Introduction
Cholangiocarcinoma (CC) is the second most common
primary liver malignancy (1). CC
arises from the biliary ductal epithelium and may be localized in
the liver biliary tract (intrahepatic CC) or the hepatic hilum
(hilar CC). Common presentations of CC include obstructive
jaundice, biliary sepsis, liver failure and pain. The treatment
goal for CC is resection with negative histological margins
(2). However, despite the advances
in radiological diagnostic modalities and interventions, only a
minority of the patients are considered eligible for curative
surgery at presentation.
The lymphatics are a common route for the metastatic
spread of CC, with the most frequent metastatic sites being the
liver, abdominal lymph nodes, peritoneum and lungs. Cutaneous
metastasis of CC is rare, and reportedly occurs most commonly
following percutaneous biliary drainage (3). Brain or skull metastasis of this tumor
is uncommon (4,5); however, these rare metastatic lesions
may occasionally be the first manifestation of the disease
(6), although scalp recurrence
following curative treatment is uncommon (4,7).
Patients with CC are evaluated preoperatively
according to their medical performance, radiological findings and
future liver remnant. Medically unfit patients, cases with distant
metastatic lesions (non-satellite hepatic lesions, lymph node
metastases beyond the portal vein or hepatic artery, or distant
site or organ metastases), patients with extensive local
involvement or inadequate future liver remnant, and cases
exhibiting major vascular invasion, are considered to be
unresectable. The CC resectability criteria (8) are listed in Table I.
 | Table I.Criteria of cholangiocarcinoma
unresectability. |
Table I.
Criteria of cholangiocarcinoma
unresectability.
| Periferal or
hilar | Distal |
|---|
| Medically unfit
patients | Medically unfit
patients |
|---|
| Distant metastatic
disease | Distant metastatic
disease |
|
Non-satellite hepatic
metastases | Distant metastasis
(liver or other organs) |
| Lymph
node metastases beyond portal vein, hepatic artery (peripancreatic
and celiac axis) distribution | Lymph node metastases
beyond portal vein, hepatic artery (peripancreaticand celiac axis)
distribution |
| Distant
metastases in other organ/sites |
|
| Extensive local
involvement | Major vascular
involvement |
| Bilateral
(or contralateral) involvement of the portal vein (some rarely
resectable) hepatic artery, secondary biliary radicals | Significant
portal/superior mesenteric vein Superior mesenteric artery |
| Inadequate FLR | Common or proper
hepatic artery |
| <30%
of FLR in a patient with normal parencyhma |
|
| <2
contiguous segments with adequate portal venous and hepatic
arterial inflow, adequate hepatic venous and biliary drainage |
|
We herein present two cases of CC patients who were
evaluated as resectable according to the CC resectability criteria
and developed scalp recurrence following curative hepatectomy.
Case reports
Case 1
A 64-year-old female patient was admitted to the
Department of General Surgery of Liv Hospital (Istanbul, Turkey)
with severe right upper quadrant abdominal pain in November 2013.
An intrahepatic CC located in the right hepatic lobe was detected
and was considered to be resectable on radiological evaluation.
Informed consent was obtained from the patient and right
hepatectomy was performed. On pathological examination,
intrahepatic CC was diagnosed, with a papillary and trabecular
pattern growth pattern. The surgical margins were tumor-free.
Follow-up without adjuvant treatment was decided by the Tumor
Board. Clinical follow-up was performed quarterly. At the 6-month
and 1-year follow-up, the thoracic and abdominal computed
tomography (CT) evaluations and positron emission tomography
(PET)/CT scans were normal. At 17 months postoperatively, the
patient was admitted to another hospital with headache and a mass
in the scalp. The lesion was localized in the posterior part of the
right parietal area, it was hard, immobile and it was sized ~2×2
cm. An incisional biopsy was performed by the surgeon and the
initial diagnosis was sebaceous cyst. Histopathological evaluation
revealed that the scalp lesion was in fact a metastasis from the
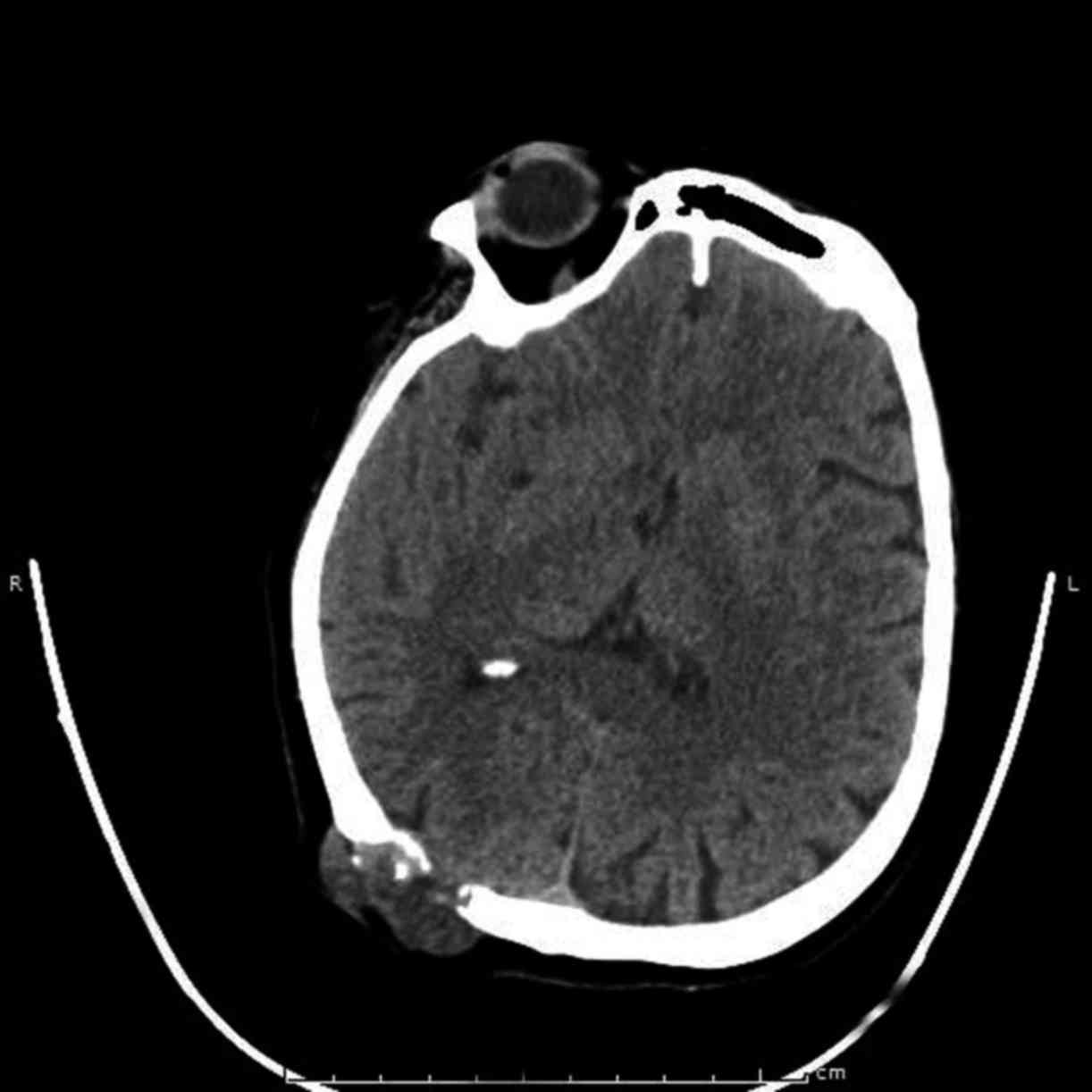
primary CC. CT scans of the brain, lung and abdomen were performed
and the mass was seen invading the skull bone and encroaching on
the dura mater (Fig. 1); in
addition, multiple lung and iliac crest metastases were detected. A
PET-CT scan also revealed involvement of the skull bone by the
mass. Radiotherapy at a total dose of 2,500 Gy was delivered to the
scalp as palliative therapy. The patient also received adjuvant
chemotherapy [6 cycles of gemcitabine and cisplatin and oxaliplatin
+ leucovorin + 5-fluorouracil (FOLFOX6)]. At 43 months after
hepatectomy, the patient had a stable skull lesion after
radiotherapy. The two lung metastases and one iliac crest
metastasis also remained stable. The last follow-up was in June
2017 and the patient remains on capecitabine treatment per os.
Case 2
A 58-year-old female patient was admitted to the
Department of Surgery of Liv Hospital (Istanbul, Turkey) with
jaundice in September 2014. Radiological evaluation revealed a
hilar CC extending to the left intrahepatic biliary system.
Informed consent was obtained from the patient, and left and
caudate lobe hepatectomy and periportal lymphadenectomy were
performed. CC was diagnosed following pathological examination of
the resected specimen. The surgical margins were tumor-free;
however, 5 of the 6 harvested lymph nodes were metastatic. Adjuvant
gemcitabine chemotherapy was administered, according to the
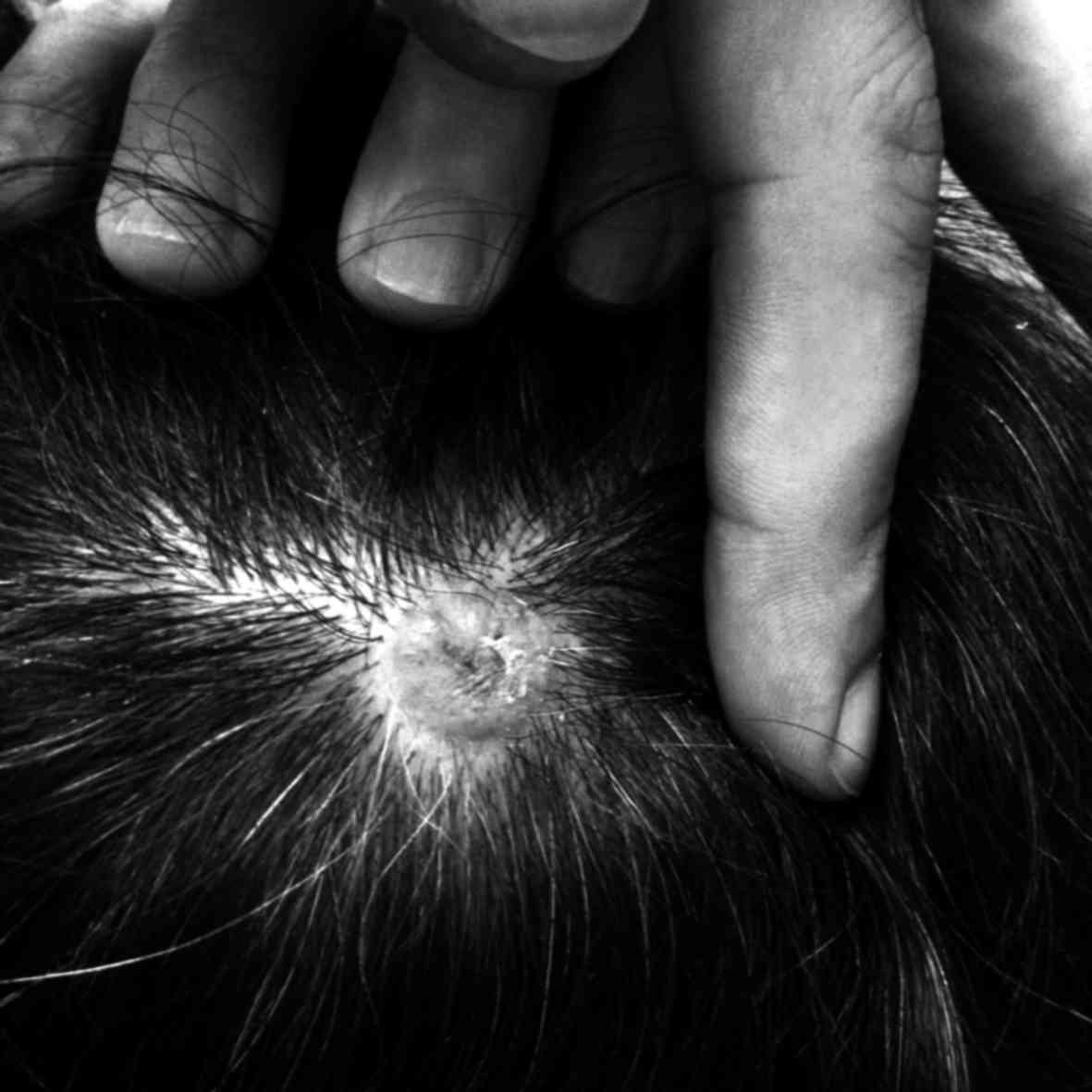
decision of the Tumor Board. At the 6-month follow-up, a painful
scalp mass was detected at the vertex (Fig. 2). The mass was excised with negative
surgical margins, and the pathological examination confirmed that
it was a metastasis from the primary CC. There were no metastatic
lesions on PET/CT. At 30 months after hepatectomy, the patient
remained on chemotherapy. At the last follow-up (March 2017) there
was no disease recurrence.
Discussion
The majority of metastatic skull lesions originate
from breast cancer (9), whereas
skull metastasis from CC is a rare entity (4). Skin metastasis of CC may develop
following percutaneous transhepatic biliary drainage or along the
tract of percutaneous transhepatic cholangiography, and its
incidence is 0.6–6% (10); however,
skull metastasis of CC is rarer than skin metastasis.
Skull metastasis may occur either via the lymphatic
route or the cerebrospinal venous system (CSVS) (11,12). The
CSVS has 2 main divisions: One includes the cortical veins, dural
sinuses and cavernous sinuses, and the other is the vertebral
venous system, which includes the vertebral venous plexus (Batson
plexus) (12). The main
characteristic of the Batson plexus is lack of venous valves. This
lack of venous valves causes bidirectional blood flow (11,13). The
increase in the intra-abdominal pressure leads to retrograde venous
flow in the CSVS, and the tumor cells may bypass the lungs and
brain and metastasize to the skull (11–13).
Port site seeding during percutaneous transhepatic
biliary drainage (PTBD) or percutaneous transhepatic
cholangiography (performed as palliative treatment in patients with
unresectable disease or to reduce serum bilirubin preoperatively in
resectable cases), is a major issue in CC.
A retrospective analysis of 67 patients who
underwent PTBD for extrahepatic CC identified 3 patients with
catheter tract implantation metastases, presenting as subcutaneous
nodules (10). In addition, there
have been reports of metastatic seeding at the abdominal wall and
peritoneum, chest wall and pleural space, and liver parenchyma,
following PTBD (14–16). Sakamoto conducted a prospective study
over a 22-year period, in which 7 of 206 (3.4%) patients who
underwent PTBD developed tumor seeding in the PTBD sinus tract as a
late complication (17). These
findings suggest that the incidence of metastatic tumor seeding
along the biliary catheter tract is not as low as initially
suspected. A report demonstrated that patients whose bile was
positive for tumor cells preoperatively were at higher risk of
developing peritoneal metastases (18), whereas a study by Mizuno et al
demonstrated that ~30–47% of patients with malignant tumors had
tumor cells in the bile (19). Based
on these findings, it is likely that metastasis in these patients
developed via the hematogenous route, through the valveless Batson
venous plexus.
Due to the lack of large case series on the
treatment of skull metastasis of CC, there is currently no
established treatment protocol for skull metastasis from primary
CC. However, there are four treatment modalities used for patients
with skull metastases: Surgery, irradiation, chemotherapy and
hormonal therapy (4). We believe
that, as the main treatment for CC is surgical resection with
negative margins, isolated skull metastasis should be resected with
clear margins whenever possible.
In conclusion, in CC patients with scalp lesions,
skull metastasis from the primary CC should be included in the
differential diagnosis and the patient should be evaluated with
cranial CT and PET/CT scans. Written consent for the publication of
the case details was obtained from the patients.
References
|
1
|
Bartella I and Dufour JF: Clinical
diagnosis and staging of intrahepatic cholangiocarcinoma. J
Gastrointestin Liver Dis. 24:481–489. 2015.PubMed/NCBI
|
|
2
|
Lafaro K, Grandhi MS, Herman JM and Pawlik
TM: The importance of surgical margins in primary malignancies of
the liver. J Surg Oncol. 113:296–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosich-Medina A, Liau SS, Jah A, Huguet E,
See TC, Jamieson N and Praseedom R: Cutaneous metastases from
cholangiocarcinoma following percutaneous transhepatic biliary
drainage: Case report and literature review. Int J Surg Case Rep.
1:33–36. 2010. View Article : Google Scholar
|
|
4
|
Fujimoto K, Kuroda J, Makino K, Hasegawa Y
and Kuratsu J: Skull metastasis from intrahepatic
cholangiocarcinoma: Report of 3 cases and review of the literature.
Neurol Med Chir (Tokyo). 53:717–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamura Y, Harada A, Maeda A, Fujioka A,
Horiba T, Ishigure K, Hirai A, Ito Y and Uesaka K: Carcinomatous
meningitis secondary to cholangiocarcinoma without other systemic
metastasis. J Hepatobiliary Pancreat Surg. 15:237–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hyun SY, Lee JH, Shin HS, Lee SW, Park YN
and Park JY: Cutaneous metastasis from cholangiocarcinoma as the
first clinical sign: A report of two cases. Gut Liver. 5:100–104.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyamoto J, Tatsuzawa K, Sasajima H and
Mineura K: Metastatic skull tumor from cholangiocarcinoma. Case
report. Neurol Med Chir (Tokyo). 47:132–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schulick RD: Criteria of unresectability
and the decision-making process. HPB (Oxford). 10:122–125. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsuya K, Nakasu Y, Horiguchi S, Harada
H, Nishimura T, Yuen S, Asakura K and Endo M: Metastatic skull
tumors: MRI features and a new conventional classification. J
Neurooncol. 104:239–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakata J, Shirai Y, Wakai T, Nomura T,
Sakata E and Hatakeyama K: Catheter tract implantation metastases
associated with percutaneous biliary drainage for extrahepatic
cholangiocarcinoma. World J Gastroenterol. 11:7024–7027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batson OV: The function of the vertebral
veins and their role in the spread of metastases. Ann Surg.
112:138–149. 1940. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tobinick E and Vega CP: The cerebrospinal
venous system: Anatomy, physiology, and clinical implications. Med
Gen Med. 8:532006.
|
|
13
|
Coman DR and deLONG RP: The role of the
vertebral venous system in the metastasis of cancer to the spinal
column; experiments with tumor-cell suspensions in rats and
rabbits. Cancer. 4:610–618. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller GA Jr, Heaston DK, Moore AV Jr,
Mills SR and Dunnick NR: Peritoneal seeding of cholangiocarcinoma
in patients with percutaneous biliary drainage. AJR Am J
Roentgenol. 141:561–562. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uenishi T, Hirohashi K, Inoue K, Tanaka H,
Kubo S, Shuto T, Yamamoto T, Kaneko M and Kinoshita H: Pleural
dissemination as a complication of preoperative percutaneous
transhepatic biliary drainage for hilar cholangiocarcinoma: Report
of a case. Surg Today. 31:174–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu Y, Yasui K, Kato T, Yamamura Y,
Hirai T, Kodera Y, Kanemitsu Y, Ito S, Shibata N, Yamao K and
Ohhashi K: Implantation metastasis along the percutaneous
transhepatic biliary drainage sinus tract. Hepatogastroenterology.
51:365–367. 2004.PubMed/NCBI
|
|
17
|
Sakamoto E, Hayakawa N, Kamiya J, Kondo S,
Nagino M, Kanai M, Miyachi M, Uesaka K and Nimura Y: Treatment
strategy for mucin-producing intrahepatic cholangiocarcinoma: Value
of percutaneous transhepatic biliary drainage and cholangioscopy.
World J Surg. 23:1038–1044. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka N, Nobori M and Suzuki Y: Does bile
spillage during an operation present a risk for peritoneal
metastasis in bile duct carcinoma? Surg Today. 27:1010–1014. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizuno T, Ishizaki Y, Komuro Y, Yoshimoto
J, Sugo H, Miwa K and Kawasaki S: Surgical treatment of abdominal
wall tumor seeding after percutaneous transhepatic biliary
drainage. Am J Surg. 193:511–513. 2007. View Article : Google Scholar : PubMed/NCBI
|