Introduction
Extrahepatic bile duct carcinoma
(cholangiocarcinoma) is an epithelial cancer that originates from
the bile ducts and exhibits features of cholangiocytic
differentiation. Its incidence rate has no significant geographical
variation. It accounts for 0.16 and 0.15% of all invasive cancers
in males and females, respectively, in the USA (1). Despite recent advances in diagnostic
and therapeutic techniques, complete surgical resection of the
tumor remains the best way to cure extrahepatic bile duct
carcinoma; however, even in patients who have undergone curative
resection, poor prognosis is extremely common due to the high
recurrence rate of this tumor (2–4). In a
recent study, the biliary-type histological phenotype was reported
to be a factor for poor prognosis in diseases such as intraductal
papillary mucinous neoplasm (IPMN) (5) and gallbladder cancer (6). The classification of histological
phenotypic subtype of IPMN is performed based on pancreatic IPMN;
tumors are classified into four types according to histological
cell morphology: Pancreaticobiliary type, intestinal type, gastric
type, and oncocytic type (7).
Different histological subtypes have a tendency to occur at
different primary sites, such as branch-duct type and main-duct
type, and have varying incidence rates of malignant transformation
(8). On the other hand, intraductal
papillary neoplasm of the bile duct has also been accepted as a
counterpart of pancreatic IPMN, and the concept of the phenotypic
classification has now been introduced for bile duct tumors
(3,9). However, the clinicopathological
features and prognosis associated with the phenotype of
extrahepatic cholangiocarcinoma have not been clarified. Therefore,
in the present study, the phenotypes of patients with extrahepatic
cholangiocarcinoma who underwent macroscopic curative resection
were classified, and the clinicopathological features and prognosis
were examined accordingly in order to clarify the significance of
phenotypic classification.
Patients and methods
Ethics statement
The ethics committee of the Hirosaki University
Graduate School of Medicine approved the current study (approval
number. 2017-1006).
Patients and samples
A total of 99 consecutive bile duct carcinoma
surgical cases treated between January 2005 and December 2011 were
investigated, after obtaining each patient's informed consent for
use of their clinical records and pathological specimens at
Hirosaki University Hospital. The series consisted of 72 men and 27
women with a median age of 68 years (range, 31–83 years). The
carcinomas were located in the perihilar (32 cases) and distal bile
duct (67 cases). The clinicopathological features of the patients
are summarized in Table I. Curative
resection and regional lymph node dissection were dependent on the
location of the primary tumor: Pancreaticoduodenectomy or
pylorus-preserving pancreaticoduodenectomy was performed in 61
patients, bile duct resection in 1 patient, combined hepatectomy
with bile duct resection in 30 patients, and combined hepatectomy
and pancreaticoduodenectomy in 7 patients. Survival data were
obtained from hospital medical charts, and the median observation
period was 31 months.
 | Table I.Patient characteristics (n=99). |
Table I.
Patient characteristics (n=99).
| Characteristic | Value |
|---|
| Sex, n |
|
| Male | 72 |
|
Female | 27 |
| Age (years), n |
|
| ≥70 | 44 |
|
<70 | 55 |
| Location, n |
|
|
Hilar | 32 |
|
Distal | 67 |
| Size (mm) |
|
| Mean | 33 |
|
Range | 10–85 |
| Carcinoembryonic
antigen (ng/ml), n |
|
|
<5 | 81 |
| ≥5 | 18 |
| Carbohydrate antigen
19-9 (U/ml), n |
|
|
<100 | 69 |
| ≥100 | 30 |
| Superficial
spreading |
|
|
Positive | 45 |
|
Negative | 54 |
| Histological
differentiation |
|
| Pap,
well, mod | 83 |
| Por,
others | 16 |
| Phenotype |
|
| Biliary
type | 56 |
| Gastric
type | 42 |
|
Intestinal type | 1 |
| pT classification,
n |
|
|
pT1-2 | 49 |
|
pT3-4 | 50 |
| pN classification,
n |
|
| pN0 | 64 |
| pN1 | 35 |
| pM classification,
n |
|
| pM0 | 94 |
| pM1 | 5 |
| Lymphatic invasion,
n |
|
|
ly0-1 | 55 |
|
ly2-3 | 44 |
| Venous vessel
invasion, n |
|
| v0-1 | 53 |
| v2-3 | 46 |
| Neural invasion,
n |
|
|
ne0-1 | 34 |
|
ne2-3 | 65 |
Pathological analysis
All surgically resected specimens were routinely
fixed with 10% formalin, then embedded in paraffin and stained with
hematoxylin and eosin for pathological evaluation. The following
histological features were assessed: Depth of invasion (T-stage),
histological differentiation, lymphovascular invasion (ly), venous
vessel invasion (v), perineural invasion (ne), lymph node
metastasis (N) and histological phenotype. Histological phenotype
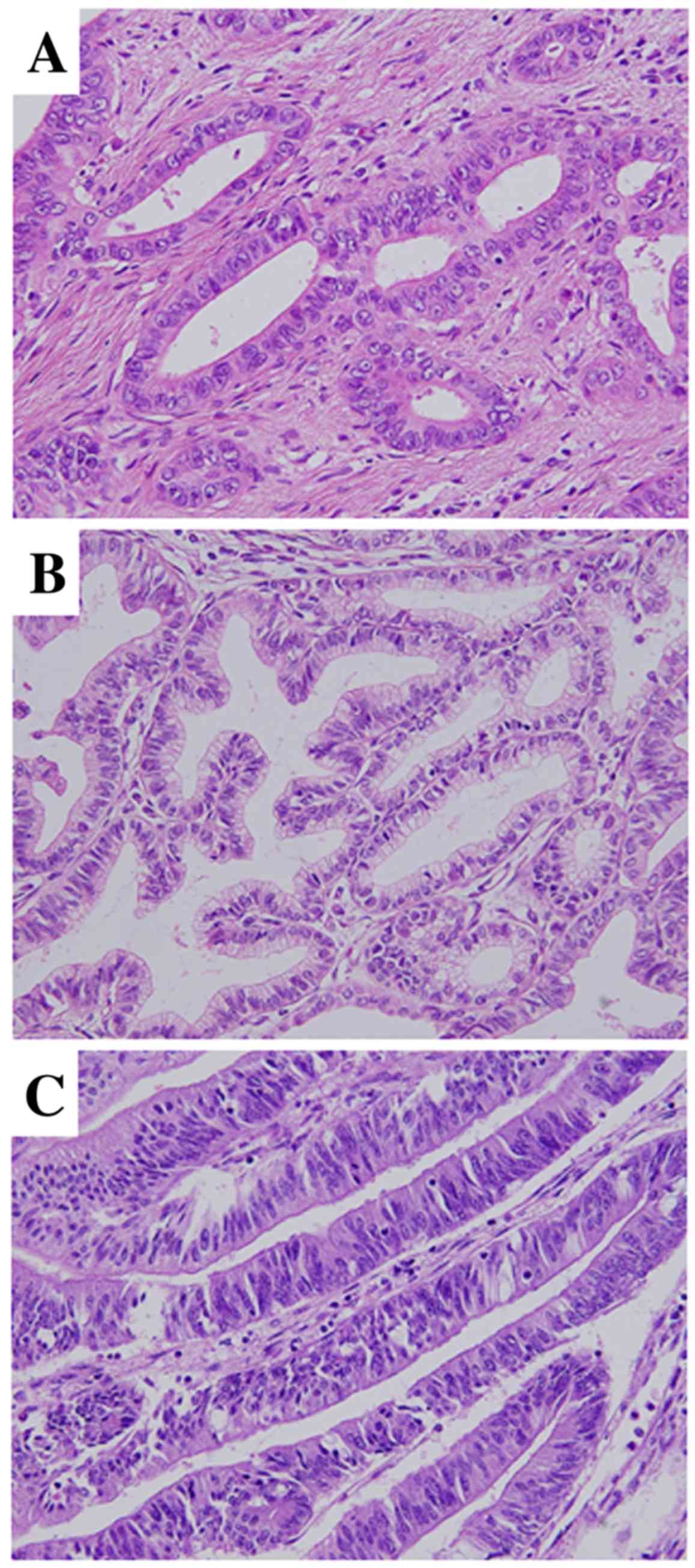
was defined as biliary type (BT) or metaplastic type (MT), as
follows: BT is composed of short or long tubular glands lined by
cells that vary in height from cuboidal to tall columnar,
superficially resembling biliary epithelium (Fig. 1A); and MT comprises gastric-type [GT;
composed of tall columnar cells with basally oriented nuclei and
abundant mucin-containing cytoplasm (Fig. 1B)] and intestinal-type [IT; composed
of tubular glands closely resembling those of colonic
adenocarcinomas (Fig. 1C); the
glands are lined predominantly by columnar cells with
pseudostratified ovoid or elongated nuclei]. These data were
evaluated according to the General Rules for Surgical and
Pathological Studies on Cancer of the Biliary Tract (10) with reference to the World Health
Organization classification (11),
and were staged according to the Tumor-Node-Metastasis
classification of the International Union Against Cancer (12).
Immunohistochemistry
For histological examination, extrahepatic bile duct
carcinoma specimens were routinely fixed with formalin, embedded in
paraffin, sectioned to a thickness of 4-µm, and mounted on
saline-coated glass slides. Immunohistochemical examination was
performed on deparaffinized sections using the standard
avidin-biotin-peroxidase complex method with a BenchMark XT
automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ,
USA). The different phenotypes were investigated for mucin (MUC)
expression using primary antibodies against MUC1 (#NCL-MUC-1,
dilution, 1:50; clone Ma696), MUC2 (#NCL-MUC-2, dilution, 1:50;
clone Ccp), MUC5AC (#NCL-MUC-5AC, dilution, 1:100; clone CLH2) and
MUC6 (#NCL-MUC-6, dilution, 1:100; clone CLH5), all purchased from
Novocastra (Leica Biosystems, Newcastle, UK). After washing in PBS
three times, secondary immunostaining was performed with an i-VIEW
DAB Universal Kit (Roche Diagnostics, Tokyo, Japan) for 28 min at
42°C.
Evaluation of
immunohistochemistry
Three evaluators, who were blinded to the clinical
characteristics of the patients, assessed all 99 specimens. MUC1
was determined to be positive in the presence of luminal membranous
immunoreactivity of the tumor, whereas the cytoplasmic
immunoreactivities were considered when determining MUC2, MUC5AC
and MUC6 positivity. The results were classified into groups based
on the percentage of positively stained cells, as follows: Negative
group, <5% of cancer cells stained; and positive group, ≥5% of
cells stained.
Statistical analysis
Statistical comparisons between two groups were
analyzed using the Pearson's χ2 test for categorical
data and the Student's t-test for continuous data. Survival curves
were constructed using the Kaplan-Meier method. The Cox
proportional hazards model was used for multivariate analysis.
Differences were considered to be statistically significant when
P<0.05. All statistical evaluations were performed using SPSS
software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Clinicopathological features according
to cholangiocarcinoma phenotype
The clinicopathological findings pertaining to
patients with BT and MT tumors are summarized in Table II. In total, 56 patients had BT
cholangiocarcinoma and 43 patients had MT cholangiocarcinoma (42
patients with GT and 1 patient with IT). The mean tumor diameter
was 37.8 mm (range, 10–75 mm) in BT, and 34.4 mm (range, 13–85 mm)
in MT, with no significant difference observed (P=0.307). Carcinoma
in situ developed in 26 patients with BT (46.4%), and 19
patients with MT (44.2%; P=0.826). No significant differences were
observed in the levels of carcinoembryonic antigen (cut-off value,
5 ng/ml; P=0.950), and carbohydrate antigen 19-9 (cut-off value,
100 U/ml; P=0.673) between the BT and MT groups. With regard to
T-stage, pT3-4 cancer was observed in 32 patients with BT (57.1% of
group), and 17 patients with MT (39.5% of group), with no
significant difference observed (P=0.084). Regarding lymphatic
invasion, ly2-3 was observed in 29 patients with BT (51.8% of
group), and 15 patients with MT (34.9% of group), with no
significant difference observed (P=0.095). However, significant
differences between the two groups were observed for four factors:
Histological differentiation [papillary adenocarcinoma or
well/moderately differentiated adenocarcinoma observed in 45
patients with BT (80.4%) and 38 patients with MT (88.4%); P=0.018];
N stage [pN1 observed in 24 patients with BT (42.9%) and 13 patents
with MT (30.2%); P=0.042]; venous invasion [v2-3 observed in 36
patients with BT (64.3%) and 10 patients with MT (23.3%);
P<0.001]; and perineural invasion [ne2-3 observed in 44 patients
with BT (78.6%) and 21 patients with MT (48.8%); P=0.002].
 | Table II.Clinicopathological features according
to histological phenotype in cholangiocarcinoma. |
Table II.
Clinicopathological features according
to histological phenotype in cholangiocarcinoma.
| Feature | Biliary type | Metaplastic type | P-value |
|---|
| Total patients,
n | 56 | 43 | – |
| Sex, n (%) |
|
| 0.206 |
| Male | 43 (76.8) | 29 (67.4) |
|
|
Female | 13 (23.2) | 14 (32.6) |
|
| Age (years), n
(%) |
|
| 0.230 |
| ≥70 | 29 (51.8) | 17 (39.5) |
|
|
<70 | 27 (48.2) | 26 (60.5) |
|
| Location, n (%) |
|
| 0.770 |
|
Hilar | 17 (30.4) | 15 (34.9) |
|
|
Distal | 39 (69.6) | 28 (65.1) |
|
| Size (mm) |
|
|
|
|
Mean | 37.8 | 34.4 | 0.307 |
|
Range | 10–75 | 13–85 |
|
| Carcinoembryonic
antigen (ng/ml), n (%) |
|
| 0.950 |
|
<5 | 51 (91.1) | 39 (90.7) |
|
| ≥5 | 5 (8.9) | 4 (9.3) |
|
| Carbohydrate
antigen 19-9 (U/ml), n (%) |
|
| 0.673 |
|
<100 | 40 (71.4) | 29 (67.4) |
|
|
≥100 | 16 (28.6) | 14 (32.6) |
|
| Carcinoma in
situ, n (%) |
|
| 0.826 |
|
Positive | 26 (46.4) | 19 (44.2) |
|
|
Negative | 30 (53.6) | 24 (55.8) |
|
| Histological
differentiationa, n (%) |
|
| 0.018 |
| Pap,
well, mod | 45 (80.4) | 38 (88.4) |
|
| Poor,
other | 11 (19.6) | 5 (11.6) |
|
| T classification, n
(%) |
|
| 0.084 |
|
pT1-2 | 24 (42.9) | 26 (60.5) |
|
|
pT3-4 | 32 (57.1) | 17 (39.5) |
|
| N classification, n
(%) |
|
| 0.042 |
|
pN0 | 32 (57.1) | 33 (76.7) |
|
|
pN1 | 24 (42.9) | 10 (23.3) |
|
| Lymphatic invasion,
n (%) |
|
| 0.095 |
|
ly0-1 | 27 (48.2) | 28 (65.1) |
|
|
ly2-3 | 29 (51.8) | 15 (34.9) |
|
| Venous vessel
invasion, n (%) |
|
| <0.001 |
|
v1-2 | 20 (35.7) | 33 (76.7) |
|
|
v2-3 | 36 (64.3) | 10 (23.3) |
|
| Perineural
invasion, n (%) |
|
| 0.002 |
|
ne0-1 | 12 (21.4) | 22 (51.2) |
|
|
ne2-3 | 44 (78.6) | 21 (48.8) |
|
MUC immunostaining according to
cholangiocarcinoma phenotype
Immunostaining for MUC1, MUC2, MUC5AC and MUC6 was
performed in three groups divided according to phenotype (BT, GT
and IT; summarized in Table III).
MUC1-positivity was observed in 45 patients (80.3%) with BT, 23
patients (54.3%) with GT, and 0 patients (0%) with IT.
MUC2-positivity was observed in 7 patients (12.5%) with BT, 7
patients (16.6%) with GT, and 1 patient (100%) with IT.
MUC5AC-positivity was observed in 18 patients (32.1%) with BT, 33
patients (78.6%) with GT, and 1 patient (100%) with IT.
MUC6-positivity was observed in 20 patients (35.7%) with BT, 27
patients (64.3%) with GT, and 0 patients (0%) with IT. Significant
differences in the ratios of tumors positively expressing MUC1,
MUC5AC and MUC6 were observed between the BT and MT groups
(P=0.004, P<0.001 and P=0.008, respectively).
 | Table III.MUC expression in cases of
cholangiocarcinoma. |
Table III.
MUC expression in cases of
cholangiocarcinoma.
|
| Cases exhibiting
positive expression, n (%) |
|
|---|
|
|
|
|
|---|
| MUC type | Metaplastic
typea (n=43) | Biliary type
(n=56) | P-value |
|---|
| MUC1 | 23 (53.5) | 45 (80.4) | 0.004 |
| MUC2 | 8
(18.6) | 7
(12.5) | 0.406 |
| MUC5AC | 34 (79.1) | 18 (32.1) | <0.001 |
| MUC6 | 27 (62.8) | 20 (35.7) | 0.008 |
Survival according to
cholangiocarcinoma phenotype
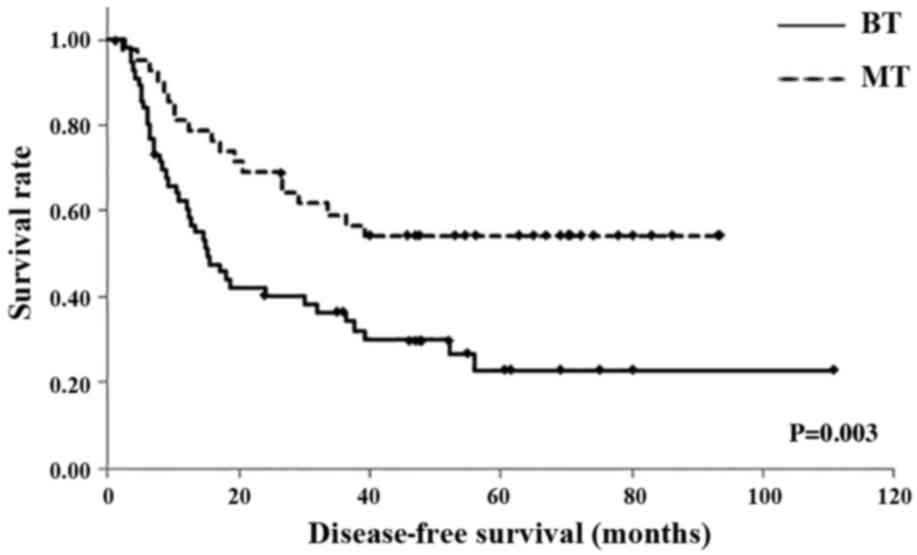
Overall survival (OS) and disease-free survival
(DFS) were evaluated in the BT and MT groups using the Kaplan-Meier
method. The 1-year DFS rates were 32.2% in the BT group and 81.0%
in the MT group; the 3-year DFS rates were 36.4% in the BT group
and 59.2% in the MT group; and the 5-year DFS rates were 22.8% in
the BT group and 54.3% in the MT group. The mean DFS times were
38.6 months [95% confidence interval (CI), 27.06–50.32 months] in
the BT group and 58.9 months (95% CI, 47.24–70.62 months) in the MT
group; the BT group exhibited a significantly shorter DFS than the
MT group (P=0.003; Fig. 2).
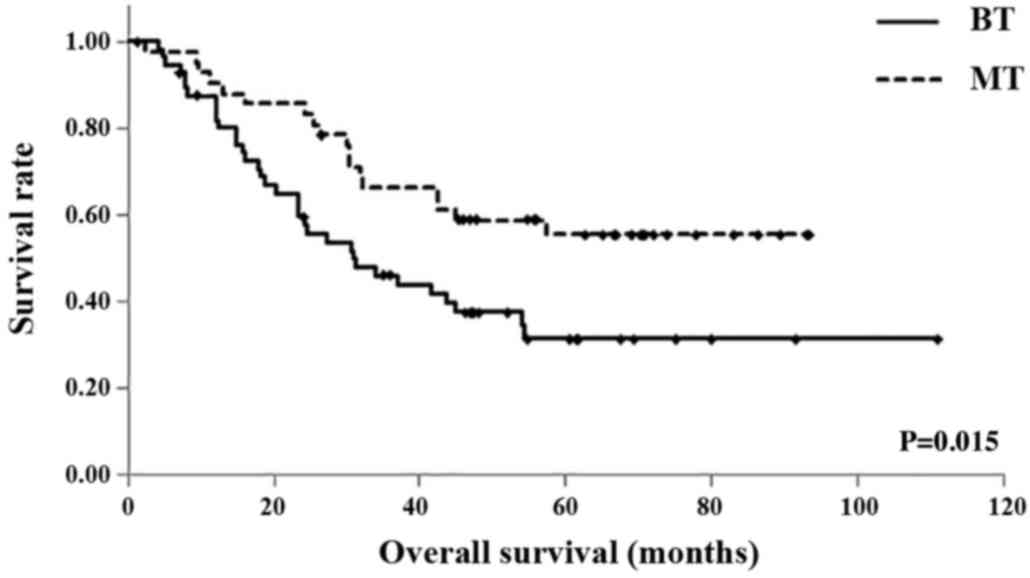
In the BT and MT groups, respectively, the 1-year OS
rates were 87.3 and 90.5%, the 3-year OS rates were 46.1 and 66.3%,
and the 5-year OS rates were 31.4 and 55.5%. The mean OS times were
51.2 months (95% CI, 39.43–62.91 months) in the BT group, and 64.0
months (95% CI, 53.55–74.51 months) in the MT group. Similarly, OS
was significantly shorter in the BT group compared with the MT
group (P=0.015; Fig. 3).
Univariate and multivariate analyses
of survival
Univariate analysis of overall survival time
following surgery using the log-rank test was performed for the 99
patients with extrahepatic cholangiocarcinoma. In addition to the
phenotype of the tumor (BT; P=0.012), the histological grade (G3-4;
P=0.018), N classification (N1; P<0.001), extent of venous
invasion (v2-3; P<0.001) and perineural invasion (ne2/3;
P=0.030) were identified as variables that were significantly
associated with poor prognosis. On multivariate analysis, N
classification [N1; P=0.020; hazard ratio (HR)=2.02 (95% CI,
1.13–3.62)] was identified as an independent prognostic factor.
Multivariate analysis of survival showed that the BT phenotype had
a HR of 0.82 (95% CI, 0.45–1.52; P=0.532, and therefore it was not
considered to be an independent prognostic factor in patients with
extrahepatic cholangiocarcinoma (Table
IV).
 | Table IV.Univariate and multivariate analyses
of overall survival. |
Table IV.
Univariate and multivariate analyses
of overall survival.
|
| Univariate
analysis | Cox proportional
hazards analysis |
|---|
|
|
|
|
|---|
| Variable | Cases, n (%) | MST (months) |
P-valuea | HR | 95% CI | P-value |
|---|
| Phenotype of
tumor |
|
| 0.015 |
|
|
|
|
Metaplastic type | 43 (43.4) | n.a. |
| – | – | – |
| Biliary
type | 56 (56.6) | 32.0 |
| 0.82 | 0.45–1.52 | 0.532 |
| Histological
grade |
|
| 0.018 |
|
|
|
|
G1-2 | 28 (28.3) | n.a. |
| – | – | – |
|
G3-4 | 71 (71.7) | 31.0 |
| 1.86 | 0.82–4.30 | 0.137 |
| N
classification |
|
| <0.001 |
|
|
|
| N0 | 65 (65.7) | n.a. |
| – | – | – |
| N1 | 34 (34.3) | 23.0 |
| 2.02 | 1.13–3.62 | 0.018 |
| Venous
infiltration |
|
| <0.001 |
|
|
|
|
v0-1 | 53 (53.5) | n.a. |
| – | – | – |
|
v2-3 | 46 (46.5) | 30.0 |
| 1.73 | 0.90–3.36 | 0.105 |
| Perineural
invasion |
|
| 0.030 |
|
|
|
|
ne0-1 | 34 (34.3) | n.a. |
| – | – | – |
|
ne2-3 | 65 (65.7) | 32.0 |
| 1.01 | 0.52–2.18 | 0.862 |
Discussion
In the present study, cholangiocarcinoma specimens
were classified into two phenotypes (BT and MT), and examined
according to the clinicopathological features and prognosis of the
patients. Compared with MT, BT tumors tended to have higher T
stages, and this was also found to be associated with increased
rates of lymph node metastasis, severe venous invasion, and severe
perineural invasion. Furthermore, with regard to OS and DFS,
survival was significantly decreased in patients with BT compared
with those with MT tumors.
In a previous study, Furukawa et al (5) reported that patients with
pancreatobiliary-type IPMN have a significantly poorer prognosis
than those with GT or IT tumors. Yamamoto et al (13) classified gallbladder carcinoma into
non-metaplastic and metaplastic types, and reported that patients
with non-metaplastic-type tumors exhibited a higher incidence of
direct invasion to the liver and significantly shorter survival
times (13). Previously, our
colleagues reported that carcinogenesis of cholangiocarcinoma can
occur via two pathways: One originating from the biliary
epithelium, in which a biliary phenotype is expressed; and one
originating from the metaplastic epithelium, in which gastric and
intestinal phenotypes are expressed (14).
In the present study, 99 specimens were classified
into three phenotypes: 56 cases of BT, 42 cases of GT, and 1 case
of IT. Focusing on the malignant potential of BT, the GT and IT
groups were combined as an MT group, as reported by Yamamoto et
al (13), and the survival
differences between the BT and MT groups were then investigated. As
a result, it was revealed that extrahepatic cholangiocarcinomas
with a BT phenotype had greater malignant potential compared with
those with the MT phenotype. However, multivariate analysis using a
Cox proportional hazards model showed that BT phenotype expression
was not an independent prognostic factor for OS, and lymph node
metastasis and venous infiltration were found to have a greater
influence on prognosis. In the BT phenotype group, features of
locally advanced disease (pN1, v2-3 and ne2-3) were more commonly
observed. Thus, it was hypothesized that the BT phenotype may be
strongly associated with multiple prognostic factors, and could not
be considered an independent prognostic factor.
In order to examine the immunohistological
differences between BT and MT, MUC protein expression was
investigated in the tumor tissues. BT tumors exhibited a
significantly higher rate of MUC1 positivity (80.4%) compared with
MT (P=0.004). Thus, there appears to be a strong association
between BT and MUC1 expression. The MUC1 protein is a MUC core
protein responsible for the mucous lining of inner cavities, such
as the gastrointestinal and respiratory tracts. MUCs are divided
into secretory MUCs and membrane-bound MUCs according to the type
of core protein. The former is a major component of mucous secreted
from epithelial cells, and primarily includes the core proteins
MUC2, MUC5AC and MUC6. On the other hand, MUC molecules of the
latter have an extracellular domain, transmembrane domain, and
intracellular domain. Membrane-bound mucins can pass through the
cell membrane, and the main core proteins include MUC1, MUC3 and
MUC4. Of particular note, membrane-bound MUC1 acts as an adhesion
molecule for cancer cells (15–17), and
is considered to contribute to extravascular migration of cancer
cells and metastasis, such as in lung, breast, gastric, pancreatic
and colorectal cancers (18).
Furthermore, research has suggested the application of MUC1 as a
tumor marker in several malignant neoplasms (19,20), as
well as a target in immunotherapy (21–23).
Park et al (24) examined the
expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma,
and reported that MUC1-positive patients exhibited considerably
advanced histological differentiation, T stage, perineural
invasion, and venous invasion; thus, MUC1 expression within tumor
tissue was reported to be a potential factor for poor
prognosis.
In the present study, it was demonstrated that BT
was strongly associated with an increased ratio of tumors with
positive MUC1 expression. Therefore, it was suggested that the
function of MUC1 as a cancer cell adhesion molecule and its
properties as a metastasis inducer contributed to high-grade
extrahepatic BT cholangiocarcinoma. Furthermore, compared with MT
tumors, it was revealed that patients with BT tumors had
significantly shorter DFS and OS times, and thus it was
hypothesized that BT could be a predictive factor for prognosis in
cases of extrahepatic cholangiocarcinoma. To the best of our
knowledge, no studies reported to date have investigated the
difference in clinicopathological features and prognosis according
to extrahepatic cholangiocarcinoma phenotype, and this is the first
study to do so.
The present study had certain limitations. First, it
was a retrospective study involving a limited number of cases.
Second, BT phenotype expression was not determined to be an
independent prognostic factor for extrahepatic cholangiocarcinoma
on multivariate analysis, and multivariate analysis indicated that
lymph node metastasis and venous infiltration had a greater
influence on prognosis. Therefore, the correlation between the
malignant potential of BT and those prognostic factors should be
clarified in future.
In conclusion, extrahepatic cholangiocarcinoma may
be classified into BT and MT phenotypes, and tumors with the BT
phenotype appear to have a higher malignant potential. Thus, the BT
phenotype could potentially be an important factor associated with
poor prognosis.
Acknowledgements
The present study was supported by Grants-in Aid for
Science from the Ministry of Education, Culture, Sports, Science
and Technology in Japan, and a Grant for Hirosaki University
Institutional Research.
References
|
1
|
World health Organization Classification
of Tumors of the Digesive System. IARC Press; Lyon: 2012
|
|
2
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higuchi R, Ota T, Araida T, Kobayashi M,
Furukawa T and Yamamoto M: Prognostic relevance of ductal margins
in operative resection of bile duct cancer. Surgery. 148:7–14.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagino M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Takahashi Y and Nimura Y: Evolution of surgical
treatment for perihilar cholangiocarcinoma: A single-center 34-year
review of 574 consecutive resections. Ann Surg. 258:129–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furukawa T, Hatori T, Fujita I, Yamamoto
M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, et
al: Prognostic relevance of morphological types of intraductal
papillary mucinous neoplasms of the pancreas. Gut. 60:509–516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toba T, Kijima H, Hakamada K and Igarashi
Y: Histological phenotype is correlated with the wall-invasion
pattern of gallbladder adenocarcinoma. Biomed Res. 35:295–302.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan
YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K, et al: Intraductal
papillary neoplasia of the liver associated with hepatolithiasis.
Hepatology. 34:651–658. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki Y, Atomi Y, Sugiyama M, Isaji S,
Inui K, Kimura W, Sunamura M, Furukawa T, Yanagisawa A, Ariyama J,
et al: Cystic neoplasm of the pancreas: A Japanese
multiinstitutional study of intraductal papillary mucinous tumor
and mucinous cystic tumor. Pancreas. 28:241–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furukawa T, Klöppel G, Adsay N Volkan,
Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y,
Klimstra DS, Longnecker DS, et al: Classification of types of
intraductal papillary-mucinous neoplasm of the pancreas: A
consensus study. Virchows Arch. 447:794–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surgery JSoB: Classification of biliary
tract carcinoma. 2nd. Kanehara and Co., Ltd.; Tokyo: 2004
|
|
11
|
Albores-Saavedra J, Adsay NV, Crawford JM,
Klimstra DS and Kloppel G: World health organization of
classification of tumors of the digestive system. IARC; Lyon:
2010
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours (UICC). 7th.
Wiler-Liss; New York: 2009
|
|
13
|
Yamamoto M, Nakajo S and Tahara E:
Carcinoma of the gallbladder: The correlation between histogenesis
and prognosis. Virchows Arch A Pathol Anat Histopathol. 414:83–90.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haga T, Yoshizawa T, Morohashi S, Hirai H,
Saitou K, Ota R, Takatsuna A, Wu Y, Fukuda S and Kijima H:
Phenotypic characterization of early biliarytract carcinomas
proposes two carcinogenesis pathways. Hirosaki Med J. 67:28–38.
2016.
|
|
15
|
Sawada T, Ho JJ, Chung YS, Sowa M and Kim
YS: E-selectin binding by pancreatic tumor cells is inhibited by
cancer sera. Int J Cancer. 57:901–907. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wesseling J, van der Valk SW, Vos HL,
Sonnenberg A and Hilkens J: Episialin (MUC1) overexpression
inhibits integrin-mediated cell adhesion to extracellular matrix
components. J Cell Biol. 129:255–265. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hudson MJ, Stamp GW, Chaudhary KS, Hewitt
R, Stubbs AP, Abel PD and Lalani EN: Human MUC1 mucin: A potent
glandular morphogen. J Pathol. 194:373–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu F, Liu F, Zhao H, An G and Feng G:
Prognostic significance of mucin antigen MUC1 in various human
epithelial cancers: A meta-analysis. Medicine (Baltimore).
94:e22862015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campos LC, Silva JO, Santos FS, Araújo MR,
Lavalle GE, Ferreira E and Cassali GD: Prognostic significance of
tissue and serum HER2 and MUC1 in canine mammary cancer. J Vet
Diagn Invest. 27:531–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Hu YM, Du YJ, Zhu LR, Qian H, Wu Y
and Shi WL: Expressions of MUC1 and vascular endothelial growth
factor mRNA in blood are biomarkers for predicting efficacy of
gefitinib treatment in non-small cell lung cancer. Bmc Cancer.
14:8482014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jerome KR, Barnd DL, Bendt KM, Boyer CM,
Taylor-Papadimitriou J, McKenzie IF, Bast RC Jr and Finn OJ:
Cytotoxic T-lymphocytes derived from patients with breast
adenocarcinoma recognize an epitope present on the protein core of
a mucin molecule preferentially expressed by malignant cells.
Cancer Res. 51:2908–2916. 1991.PubMed/NCBI
|
|
22
|
Kontani K, Taguchi O, Narita T, Izawa M,
Hiraiwa N, Zenita K, Takeuchi T, Murai H, Miura S and Kannagi R:
Modulation of MUC1 mucin as an escape mechanism of breast cancer
cells from autologous cytotoxic T-lymphocytes. Br J Cancer.
84:1258–1264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato Y: Efficacy of WT1 peptide-/MUC-1
peptide-pulsed dendritic cell therapy in 313 patients with a wide
range of cancers. Gan To Kagaku Ryoho. 41:1280–1282. 2014.(In
Japanese). PubMed/NCBI
|
|
24
|
Park SY, Roh SJ, Kim YN, Kim SZ, Park HS,
Jang KY, Chung MJ, Kang MJ, Lee DG and Moon WS: Expression of MUC1,
MUC2, MUC5AC and MUC6 in cholangiocarcinoma: Prognostic impact.
Oncol Rep. 22:649–657. 2009.PubMed/NCBI
|