Introduction
Recently, the incidences of ovarian carcinoma have
been increasing (1). The prognoses
of ovarian carcinoma have not been improved in spite of development
for anti-cancer treatment, particularly in advanced stages
(2). Several predictive factors for
prognoses in ovarian carcinomas have been identified: FIGO stage,
residual tumor diameter, and histological subtypes, and so on
(3,4). Among all histological subtypes, clear
cell carcinoma (CCC) has been recognized as a subtype showing worse
prognoses (3). Additionally, CCC is
a distinct subtype with lower response and short response duration
even in responder against chemotherapy (3,5,6).
Endometriosis is well-known as precursor of CCC
(7,8), and there was a report suggesting that
genetic back ground in CCC derived from endometriosis was different
from those without endometriosis (7). Some reports have shown negative
correlation of endometriosis with prognoses in CCC (9–11), and
other investigations have shown positive association with better
prognoses (12–15). So the clinical impact of the
complication with endometriosis in upon prognosis of CCC has not
been determined.
Recently, it has been pointed out that
post-progression survival (PPS), defined as duration from the date
of recurrence to the date of death, was relatively longer in
ovarian cancers (16). Herein, we
investigated the relationship with complication with endometriosis,
and clinicopathological factors in CCC, including evaluation of
PPS.
Patients and methods
Patients and treatment
The cases with CCC who received primary debulking
surgery and adjuvant chemotherapy at our institution between 199 0
and 2013 were identified, and medical charts of the cases were
retrospectively reviewed.
The patients with endometriosis was defined as the
coexistence with CCC and endometriosis in the same and/or
heterolateral ovary, and/or coexistence with CCC and extraovarian
endometriosis (EM-group) (9,11,15). The
patients without endometriosis were defined as non-EM group.
Staging was evaluated according to International Federation of
Gynecology and Obstetrics (FIGO) staging system 2014. Adjuvant
chemotherapy was classified into two categories: Conventional
platinum-based, and taxane-platinum chemotherapy. The regimens of
conventional platinum-based chemotherapy included cyclophosphamide
plus platinum (CP), CP plus doxorubicin (CAP), epirubicin plus
platinum (EP), and irinotecan plus platinum. Taxane-platinum
chemotherapy included paclitaxel plus carboplatin, and docetaxel
plus carboplatin.
All cases received chemotherapy after primary
debulking surgery. Patients were considered to be
platinum-sensitive if the time from completion of primary
chemotherapy regimen to disease recurrence/progression was more
than six months, and the cases were regarded as platinum-resistant
when the time from completion of primary chemotherapy to disease
recurrence/progression was less than 6 months. Serum levels of
tumor markers including CA125 were not used for judgement of
response to chemotherapy. This study was approved by the
institution review board of National Defense Medical College.
Statistical methods
The STAT View software ver 5.0 (SAS Institution,
Cary, NC, USA) was used for statistical analysis. The
χ2-test, Fisher's exact test, and Mann-Whitney U test
were used to evaluate clinical significance of clinicopathological
factors. Progression-free survival (PFS) was defined as the
duration from the date of the primary surgery to the date of death
or recurrence/progression of the diseases. Overall survival (OS)
was defined as the duration from the date of the primary surgery to
the date of death. Post-progression survival (PPS) was defined as
duration from the date of recurrence/progression of the disease
until the date of death. PFS, OS, and PPS curves were generated
using the method of Kaplan-Meier. Comparisons of the survival
distribution were made with log-rank test. Cox proportional hazards
model was used for multivariate analysis of PFS, OS and PPS. A
P-value of <0.05 was considered to be statistically
significant.
Results
In this study, a total of 105 cases were enrolled.
The median follow-up time was 60 months (range, 2–276 months). Of
all cases, 45 cases (42.8%) were classified into EM-group, and 60
cases (57.1%) were in non-EM group (Table I). The clinicopathological factors of
EM and non-EM patients were shown in Table I. EM-group patients were diagnosed at
younger ages (P=0.03), and at earlier stages (P<0.01), compared
with non-EM cases. Other factors such as residual tumor and
adjuvant therapy regimens were not statistically different between
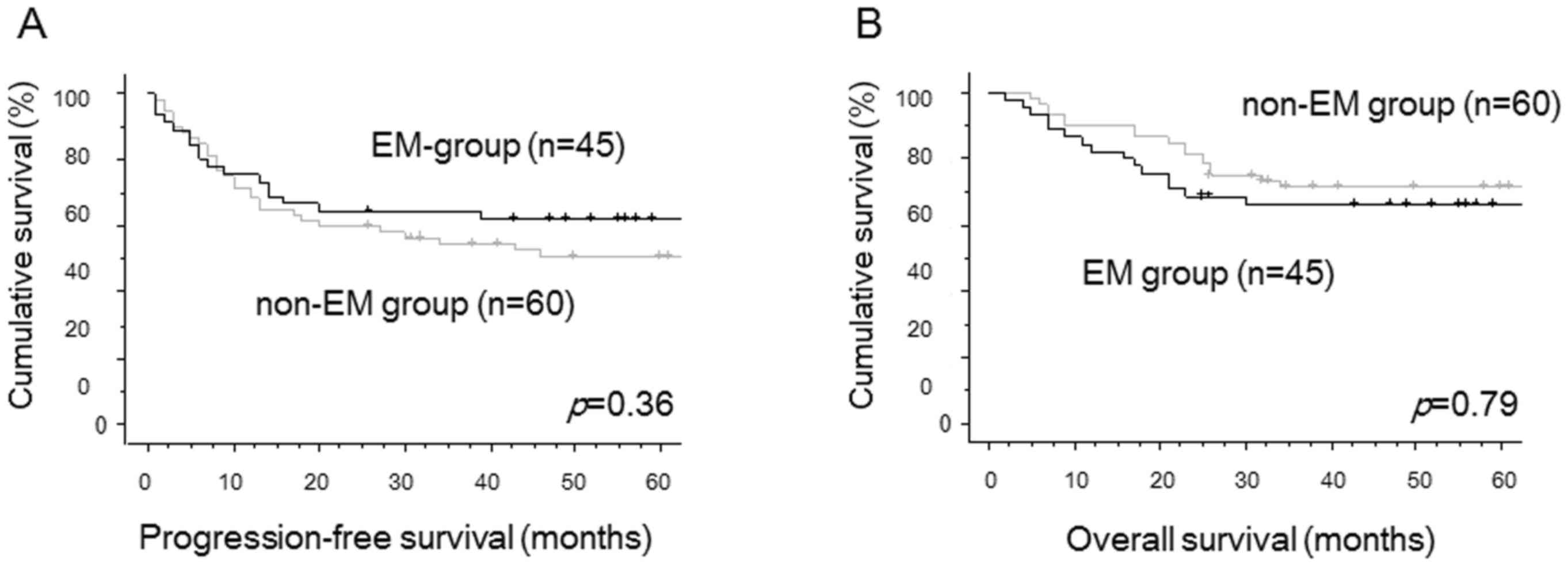
two groups. There were no significant differences in PFS (P=0.36,
Fig. 1A) and OS (P=0.79, Fig. 1B) between two groups by univariate
analysis. Using multivariate analyses for PFS, FIGO stage was
identified as the only independent prognostic factor. The
complication with endometriosis was selected as the only
independent prognostic factor for OS in multivariate analyses
(Table II).
 | Table I.Characteristics of the patients with
ovarian clear cell carcinoma. |
Table I.
Characteristics of the patients with
ovarian clear cell carcinoma.
| Variables | Patients associated
with endometriosis n=45 | Patients without
endometriosis n=60 | P-value |
|---|
| Age, mean ± SD
(range) | 51.0±9.5 (35–74) | 55.5±9.0 (32–75) | 0.03 |
| FIGO stage (%) |
|
| <0.01 |
| I | 36 (80.0) | 28 (46.6) |
|
| II | 1 (2.2) | 10 (16.7) |
|
| III | 7 (15.6) | 19 (31.7) |
|
| IV | 1 (2.2) | 3 (5.0) |
|
| Residual tumor
diameter at PDS |
|
| 0.20 |
| None
(%) | 38 (84.5) | 42 (70.0) |
|
| <1.0
cm | 1 (2.2) | 5 (8.3) |
|
| ≥1.0
cm | 6 (13.3) | 13 (21.7) |
|
| Recurrence (%) |
|
| 0.33 |
| Yes | 18 (40.0) | 30 (50.0) |
|
| No | 27 (60.0) | 30 (50.0) |
|
| Adjuvant chemotherapy
(%) |
|
| 0.40 |
|
Taxane-platinum
therapya | 16 (35.6) | 16 (26.7) |
|
|
Platinum-based
therapyb | 29 (64.4) | 44 (73.3) |
|
 | Table II.Multivariate analyses for
progression-free survival and overall survival in all patients with
ovarian clear cell carcinoma. |
Table II.
Multivariate analyses for
progression-free survival and overall survival in all patients with
ovarian clear cell carcinoma.
|
| Progression-free
survival | Overall survival |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
|
|
| 0.69 |
| >53
vs. ≤53 | 1.00 (0.44–2.30) | 0.99 | 0.69 (0.51–2.72) |
|
| FIGO stage |
|
|
| 0.12 |
| I, II vs.
III, IV | 0.45 (0.20–1.00) | 0.05 | 0.5 (0.20–1.21) |
|
| Residual tumor at
PDS |
|
|
| 0.27 |
| None vs.
macroscopic disease | 1.58 (0.70–3.58) | 0.27 | 1.64 (0.68–4.03) |
|
| Endometriosis |
|
|
| <0.01 |
|
EMa vs. non-EMb | 1.37 (0.69–2.71) | 0.36 | 3.02 (1.41–6.52) |
|
| Adjuvant
chemotherapy |
|
|
| 0.58 |
|
Taxane-platinumc vs. platinum-basedd | 1.28 (0.53–3.03) | 0.58 | 0.80 (0.29–1.91) |
|
During the study period, 48 cases developed
recurrence: 18 (40%) in 45 EM-group cases and 30 (50%) in 60 non-EM
cases. The clinicopathological features of recurrent cases were
shown in Table III. No cases
received radiation therapy and most of the cases received
chemotherapy. Some cases received secondary debulking surgery for
recurrent tumors. There were no significant differences of
clinicopathological factors including age, FIGO stage, debulking
status at primary debulking surgery, and platinum-sensitivity
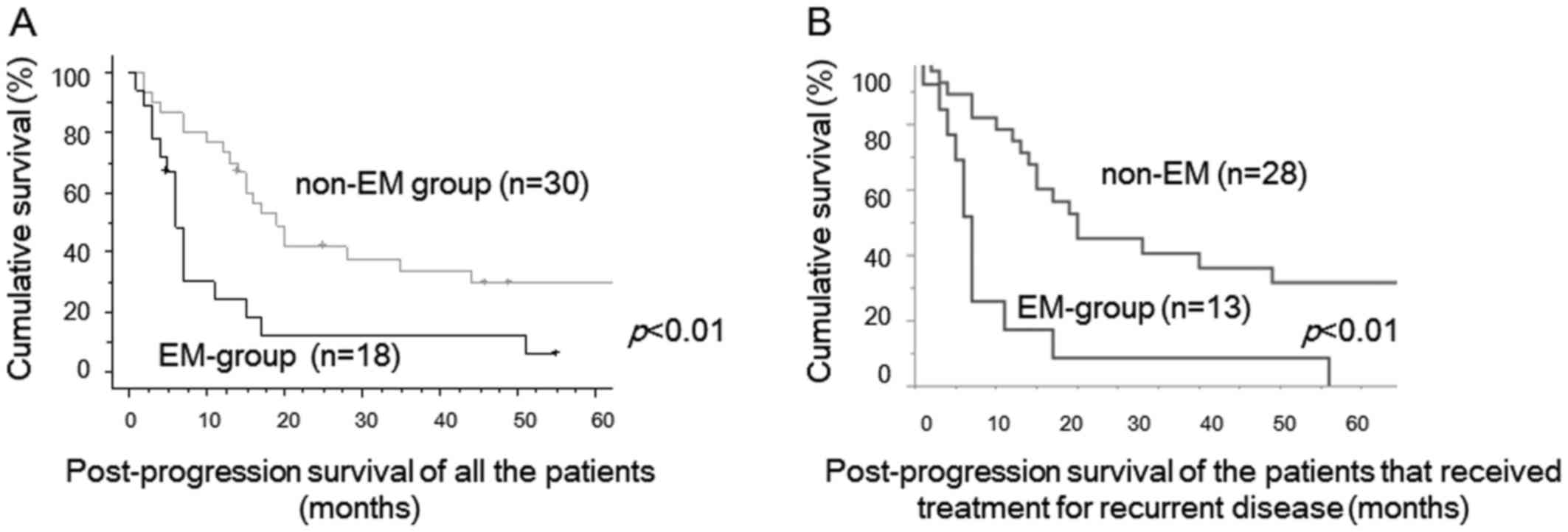
between two groups. PPS of recurrent EM-group cases was
significantly worse than that of recurrent non-EM cases (P<0.01,
Fig. 2A). Median PPS was 6 months in
recurrent EM-group, 18 months in recurrent non-EM cases,
respectively. PPS of the patients that received therapeutic
modality for recurrent tumors were shown in Figure 2B. PPS of EM-group cases was also
significantly worse than that of non-EM cases (p<0.01, Fig. 2B).
 | Table III.Characteristics of patients with
recurrent ovarian clear cell carcinoma. |
Table III.
Characteristics of patients with
recurrent ovarian clear cell carcinoma.
| Variables | Recurrent patients
associated with endometriosis n=18 | Recurrent patients
without endometriosis n=30 | P-value |
|---|
| Age, mean ± SD
(range) | 51.0±7.0
(38–63) | 55.0±9.0
(36–75) | 0.05 |
| FIGO stage (%) |
|
| 0.49 |
| I | 9 (50.0) | 9 (30.0) |
|
| II | 1 (5.6) | 5 (16.7) |
|
|
III | 7 (38.8) | 14 (46.7) |
|
| IV | 1 (5.6) | 2 (6.6) |
|
| Residual tumor
diameter at PDS (%) |
|
| 0.99 |
|
None | 11 (61.1) | 18 (60.0) |
|
| <1.0
cm | 1 (5.6) | 2 (6.7) |
|
| ≥1.0
cm | 6 (33.3) | 10 (33.3) |
|
| Treatment at
recurrence (%) |
|
| 0.08 |
|
Chemotherapya | 11 (61.1) | 26 (86.6) |
|
|
Secondary debulking
surgery | 2 (11.1) | 2 (6.7) |
|
| Not
done | 5 (27.8) | 2 (6.7) |
|
|
Platinum-sensitivityb (%) |
|
| 0.99 |
|
Sensitive | 8 (44.4) | 14 (46.7) |
|
|
Resistant | 10 (55.6) | 16 (53.3) |
|
Multivariate analysis for PPS revealed that
complication with endometriosis was an independent worse prognostic
factor (Hazard ratio, 4.57; 95% Confidence Interval, 1.93–10.97;
P<0.01), in addition to platinum-sensitivity (Table IV).
 | Table IV.Multivariate analysis for
post-progression survival in recurrent cases with ovarian clear
cell carcinoma. |
Table IV.
Multivariate analysis for
post-progression survival in recurrent cases with ovarian clear
cell carcinoma.
|
| Post-progression
survival |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Age (years) |
| 0.71 |
| >53
vs. ≤53 | 0.87
(0.43–1.76) |
|
| FIGO stage |
| 0.24 |
| I, II
vs. III, IV | 0.57
(0.22–1.47) |
|
| Residual tumor at
PDS |
| 0.53 |
| None
vs. macroscopic disease | 0.74
(0.29–1.88) |
|
| Endometriosis |
| <0.01 |
|
EMa vs. non-EMb | 4.57
(1.93–10.97) |
|
|
Platinum-sensitivityc |
| <0.01 |
|
Sensitive vs. resistant | 0.32
(0.14–0.73) |
|
| Treatment at
recurrence |
| 0.43 |
| Done
vs. not done | 1.46
(0.59–4.18) |
|
Discussion
In the present study, the cases with EM were
diagnosed at younger age and at earlier stages. Nevertheless, the
complication with endometriosis was not a predictive factor of
prognosis including PFS and OS. Until now, several studies had
evaluated clinical significance of the correlation with
endometriosis in prognoses of CCC, however, the conclusions have
not been determined (7,11,14,16).
According to recent meta-analysis conducted by Kim et al,
the complication with endometriosis was not related with prognosis
in all subtypes of ovarian cancers (7).
PPS of clear cell carcinoma was considered to be
shorter than that of serous carcinoma (17). This reason was caused by not only
lower response rate, regardless of platinum-sensitivity status, but
also short response duration in cases with recurrent CCC (3,5,9). In general, PPS of ovarian carcinomas
including all histological subtypes was >20 months (18). However, PPS of CCC was much shorter,
especially in the cases with endometriosis. A report suggested that
PPS was more highly associated than PFS with OS in the first-line
chemotherapy for advanced epithelial ovarian cancer (18). Short PPS in CCC could have led to
shorter OS in CCC cases, especially in EM-group patients. Although
genetic difference in the tissue specimens was not evaluated in the
present study, a report suggested that some patients carried driver
mutations in ARID1A, PIK3CA, KRAS genes in benign deep infiltrating
endometriosis (19). It is
speculated that tumor samples of EM-group had potentially
aggressive phenotype compared with non-EM group tumors.
Although there was no difference in PFS between
EM-group and non-EM group, PPS was significantly shorter in
EM-group patients. Primary therapy using anti-cancer agents might
alter genetic profile in CCC tumors, and cause refractory phenotype
after recurrence. A report suggested CCC patients with Met
gene amplification showed chemoresistant phenotype and worse
prognosis (19). Thus, the
alteration of genetic profiles affected by primary therapy might
influence PPS in the patients with CCC. Additionally,
phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway, and the
receptor tyrosine kinase (RTK)/Ras signaling pathway were
identified as prognostic biomarkers for CCC tumors using
whole-genome sequencing (21). Novel
chemotherapeutic agents inhibiting these pathways might improve
survival of the CCC patients.
The limitation of this study included a
retrospective study and a small number of the patients enrolled in
a single-institutional analysis. Further prospective investigation
is needed to confirm the impact of complication with endometriosis
upon PPS of CCC patients.
In conclusion, the complication with endometriosis
was the independent poor prognostic factor for PPS in CCC. Longer
PPS could have led to longer OS in EM-group CCC. Further
prospective investigation is necessary to confirm the significance
of complication with endometriosis on PPS in CCC and develop the
new therapy for recurrent CCC with endometriosis should also be
considered.
Acknowledgements
The present study has been edited and corrected by
an experienced proofreader who is a native speaker of English and
who is under the direct supervision of Honyaku Center Inc. (Osaka,
Japan).
References
|
1
|
Permuth-Wey J and Sellers TA: Epidemiology
of ovarian cancer. Methods Mol Biol. 472:413–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynecol Obstet. 95 Suppl
1:S161–S192. 2006. View Article : Google Scholar
|
|
3
|
Takano M, Sugiyama T, Yaegashi N, Sakuma
M, Suzuki M, Saga Y, Kuzuya K, Kigawa J, Shimada M, Tsuda H, et al:
Low response rate of second-line chemotherapy for recurrent or
refractory clear cell carcinoma of the ovary: A retrospective Japan
clear cell carcinoma study. Int J Gynecol Cancer. 18:937–942. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo PE, Mourregot A, Fabbro M,
Gutowski M, Saint-Aubert B, Quenet F, Gourgou S and Rouanet P:
Aggressive surgical strategies in advanced ovarian cancer: A
monocentric study of 203 stage IIIC and IV patients. Eur J Surg
Oncol. 35:135–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takano M, Goto T, Kato M, Sasaki N,
Miyamoto M and Furuya K: Short response duration even in responders
to chemotherapy using conventional cytotoxic agents in recurrent or
refractory clear cell carcinomas of the ovary. Int J Clin Oncol.
18:556–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyamoto M, Takano M, Goto T, Kato M,
Sasaki N, Tsuda H and Furuya K: Clear cell histology as a poor
prognostic factor for advanced epithelial ovarian cancer: A single
institutional case series through central pathologic review. J
Gynecol Oncol. 24:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HS, Kim TH, Chung HH and Song YS: Risk
and prognosis of ovarian cancer in women with endometriosis: A
meta-analysis. Br J Cancer. 110:1878–1890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HS, Kim MA, Lee M, Suh DH, Kim K, No
JH, Chung HH, Kim YB and Song YS: Effect of endometriosis on the
prognosis of ovarian clear cell carcinoma: A two-center cohort
study and meta-analysis. Ann Surg Oncol. 22:2738–2745. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar S, Munkarah A, Arabi H,
Bandyopadhyay S, Semaan A, Hayek K, Garg G, Morris R and Ali-Fehmi
R: Prognostic analysis of ovarian cancer associated with
endometriosis. Am J Obstet Gynecol. 204(63): e1–e7. 2011.
|
|
11
|
Orezzoli JP, Russell AH, Oliva E, Del
Carmen MG, Eichhorn J and Fuller AF: Prognostic implication of
endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol.
110:336–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komiyama S, Aoki D, Tominaga E, Susumu N,
Udagawa Y and Nozawa S: Prognosis of Japanese patients with ovarian
clear cell carcinoma associated with pelvic endometriosis:
Clinicopathologic evaluation. Gynecol Oncol. 72:342–346. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erzen M, Rakar S, Klancnik B and Syrjänen
K: Endometriosis-associated ovarian carcinoma (EAOC): An entity
distinct from other ovarian carcinomas as suggested by a nested
case-control study. Gynecol Oncol. 83:100–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melin A, Lundholm C, Malki N, Swahn ML,
Sparen P and Bergqvist A: Endometriosis as a prognostic factor for
cancer survival. Int J Cancer. 129:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai H, Cao D, Yuan F, Sha G, Yang J, Chen
J, Wang Y, Zhang Z and Shen K: Prognostic value of endometriosis in
patients with stage I ovarian clear cell carcinoma: Experiences at
three academic institutions. Gynecol Oncol. 143:526–531. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye S, Yang J, You Y, Cao D, Bai H, Lang J,
Chen J and Shen K: Comparative study of ovarian clear cell
carcinoma with and without endometriosis in People's Republic of
China. Fertil Steril. 102:1656–1662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kajiyama H, Shibata K, Mizuno M, Yamamoto
E, Fujiwara S, Umezu T, Suzuki S, Nakanishi T, Nagasaka T and
Kikkawa F: Postrecurrent oncologic outcome of patients with ovarian
clear cell carcinoma. Int J Gynecol Cancer. 22:801–806. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimokawa M, Ohki M and Kaku T:
Correlation of progression-free and post-progression survival with
overall survival in phase III trials of first-line chemotherapy for
advanced epithelial ovarian cancer. Eur J Gynaecol Oncol.
36:370–375. 2015.PubMed/NCBI
|
|
19
|
Anglesio MS, Papadopoulos N, Ayhan A,
Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T,
Ho J, Wu RC, Lac V, Ogawa H, Tessier-Cloutier B, Alhassan R, Wang
A, Wang Y, Cohen JD, Wong F, Hasanovic A, Orr N, Zhang M, Popoli M,
McMahon W, Wood LD, Mattox A, Allaire C, Segars J, Williams C,
Tomasetti C, Boyd N, Kinzler KW, Gilks CB, Diaz L, Wang TL,
Vogelstein B, Yong PJ, Huntsman DG and Shih IM: Cancer-Associated
Mutations in Endometriosis without Cancer. N Engl J Med.
376:1835–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita Y, Akatsuka S, Shinjo K, Yatabe
Y, Kobayashi H, Seko H, Kajiyama H, Kikkawa F, Takahashi T and
Toyokuni S: Met is the most frequently amplified gene in
endometriosis-associated ovarian clear cell adenocarcinoma and
correlates with worsened prognosis. PLoS One. 8:e577242013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Itamochi H, Oishi T, Oumi N, Takeuchi S,
Yoshihara K, Mikami M, Yaegashi N, Terao Y, Takehara K, Ushijima K,
Watari H, Aoki D, Kimura T, Nakamura T, Yokoyama Y, Kigawa J and
Sugiyama T: Br J Cancer. 2017.doi: 10.1038/bjc.2017.228 (Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|