Introduction
Salivary glands give rise to no fewer than 30
histologically distinct tumor types. Over the years there has been
some progress in clarifying specific causes of salivary gland
cancer. The best known risk factor is that of radiation exposure as
is evident in the increased risk of post-atomic bomb survivors and
in patients who received therapeutic radiation (1). In the second half of the 20th century a
considerable number of new entities of salivary gland tumor were
added. These neoplasms are relatively uncommon, comprising <2%
of all tumors in humans. Approximately 65–80% arise within the
parotid gland, 10% within the submandibular gland and the remainder
in the minor salivary glands (2–5).
In the present study we investigated pleomorphic
adenoma (benign tumor), mucoepidermoid carcinoma, acinic cell
carcinoma, and carcinoma ex-pleomorphic adenoma. The histological
diagnosis of salivary gland tumors was performed using hematoxylin
and eosin (H&E)-stained slides. The results showed different
expression levels of Topo II-α in benign and malignant salivary
gland tumors. These differing expression levels may act as valuable
biomarkers for the correct histological diagnosis.
Materials and methods
Topoisomerase II-α
Topoisomerase is a nuclear enzyme important for DNA
replication, transcription, recombination, and mitosis.
Topoisomerases introduce supercoils that release the strain caused
by over winding. Type I Topoisomerases relax supercoiled structures
while type II Topoisomerase (also known as DNA gyrase) can
introduce negative supercoiling through coupling of ATP hydrolysis.
Therefore, negative supercoiling is introduced by Topoisomerase II
and relaxed by Topoisomerase I (6).
Topoisomerase II has two isoenzymes, isoenzyme α exists only in
proliferating cells and has been shown to be a marker for cell
proliferation in normal and neoplastic cells (6–8). In
addition, it is involved in different signaling pathways related to
the cell cycle and apoptosis, such as extracellular
signal-regulated kinase (ERK2) (9),
NF-κB (10), and others.
Topoisomerase II-α (Topo II-α) has been extensively researched for
its role as a molecular target of chemotherapy agents. Several
studies have suggested that Topo II-α expression is related to
response to anthracycline treatment for breast cancer (11–13),
renal medullary carcinoma (14),
ovarian carcinoma (15), salivary
gland tumors (16,17), acute myeloid leukemia (18), Hodgkin's lymphoma (19), colorectal cancer (20), hepatocellular carcinoma (21), gallbladder carcinoma (22), prostate carcinoma (23), and other malignant tumors (24). Thus, these cancer types benefit from
using Topo II-α as a prognostic marker, and also for individual
tailored therapy.
Few articles available regarding the expression of
Topo II-α in salivary gland tumors. The purpose of the current
study was to determine whether the expression of Topo II-α can be
used as a marker to differentiate between benign and malignant
salivary gland tumors, and as a prognostic marker. To the best of
our knowledge, our research is the most extensive one to examine
the expression of Topo II-α in salivary gland tumors.
Patients
A total of 59 consecutive cases of salivary gland
tumors, 34 women and 25 men (median age, 57 years), diagnosed at
‘Rabin Medical Center’ during the years 1991–2010, were examined.
All the tumors were diagnosed following a superficial or radical
parotidectomy, except one which was diagnosed after a base of
tongue biopsy. Surgical specimens were obtained directly from the
operation room, fixed in 4% buffered formaldehyde and embedded in
paraffin for examination in conventional H&E staining. The
specimens were reviewed in order to confirm the diagnosis.
Immunohistochemistry
The representative paraffin embedded blocks were cut
into 3–5 µm sections. Immunostaining was carried out using an
antibody commercial kit (cat. no. NCL-TOPOIIAp; Novocastra
Laboratories Ltd., Newcastle upon Tyne, England), containing a
mouse anti-rabbit polyclonal antibody against a recombinant protein
corresponding to the C-terminal region of the Topo II-α molecule.
The procedure was performed with an automated immunohistochemistry
system (Ventana medical systems Inc., Tucson, AZ, USA) in
accordance with the manufacturer's instructions. High-grade colon
carcinoma specimens were used as control for stain adequacy.
Sections of positive and negative control tissues and non-malignant
tissue of the resected salivary gland specimen were also
included.
Microscopy and staining
evaluation
To assess the level of Topo II-α expression, five
representative high power fields (×400) were examined. For each
field, the percentage of positively stained nuclei was registered
along with the intensity of the stain. The intensity was rated 0–3
(0, for no nuclear stain; 1, for the weakest nuclear stain; and 3,
for the strongest nuclear stain) in comparison with the control
stain. A score was calculated by multiplying the intensity of each
field with the percentage of positively stained cells and then by a
factor of 100 (i.e., if 20% of the nuclei in the slide was stained
with an intensity of 2 the slide got a score of 40) (25). The mean score of five fields was
named ‘Topo II-α index’ of the specimen. This was conducted by two
independent observers (L.R.W and R.K.), and inter-observer
concordance was >95%.
Statistical analysis
Our main aim was to assess Topo II-α expression as a
diagnostic and prognostic marker. Sensitivity and specificity of
the method in diagnosing malignant tumors were calculated, using
the pathologic diagnosis as the ‘gold standard’.
Survival curves were calculated for two groups
divided by a cut-off Topo II-α index. One group comprised malignant
tumors with an index above the cut-off index, while the second
group comprised cases of malignant tumors with an index below the
cut-off index. Survival curves were calculated using the Kaplan
Meier method and the log rank test was used to test the difference
between them to find the most significant cut-off index.
Comparisons between the Topo II-α index of the two different groups
(malignant vs. benign) were carried out using the Mann-Whitney U
test. Multivariate logistic regression analysis was conducted to
determine clinicopathological characteristics associated with a
high expression of Topo II-α. Data were arranged and analyzed using
SPSS for Windows version 16 (SPSS, Inc., Chicago, IL, USA). The
results are presented as a mean and standard deviation. The
statistical significance level was set to 0.05 (two-tailed).
Ethics approval
The present study was approved by the local Ethics
Committee in Rabin Medical Center, Petah Tikva, Israel (ministry of
health approval no. 920100120). Procedures performed in studies
involving human participants were in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. For retrospective studies formal
consent is not required.
Results
Patients
Clinicopathological data from 59 patients (34 men
and 25 women) with salivary gland tumors were included in the
present study, and the corresponding specimens were investigated
for Topo II-α expression. The median age of the overall patient
group was 57 years (interquartile range, 44–68 years) (Table I).
 | Table I.Clinicopathological data of the study
cohort. |
Table I.
Clinicopathological data of the study
cohort.
| Histological
type | No. of cases | Sex (% male) | Age, years (median,
IQR) | Tumor size, cm (mean
± SD) | Positive surgical
margins (%) |
|---|
| Pleomorphic
adenoma | 18 | 44 | 56, 42–65.3 | 2.8±1.2 | 0 |
| Acinic cell
carcinoma | 13 | 33 | 59, 43.5–69 | 3.1±0.7 | 50 |
| Mucoepidermoid
carcinoma | 15 | 23 | 52, 33.5–61.5 | 2.1±1.5 | 25 |
| Carcinoma
ex-pleomorphic adenoma | 13 | 69 | 62, 54–69 | 3.0±1.0 | 100 |
| Total | 59 | 42 | 57, 44–68 | 2.8±1.2 | 25 |
Histology
Of the total 59 tumors, 18 (30%) were pleomorphic
adenoma, 15 (24%) were mucoepidermoid carcinoma, 13 (22%) were
acinic cell carcinoma, and 13 (22%) were carcinoma ex-pleomorphic
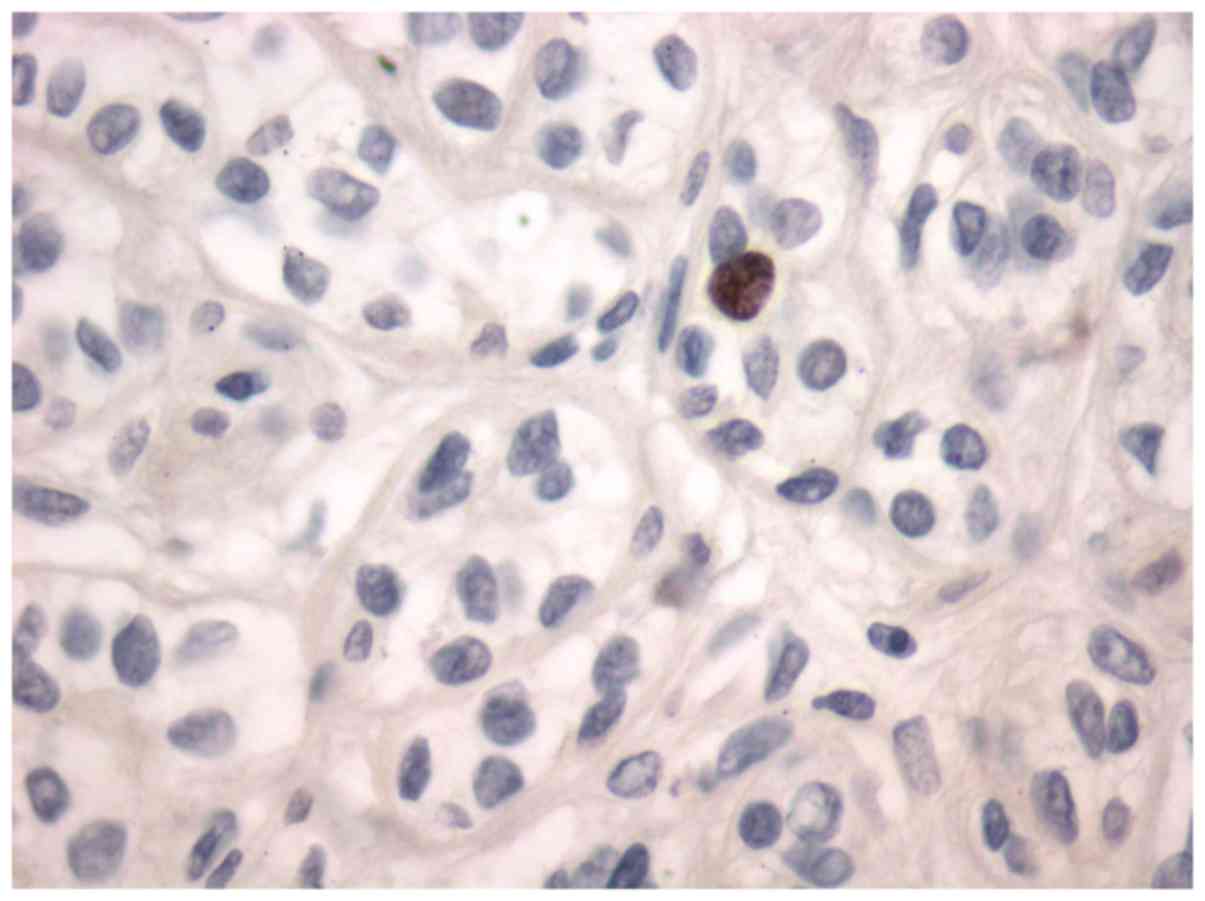
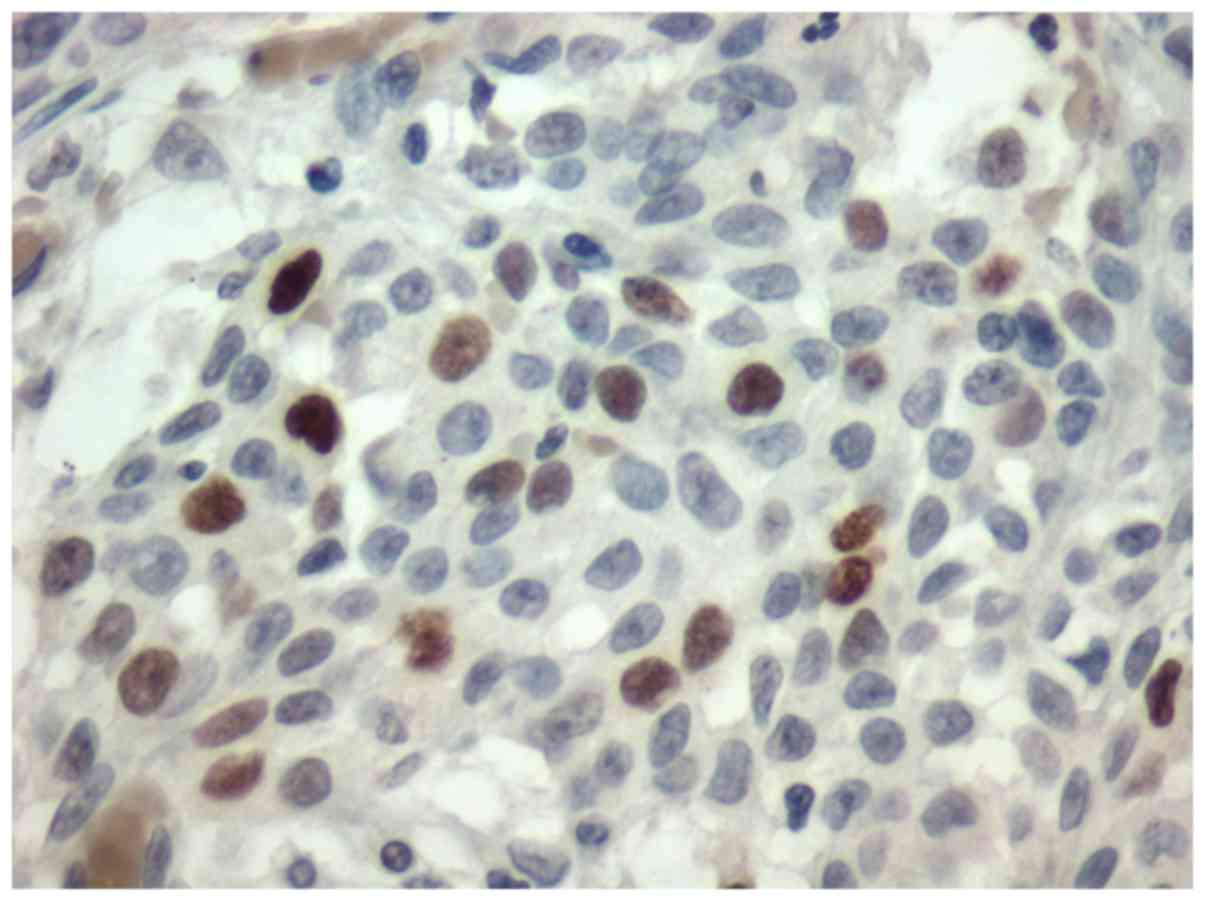
adenoma. All the tumors were stained for Topo II-α. Nuclear stain
was considered positive (Figs. 1 and
2).
Topo II-α expression
The mean expression levels of Topo II-α, presented
as the Topo II-α index, are provided in Table II. The highest expression of Topo
II-α was in the carcinoma ex-pleomorphic adenoma tumors, with a
range of 0.4–38.0. A significant difference (P<0.05) was found
between the mean expression of Topo II-α in the normal salivary
gland tissue and pleomorphic adenoma (Fig. 1) and mucoepidermoid carcinoma
(P<0.001) (Fig. 2), acinic cell
carcinoma (P<0.005), carcinoma ex-pleomorphic adenoma
(P<0.001), and a group comprising all the malignant tumors
(P<0.001). A Topo II-α index of 0.4 yielded a sensitivity of 76%
and specificity of 94% in differentiating the pleomorphic adenoma
group from the group comprising all the malignant tumors.
 | Table II.Topo II-α index according to the
histological type. |
Table II.
Topo II-α index according to the
histological type.
| Histological
type | n | Topo II-α index (mean
± SD) |
|---|
| Normal salivary
gland | 16 | 0.50±0.30 |
| Pleomorphic
adenoma | 18 | 0.13±0.20 |
| Acinic cell
carcinoma | 13 | 1.81±2.58 |
| Mucoepidermoid
carcinoma | 15 | 6.41±10.91 |
| Carcinoma
ex-pleomorphic adenoma | 13 | 8.16±12.55 |
Association between tumor grade and Topo II-α index.
Table III shows the association
between tumor grade and Topo II-α index. As the acinic cell
carcinoma grading was not uniformly accepted, this tumor was
excluded from this part of the analysis.
 | Table III.Grade and Topo II-α index summation in
mucoepidermoid carcinoma and carcinoma ex-pleomorphic adenoma. |
Table III.
Grade and Topo II-α index summation in
mucoepidermoid carcinoma and carcinoma ex-pleomorphic adenoma.
| Histological
type | Grade | No. of cases | Mean topo II-α
index |
|---|
| Mucoepidermoid | Low | 6 | 2.13 |
| Carcinoma | Intermediate and
high | 9 | 8.91 |
| Carcinoma
ex-pleomorphic | Low | 2 | 1.1 |
| adenoma | High | 11 | 9.45 |
Survival
The mean follow-up for the entire cohort with
malignant tumors was 9.1 years (range, 1.5–15). A total of 13
patients with malignant tumors died of their disease after an
average of 4.5 years (range, 0.7–12.8). We searched for the most
significant cut-off Topo II-α index to yield prognostic
significance but we were limited by the relatively small study
group and by other confounding clinic-pathological parameters known
to have prognostic significance (like age and tumor size). Serial
calculations were performed in order to find the most significant
cut-off index thich was 2.0. Between the two groups (above and
below the cut-off index) there was no significant difference in all
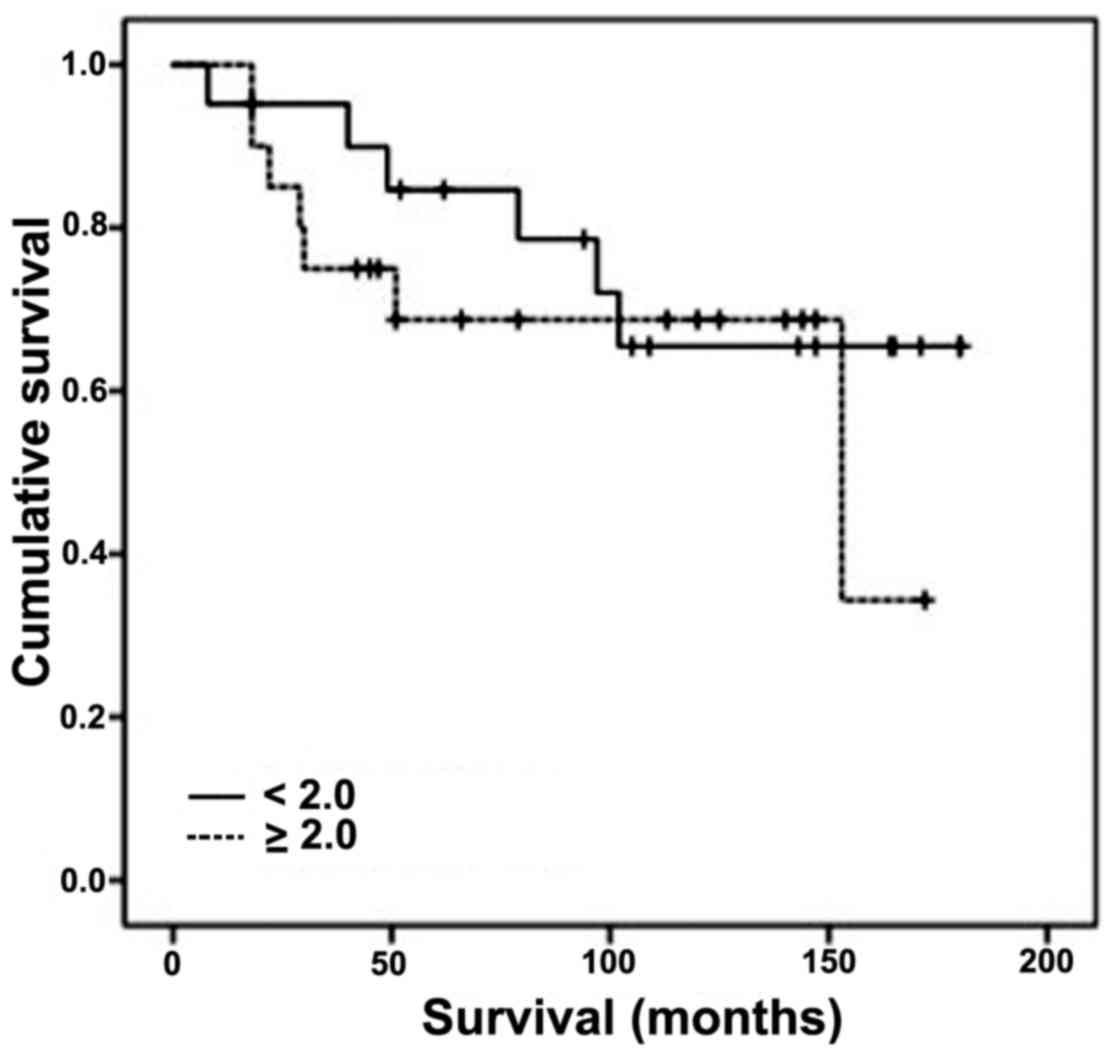
tested clinicopathologic parameters (Table IV). The 5-year cancer-specific
survival among the patients with an index ≥2.0 was 68% (20
patients), compared with 85% for those with an index <2.0 (21
patients) (Fig. 3). This difference
was not statistically significant (log rank test, P=0.464).
 | Table IV.Clinicopathological data of low and
high Topo II-α index among malignant tumors. |
Table IV.
Clinicopathological data of low and
high Topo II-α index among malignant tumors.
|
| Topo II- α index |
|
|---|
|
|
|
|
|---|
|
| <2.0 | ≥2.0 | P-value |
|---|
| Number of
patientsa | 21 | 20 |
|
| Age (average in
years) | 52.1 | 59.5 | 0.164 |
| Topo II-α index
(average ± SD) | 0.62±0.52 | 10.6±12.3 | <0.001 |
| Tumor size (average
in cm ± SD) | 2.5±0.94 | 2.9±1.2 | 0.241 |
| Positive surgical
margins | 61% | 61% | 0.981 |
Discussion
In this research, we set out to study the expression
of Topo II-α, in several salivary gland tumors. We found a
statistically significant higher expression of Topo II-α in the
malignant tumors group compared with the benign tumor pleomorphic
adenoma, with an index of 0.4 having high sensitivity (76%) and
specificity (94%) for malignancy. In a study by Maruya et al
(17), a higher expression of the
enzyme was demonstrated in malignant salivary gland tumors in
comparison to common benign tumors, but the difference was not
statistically significant due to a small study group (up to 7 cases
of each malignant tumor).
Our results show that the enzyme is expressed at
different levels in each type of malignant salivary gland tumor,
the highest expression was observed in the carcinoma ex-pleomorphic
adenoma group. This tumor is considered aggressive among salivary
gland tumors, while the other two tumors we assessed are considered
low grade tumors (2), and showed
lower indices of the enzyme. Carcinoma ex-pleomorphic adenoma
originates from the benign tumor Pleomorphic Adenoma, and can
develop after decades in which the benign tumor was steady. And so,
proper and efficient follow-up of patients suffering from
pleomorphic adenoma, can lead to early diagnosis of the malignant
transformation. Furthermore, in most cases, the malignant tumor
presents with advanced disease stage III/IV, which gives us further
motivation to early detection. These findings lead us to the
conclusion that Topo II-α expression may be used as a clinical tool
for early detection of malignant transformation, similar to
previous researches conducted on p53 (26).
In addition, in the carcinoma ex-pleomorphic adenoma
group (13 cases), we found 2 cases of low grade tumors, in which
Topo II-α index was relatively low (1.1 in average) in comparison
with the other 11 cases, which were of high grade, and showed much
higher average index of 9.45. In the research of Maryua et
al (17) their 3 cases of
Carcinoma Ex-Pleomorphic Adenoma, had all very high Topo II-α
expression. The expression of the enzyme in these tumors can imply
on its biological behavior, and help us understand the nature of
the tumor. In the future, it may lead to tailored treatment plans
according to the tumor's immunohistochemical profile, and encourage
research on targeted therapy, as is already carried out in other
malignancies such as breast, colon, and lung cancer.
Mucoepidermoid carcinoma, being the most common
malignant salivary gland tumor, is a subject of vast research. Many
studies were conducted in order to clarify its genetic and
morphologic heterogeneity and several immunohistochemical and
genetic markers have been offered as prognostic and diagnostic
markers (27,28). Our results suggest Topo II-α as a
differentiation marker between this tumor and pleomorphic adenoma.
Furthermore, its expression may help us shed light on the
biological behavior of the tumor. In addition, we found Topo II-α
variable expressions in the different grades, similar to those
results published by Maruya et al. In light of these
results, it is possible that the role of Topo II-α in this type of
tumor is complex.
The expression of Topo II-α in acinic cell carcinoma
was the lowest among all malignant tumors we tested. In general,
this tumor is considered to be of low grade, with slow and indolent
course. Therefore, the relatively low level of Topo II-α expression
matches the biological behavior of the tumor. Maruya et al
(17) also found low levels of
expression in this tumor type.
Survival rates were found to be higher in malignant
tumors expressing low levels of Topo II-α (index less than 2.0),
though not statistically significant. Higher cut-off values yielded
significantly different survival rates, but also created groups
with significant differences in other clinico-pathologic parameters
known to affect prognosis (like age and tumor size). It is possible
that with more homogeneous, larger studies, the level of Topo II-α
expression may be proven to be of prognostic significance.
In the adjacent normal salivary gland, we found low
levels of Topo II-α expression, with 93% of samples exhibiting some
level of positive staining. These levels were higher than the ones
measured in pleomorphic adenoma. We did not find other comparative
studies regarding these data, and we cannot suggest any conclusions
for this finding.
The present study is limited by its retrospective
nature, its relatively small study group and by lack of homogeneity
in the study group.
To conclude, in salivary gland tumors, Topo II-α is
expressed in different levels at different histological types. It
may be used in the histological analysis of these tumors for
differentiating benign from malignant tumors. A Topo II-α index
≥2.0 is insignificantly associated with lower 5-year cancer
specific survival rates. Salivary gland tumors with high Topo II-α
index may warrant more aggressive modalities of therapy. Further
studies need to be conducted for more conclusive results.
References
|
1
|
Zarbo RJ: Salivary gland neoplasia: A
review for the practicing pathologist. Mod Pathol. 15:298–323.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheuk W and Chan JKC: Salivary Gland
TumorsFletcher CDM: Diagnostic Histopathology of Tumors. 3rd
edition. Philadelphia: Churchill Livingstone; pp. 239–326. 2007
|
|
3
|
Lingen MW: Diseases of organ systems,
salivary glandsKumar V, Abbas AK and Fausto N: Pathologic Basis of
Disease. 7th edition. Philadelphia: Elsevier Saunders; pp. 790–795.
2005
|
|
4
|
Rosai J: Major and Minor Salivary
GlandsRosai J: Surgical Pathology. 9th edition. Philadelphia:
Elsevier Mosby; pp. 873–915. 2004
|
|
5
|
Shvero A and Hadar T: Pathology of
selected tumors of salivary gland. Med Connect. 6:29–34. 2011.
|
|
6
|
Berg JM, Tymoczko JL and Stryer L: DNA
replication, recombination and repairBerg JM, Tymoczko JL and
Stryer L: Biochemistry. 5th edition. New York: W.H.Freeman &
Co; pp. 1119–1120. 2002
|
|
7
|
Turley H, Comley M, Houlbrook S, Nozaki N,
Kikuchi A, Hickson ID, Gatter K and Harris AL: The distribution and
expression of the two isophorms of DNA topoisomerase II in normal
and neoplastic human tissues. Br J Cancer. 75:1340–1346. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiang YH, Wu HY and Liu LF:
Proliferation-dependent regulation of DNA topoisomerase II in
cultured human cells. Cancer Res. 48:3230–3235. 1988.PubMed/NCBI
|
|
9
|
Shapiro PS, Whalen AM, Tolwinski NS,
Wilsbacher J, Froelich-Ammon SJ, Garcia M, Osheroff N and Ahn NG:
Extracellular signal-regulated kinase activates topoisomerase
IIalpha through a mechanism independent of phosphorylation. Mol
Cell Biol. 19:3551–3560. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabata M, Tabata R, Grabowski DR, Bukowski
RM, Ganapathi MK and Ganapathi R: Roles of NF-kappaB and 26 S
proteasome in apoptotic cell death induced by topoisomerase I and
II Poisons in human non-small cell lung carcinoma. J Biol Chem.
276:8029–8036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikolényi A, Uhercsák G, Csenki M, Hamar
S, Csörgo E, Tánczos E, Thurzó L, Brodowicz T, Wagnerova M and
Kahán Z: Tumor topoisomerase II alpha protein expression and
outcome after adjuvant dose-dense anthracycline-based chemotherapy.
Pathol Oncol Res. 18:61–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gómez HL, Pinto JA, Olivera M, Vidaurre T,
Doimi FD, Vigil CE, Velarde RG, Abugattas JE, Alarcón E and
Vallejos CS: Topoisomerase II-α as a predictive factor of response
to therapy with anthracyclines in locally advanced breast cancer.
Breast. 20:39–45. 2001. View Article : Google Scholar
|
|
13
|
Bartlett JM, Munro AF, Dunn JA, McConkey
C, Jordan S, Twelves CJ, Cameron DA, Thomas J, Campbell FM, Rea DW,
et al: Predictive markers of anthracycline benefit: A prospectively
planned analysis of the UK national epirubicin adjuvant trial
(NEAT/BR9601). Lancet Oncol. 11:266–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaeffer EM, Guzzo TJ, Furge KA, Netto G,
Westphal M, Dykema K, Yang X, Zhou M, Teh BT and Pavlovich CP:
Renal medullary carcinoma: Molecular, pathological and clinical
evidence for treatment with topoisomerase-inhibiting therapy. BJU
Int. 106:62–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrandina G, Petrillo M, Carbone A,
Zannoni G, Martinelli E, Prisco M, Pignata S, Breda E, Savarese A
and Scambia G: Prognostic role of topoisomerase-IIalpha in advanced
ovarian cancer patients. Br J Cancer. 98:1910–1915. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirabayashi S: Immunohistochemical
detection of DNA topoisomerase type II alpha and Ki-67 in adenoid
cystic carcinoma and pleomorphic adenoma of the salivary gland. J
Oral Pathol Med. 28:131–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maruya S, Shirasaki T, Nagaki T, Kakehata
S, Kurotaki H, Mizukami H and Shinkawa H: Differential expression
of topoisomerase IIalpha protein in salivary gland carcinomas:
Histogenetic and prognostic implications. BMC Cancer. 9:722009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen CC, Gau JP, You JY, Lee KD, Yu YB, Lu
CH, Lin JT, Lan C, Lo WH, Liu JM and Yang CF: Prognostic
significance of beta-catenin and topoisomerase IIalpha in de novo
acute myeloid leukemia. Am J Hematol. 84:87–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doussis-Anagnostopoulou IA,
Vassilakopoulos TP, Thymara I, Korkolopoulou P, Angelopoulou MK,
Siakantaris MP, Kokoris SI, Dimitriadou EM, Kalpadakis C,
Matzouranis M, et al: Topoisomerase IIalpha expression as an
independent prognostic factor in Hodgkin's lymphoma. Clin Cancer
Res. 14:1759–1766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coss A, Tosetto M, Fox EJ, Sapetto-Rebow
B, Gorman S, Kennedy BN, Lloyd AT, Hyland JM, O'Donoghue DP,
Sheahan K, et al: Increased topoisomerase IIalpha expression in
colorectal cancer is associated with advanced disease and
chemotherapeutic resistance via inhibition of apoptosis. Cancer
Lett. 276:228–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong N, Yeo W, Wong WL, Wong NL, Chan KY,
Mo FK, Koh J, Chan SL, Chan AT, Lai PB, et al: TOP2A overexpression
in hepatocellular carcinoma correlates with early age onset,
shorter patients survival and chemoresistance. Int J Cancer.
124:644–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Washiro M, Ohtsuka M, Kimura F, Shimizu H,
Yoshidome H, Sugimoto T, Seki N and Miyazaki M: Upregulation of
topoisomerase IIalpha expression in advanced gallbladder carcinoma:
A potential chemotherapeutic target. J Cancer Res Clin Oncol.
134:793–801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hughes C, Murphy A, Martin C, Fox E, Ring
M, Sheils O, Loftus B and O'Leary J: Topoisomerase II-alpha
expression increases with increasing Gleason score and with hormone
insensitivity in prostate carcinoma. J Clin Pathol. 59:721–724.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dekel Y, Frede T, Kugel V, Neumann G,
Rassweiler J and Koren R: Human DNA topoisomerase II-alpha
expression in laparoscopically treated renal cell carcinoma. Oncol
Rep. 14:271–274. 2005.PubMed/NCBI
|
|
25
|
Rath-Wolfson L, Purim O, Ram E,
Morgenstern S, Koren R and Brenner B: Expression of estrogen
receptor β1 in colorectal cancer: Correlation with
clinicopathological variables. Oncol Rep. 27:2017–2022.
2012.PubMed/NCBI
|
|
26
|
Ohtaké S, Cheng J, Ida H, Suzuki M,
Ohshiro K, Zhang W and Saku T: Precancerous foci in pleomorphic
adenoma of the salivary gland: Recognition of focal carcinoma and
atypical tumor cells by P53 immunohistochemistry. J Oral Pathol
Med. 31:590–597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen LH, Black MJ, Hier M, Chauvin P and
Rochon L: HER2/neu and Ki-67 as prognostic indicators in
mucoepidermoid carcinoma of salivary glands. J Otolaryngol.
32:328–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shemirani N, Osipov V, Kolker A, Khampang
P and Kerschner JE: Expression of mucin (MUC) genes in
mucoepidermoid carcinoma. Laryngoscope. 121:167–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|