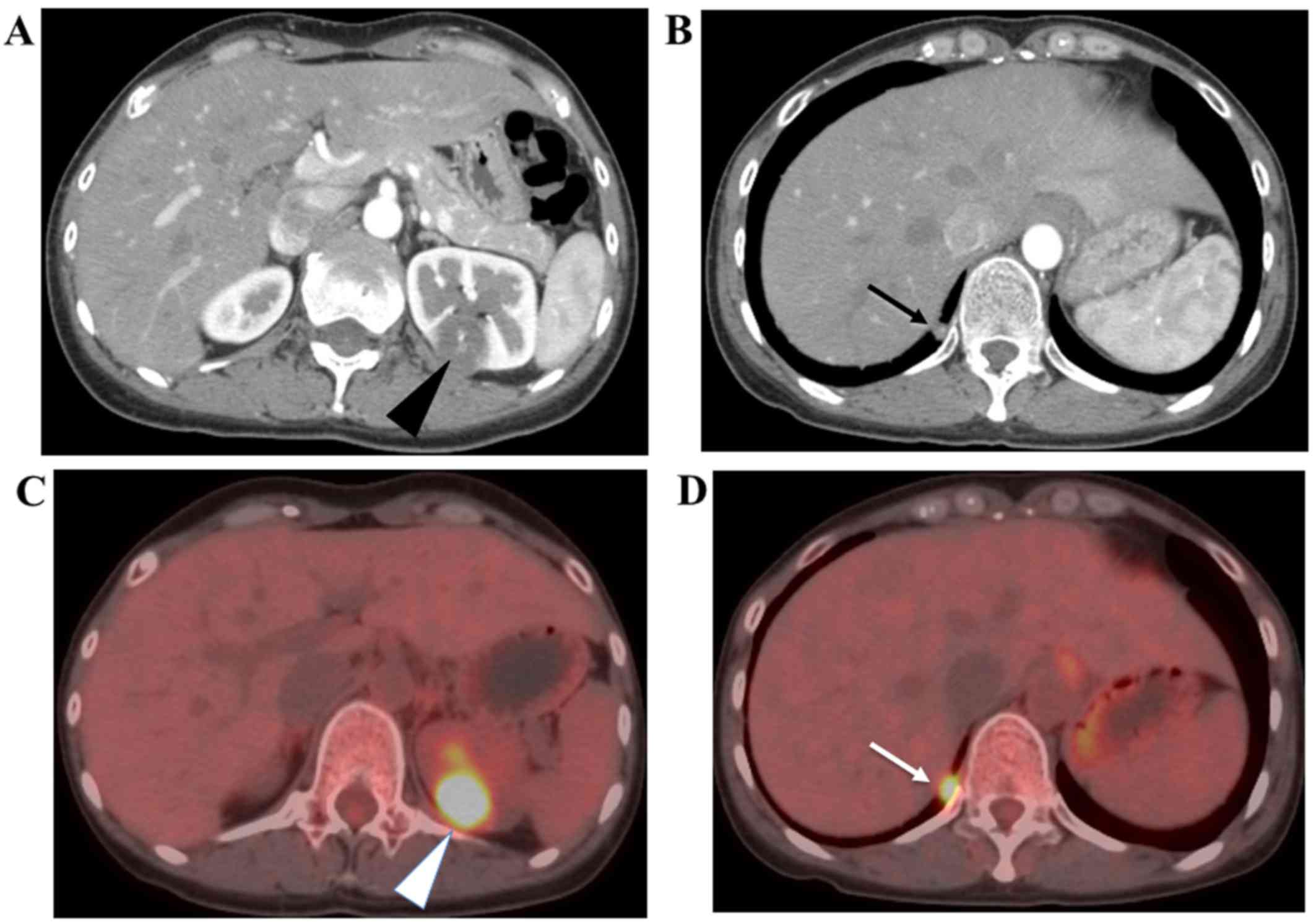
or bilateral adnexa by ultrasonography. Computed tomography (CT)
and magnetic resonance imaging (MRI) did not detect a gestational
sac in the genital tract, but showed abnormal solitary tumor
features in the left kidney and right lung (Fig. 1A). Positron emission
tomography-computed tomography (PET-CT) confirmed that both tumor
lesions showed a high uptake of 18F-FDG (Fig. 1B). Pathological examination by
endometrial curettage was negative for an intrauterine conceptus.
The ultrasonographic characterization of the renal lesion suggested
renal cell carcinoma. Therefore, to determine whether it was
metastatic trophoblastic disease or renal cell carcinoma, we
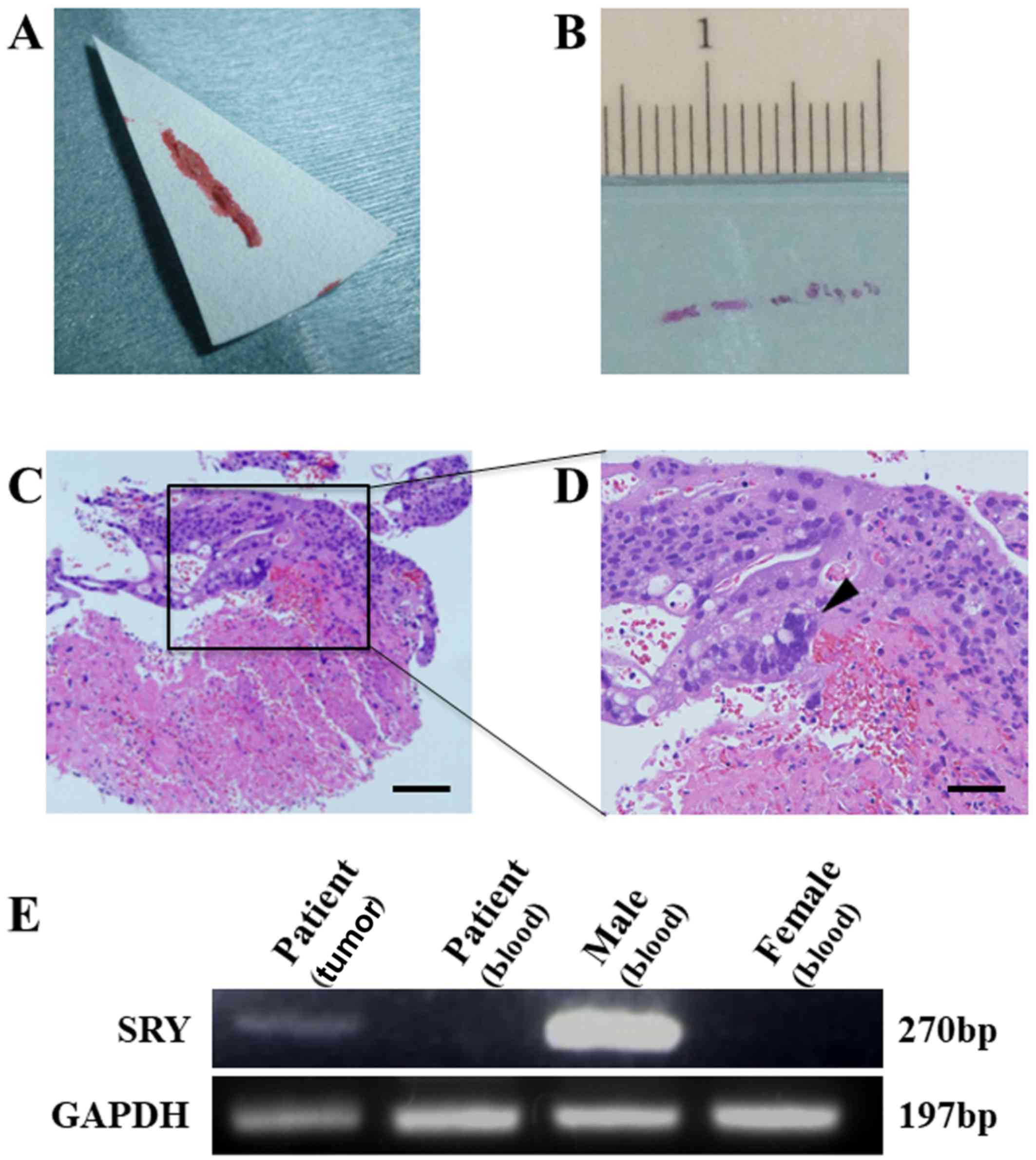
biopsied the renal lesion with a fine needle technique (Fig. 2A), and obtained a final pathological
diagnosis of choriocarcinoma (Fig. 2B
and C). We chose a chemotherapeutic regimen comprising
etoposide, methotrexate, actinomycin-D, cyclophosphamide and
vincristine (EMA/CO chemotherapy) and performed 10 courses. The
serum hCG level was successfully reduced to less than 1 mIU/ml. The
enhanced CT confirmed the size reduction of both tumors with
avascular changes. The uptake of 18F-FDG by PET-CT was
also diminished. No sign of recurrence was observed during the
18-month follow-up period.
Although the serum level of hCG has been controlled
under the detection limit, we could not obtain a complete response
based on CT. Since non-gestational choriocarcinoma was reported to
be associated with a poor prognosis (6,10), we
needed to determine whether or not these lesions were derived from
the conceptus, that is the gestational type. Accordingly, we
performed the polymerase chain reaction (PCR) to detect the SRY
gene in the needle-biopsied sample. Briefly, DNA was extracted from
formalin-fixed 4-tissue sections using TaKaRa DEXPAT Easy (Takara
Co. Kyoto, Japan). Using SRY primers
(5′-CAGTGTGAAACGGGAGAAAACAGT-3′; 5′-TATAAGTATCGACCTCGTCGGAAG-3′)
(11) and GAPDH primers
(5′-GGAGCGAGATCCCTCCAAAAT-3′; 5′-GGCTGTTGTCATACTTCTCATGG-3′)
(12), both genes were amplified in
40 cycles. The SRY gene was successfully detected (Fig. 2D), indicating that this DNA contained
Y-chromosome-specific genes.
Informed consent for the use of these tissues in
this study was obtained from the patient. Analysis of these samples
was approved by the Ethics Committee of the Kanazawa University
Graduate School of Medical Sciences.
Discussion
In this case, the malignancy-suspected solitary
lesion that can be responsible for the elevation of hCG was also
observed in the right lung by enhanced CT and PET-CT (13,14). In
addition, since the morphological characterization of the renal
lesion by ultrasound examination was compatible with that of renal
cell carcinoma, we had to rule out the possibility of independent
renal carcinoma. Consequently, we tried to biopsy the renal lesion
with a fine needle technique. Although the amount of the obtained
tissue sample was small, it contained the malignant lesion, and we
successfully achieved a final pathological diagnosis of
choriocarcinoma. However, the lesion sample was very limited,
making it difficult to extract non-contaminated
choriocarcinoma-derived DNA samples that were sufficient for the
standard analysis of gene polymorphism.
Fortunately, according to the history, this patient
had given birth to two male babies in the previous pregnancies,
along with one artificial abortion. Furthermore, although the
precise amount and purity were unclear, we could prepare DNA
samples of choriocarcinoma from the formalin-fixed tissue slices.
Consequently, we applied the above method and detected a paternal
SRY gene consensus, leading to the conclusion that this case was
gestational choriocarcinoma. Both renal and right lung lesion were
evaluated as metastases of gestational choriocarcinoma.
Although differences have been reported in the
chemotherapy response, genetic origin and prognosis, gestational
and non-gestational choriocarcinoma exhibit similar morphological
pattern, histopathological classification and biochemical markers
(4,15,16).
Even with multidrug chemotherapy, a large number of patients with
non-gestational choriocarcinoma died from the disease (16). In non-gestational choriocarcinoma,
surgery and treatment with multiple chemotherapy agents are
indicated. Therefore, distinguishing these two entities is very
important (4).
The SRY gene is one of the Y-chromosome-specific
genes. Therefore, when the SRY gene is detected in DNA samples
extracted from choriocarcinoma tissues, we can safely conclude that
this lesion is derived from the conceptus, except for the rare
cases of chromosome chimera induced by bone transplantation, twin
pregnancy, etc. In this context, fluorescence in situ
hybridization (FISH) using centromere probe of Y chromosome could
be used for this case. However, the weak point of these methods is
that they cannot be applied to patients with a history of female
delivery and sex-non-determined abortions. Accordingly, the
negative detection of the SRY gene cannot lead to a definitive
conclusion.
In conclusion, this is the first report that the
detection of the SRY gene by PCR is clinically available to
diagnose extragenital gestational choriocarcinoma. Since PCR is a
more convenient method than gene polymorphism analysis, this method
is one of the candidates for alternative techniques when the sample
is limited and marked contamination of maternal DNA is expected
(17).
Acknowledgements
The present study was supported in part by
Grants-in-Aid for Scientific Research (no. 15K10708).
References
|
1
|
Vereczkey I, Csernák E, Olasz J, Küronya
Z, Szentirmay Z and Tóth E: Renal choriocarcinoma: Gestational or
germ cell origin? Int J Surg Pathol. 20:623–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koyanagi T, Fujiwara H, Usui H, Ariga H,
Machida S, Takei Y, Saga Y, Shozu M, Fukushima N, Tóth E, et al:
Ovarian nongestational choriocarcinoma and associated
adenocarcinoma with the same germ cell origin determined by a
molecular genetic approach: A case report. Pathol Int. 66:529–534.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tempfer C, Horn LC, Ackermann S, Beckmann
MW, Dittrich R, Einenkel J, Gunthert A, Haase H, Kratzsch J,
Kreissl MC, et al: Gestational and non-gestational trophoblastic
disease. guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF
registry No. 032/049, december 2015). Geburtshilfe Frauenheilkd.
76:134–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mello JB, Cirilo Ramos PD, Michelin OC,
Domingues Custódio MA, Rudge Cunha MV, Rogatto SR and Maestá I:
Genomic profile in gestational and non-gestational
choriocarcinomas. Placenta. 50:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher RA, Newlands ES, Jeffreys AJ, Boxer
GM, Begent RH, Rustin GJ and Bagshawe KD: Gestational and
nongestational trophoblastic tumors distinguished by DNA analysis.
Cancer. 69:839–845. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Yang Y, Teng F, Zhang H and Xue F:
Pure nongestational uterine choriocarcinoma in a postmenopausal
Chinese woman confirmed with short tandem repeat analysis. Am J
Obstet Gynecol. 211:e1–e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imamura Y, Tashiro H, Saito F, Takaishi K,
Ohba T, Fukunaga M and Katabuchi H: Choriocarcinoma coexisting with
epithelioid trophoblastic tumor of the uterine horn. Gynecol Oncol
Rep. 14:31–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Guo C, Zou L, Wang Y, Song X, Ma Y
and Liu A: Clinicopathological analysis of non-gestational ovarian
choriocarcinoma: Report of two cases and review of the literature.
Oncol Lett. 11:2599–2604. 2016.PubMed/NCBI
|
|
9
|
Yamamoto E, Niimi K, Shinjo K, Yamamoto T,
Fukunaga M and Kikkawa F: Identification of causative pregnancy of
gestational trophoblastic neoplasia diagnosed during pregnancy by
short tandem repeat analysis. Gynecol Oncol Case Rep. 9:3–6. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Xiang Y, Wan XR, Feng FZ, Cui QC
and Yang XY: Molecular genetic analyses of choriocarcinoma.
Placenta. 30:816–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroki M, Okayama A, Nakamura S, Sasaki T,
Murai K, Shiba R, Shinohara M and Tsubouchi H: Detection of
maternal-fetal microchimerism in the inflammatory lesions of
patients with Sjogren's syndrome. Ann Rheum Dis. 61:1041–1046.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang G, Zhou X, Chen T, Deng Y, Yu D, Pan
S and Song Y: Hydroxysafflor yellow A inhibits
lipopolysaccharide-induced proliferation and migration of vascular
smooth muscle cells via Toll-like receptor-4 pathway. Int J Clin
Exp Med. 8:5295–5302. 2015.PubMed/NCBI
|
|
13
|
Brown J, Naumann RW, Seckl MJ and Schink
J: 15 years of progress in gestational trophoblastic disease:
Scoring, standardization and salvage. Gynecol Oncol. 144:200–207.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mangili G, Lorusso D, Brown J, Pfisterer
J, Massuger L, Vaughan M, Ngan HY, Golfier F, Sekharan PK, Charry
RC, et al: Trophoblastic disease review for diagnosis and
management: A joint report from the international society for the
study of trophoblastic disease, european organisation for the
treatment of trophoblastic disease and the gynecologic cancer
intergroup. Int J Gynecol Cancer. 24 suppl 3:S109–S116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheung AN, Zhang HJ, Xue WC and Siu MK:
Pathogenesis of choriocarcinoma: Clinical, genetic and stem cell
perspectives. Future Oncol. 5:217–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Newlands ES: The management of recurrent
and drug-resistant gestational trophoblastic neoplasia (GTN). Best
Pract Res Clin Obstet Gynaecol. 17:905–923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robino C, Barilaro MR, Gino S, Chiarle R,
Palestro G and Torre C: Incestuous paternity detected by STR-typing
of chorionic villi isolated from archival formalin-fixed
paraffin-embedded abortion material using laser microdissection. J
Forensic Sci. 51:90–92. 2006. View Article : Google Scholar : PubMed/NCBI
|