Introduction
The prevalence of obesity and being overweight,
defined by a high body mass index (BMI), have been increasing
regardless of sex, age and whether people live in developed or
developing countries (1). Health
problems related to being obese have become an issue worldwide. The
complications of obesity, such as cardiovascular disorder, diabetes
and other metabolic diseases, frequently arise at a lower BMI in
Asian nations than they do in Europe and North America (2); therefore, more attention is required
for this in Asia. Obesity has been recognized as a notable risk
factor for various malignant tumors, among which colon and rectal
cancer, endometrial cancer and postmenopausal breast cancer are
well known (3). Obesity is also a
well-known risk factor for the development of renal cell carcinoma
(RCC) (3–5).
The majority of studies investigating the
association between obesity and survival in prostate cancer and
colorectal cancer have suggested poorer rather than better survival
for obese patients (6,7). Regarding RCC, however, an inverse
relationship between BMI and prognosis has been reported, not only
in the United States and European countries (8–10), but
also in Asia (11,12). These studies demonstrated that
overweight and obese patients were less likely to present with
aggressive forms of tumors or have poor survival (9,13).
RCC may present with a wide variety of
paraneoplastic symptoms, and anorexia-cachexia syndrome (ACS) is
one of the most common (14,15). ACS is a complex metabolic disorder,
involving loss of adipose tissue due to lipolysis, loss of skeletal
muscle mass, elevation of resting energy expenditure, anorexia and
reduction in food intake (16). As a
result, patients with ACS lose weight and tend to have a low BMI.
As patients with ACS have markedly shorter survival in RCC
(14,15), we postulated that the association of
low BMI with tumor aggressiveness and poor prognosis may be due to
ACS. The purpose of the present study was to evaluate the
association of BMI with tumor aggressiveness and prognosis in RCC
while taking ACS into account.
Patients and methods
Ethics statement
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Ethics Committee of National Defense Medical College (Tokorozawa,
Japan) and with the 1964 Helsinki declaration and its later
amendments. Informed consent was obtained from all individual
participants included in the study.
Patients
A total of 503 consecutive patients (374 males and
129 females; mean age at surgery, 62.5 years) who underwent radical
or partial nephrectomy for RCC at the National Defense Medical
College Hospital between November 1983 and January 2014 were
retrospectively reviewed. All tumor tissues were evaluated for
pathological staging and histological grading according to the TNM
classification (17), and the cases
before 2010 were re-described according to the TNM classification
by the Pathology Department of our institution. Age, sex, Eastern
Cooperative Oncology Group Performance Status (ECOG-PS) scale
(18), the presence or absence of
ACS at initial presentation, BMI, regional lymph node (LN)
involvement, presence of distant metastasis and various
pathological parameters were assessed. ACS at the initial
presentation was defined as the presence of anorexia or malaise,
and/or weight loss and/or hypoalbuminemia (14,15).
Weight loss was defined as unintentional weight decrease within
several months, regardless of the amount. Anorexia was defined as
abnormal loss of the appetite for food, and malaise was defined as
fatigue or general body discomfort. Hypoalbuminemia was defined as
a preoperative serum albumin level <3.8 mg/dl. BMI was estimated
at the time of surgery by dividing the patient's weight in kg by
the square of the patient's height in m. Patients were grouped into
the following four categories based on the World Health
Organization (WHO) classification for Asian populations:
Underweight (BMI<18.5 kg/m2); normal weight
(BMI=18.5–23 kg/m2); overweight (23≤BMI<25
kg/m2), obese (BMI≥25 kg/m2) (19).
Statistical analysis
Data were presented as the mean ± standard
deviation. The Mann-Whitney U test was used for comparisons of
continuous variables among clinical and pathological parameter
groups. The Kaplan-Meier method with the log-rank test was used to
compare overall survival (OS) rates between RCC patient groups.
Univariate and multivariate Cox proportional hazard models were
used to identify which clinical and pathological parameters,
including BMI and ACS, independently predicted OS and
cancer-specific survival (CSS). All statistical analyses were
performed using JMP® 10 (SAS Institute, Inc., Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The patient characteristics and pathological
parameters are listed in Table I.
The present cohort comprised 374 males (74.4%) and 129 females
(25.6%), and the mean age at surgery was 62.5 years (range, 29–89
years). Mean follow-up duration from the date of surgery to the
last recorded follow-up was 59.3 months (range, 0.1–248.4 months),
there were 65 mortalities as a result of cancer, and there were 11
mortalities due to other causes. Anorexia or malaise (37 patients,
7.4%), weight loss (31 patients, 6.2%) and hypoalbuminemia (71
patients, 14.1%) were observed at initial presentation, and 90
patients (17.9%) were considered to have ACS according to our
definition. Of the 503 patients in this cohort, 424 (84.3%) had
clear cell type tumors, 175 (34.8%) had high-grade tumors, 398
(79.1%) had pathological T stage 1–2 tumors, 15 (3.0%) had LN
metastasis and 57 (11.3%) had distant metastasis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | n (%) |
|---|
| Total | 503 |
| Sex |
|
| Male | 374 (74.4) |
|
Female | 129 (25.6) |
| Eastern Cooperative
Oncology Group PS |
|
|
PS0-1 | 482 (95.8) |
|
PS2-4 | 21 (4.2) |
| Anorexia or
malaise |
|
|
Negative | 466 (92.6) |
|
Positive | 37 (7.4) |
| Weight loss |
|
|
Negative | 472 (93.8) |
|
Positive | 31 (6.2) |
| Hypoalbuminemia |
|
|
Negative | 432 (85.9) |
|
Positive | 71
(14.1) |
| Anorexia-cachexia
syndrome |
|
|
Negative | 413 (82.1) |
|
Positive | 90
(17.9) |
| BMI,
kg/m2 |
|
|
Underweight (<18.5) | 36 (7.2) |
| Normal
(18.5≤BMI<23) | 218 (43.3) |
|
Overweight (23≤BMI<25) | 130 (25.8) |
| Obese
(BMI≥25) | 119 (23.7) |
| Histological
type |
|
| Clear
cell | 424 (84.3) |
|
Other | 79
(15.7) |
| Grade |
|
| Low
(G1-2) | 328 (65.2) |
| High
(G3) | 175 (34.8) |
| Pathological T
stage |
|
|
pT1-2 | 398 (79.1) |
|
pT3-4 | 105 (20.9) |
| Venous
invasion |
|
|
Negative | 288 (57.3) |
|
Positive | 215 (42.7) |
| Growth pattern |
|
|
Expansive | 334 (66.4) |
|
Infiltrative | 169 (33.6) |
| Regional lymph node
involvement |
|
|
Negative | 488 (97.0) |
|
Positive | 15 (3.0) |
| Distant
metastasis |
|
|
Negative | 446 (88.7) |
|
Positive | 57
(11.3) |
Association of BMI with pathological
parameters and clinical outcome
Lower BMI has been reported to be associated with
aggressive forms of RCC and with shorter survival (9–13). The
association between BMI and clinicopathological parameters in our
cohort is presented in Table II. In
accordance with the results of previous studies, BMI was
significantly lower in patients with high-grade tumors (P=0.0027),
patients with distant metastasis at the time of surgery (P=0.0025)
and patients having tumors with an infiltrative pattern (P=0.0453).
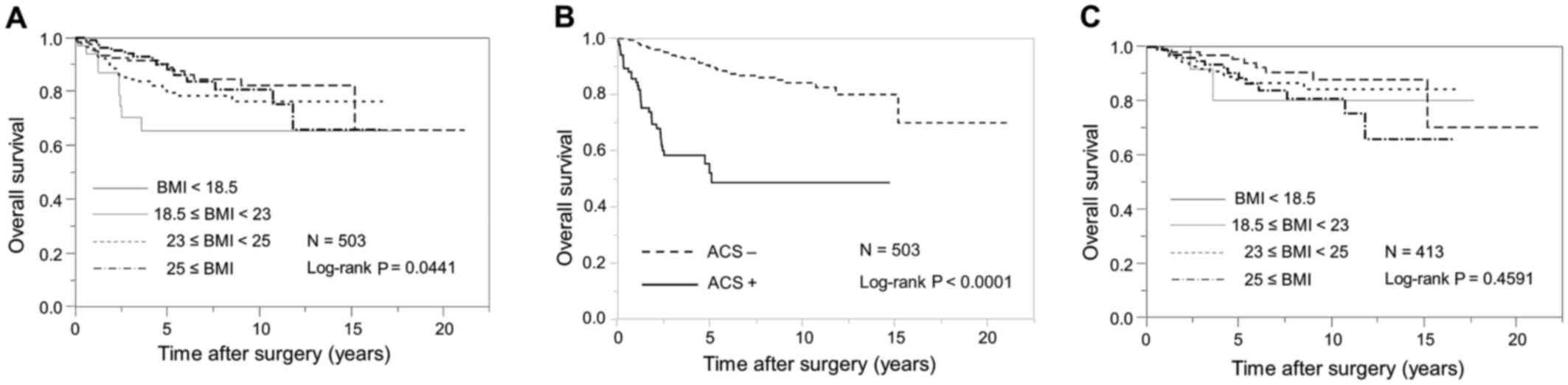
Kaplan-Meier curves for OS stratified according to WHO BMI
categories for Asian populations are demonstrated in Fig. 1A. Patients with a lower BMI had
significantly shorter OS than those with a higher BMI (log-rank,
P=0.0441).
 | Table II.Association between BMI and
clinicopathological parameters in all patients and in patients
without ACS. |
Table II.
Association between BMI and
clinicopathological parameters in all patients and in patients
without ACS.
|
| All patients | Patients without
ACS |
|---|
|
|
|
|
|---|
| Parameter | n | BMI, kg/m2 | P-value | n | BMI,
kg/m2 | P-value |
|---|
| Grade |
|
| 0.0027 |
|
| 0.0616 |
|
G1-2 | 328 |
23.4±3.57 |
| 301 |
23.6±3.58 |
|
| G3 | 175 |
22.5±3.39 |
| 112 |
23±3.45 |
|
| Pathological T
stage |
|
| 0.0789 |
|
| 0.5415 |
|
pT1-2 | 398 |
23.3±3.64 |
| 352 |
23.5±3.65 |
|
|
pT3-4 | 105 |
22.4±3.02 |
| 61 |
23.0±2.86 |
|
| Regional lymph node
involvement |
|
| 0.2167 |
|
| 0.7041 |
|
Negative | 488 |
23.1±3.52 |
| 405 |
23.5±3.56 |
|
|
Positive | 15 |
21.6±3.90 |
| 8 |
23.4±3.35 |
|
| Distant
metastasis |
|
| 0.0025 |
|
| 0.0807 |
|
Negative | 446 |
23.3±3.59 |
| 386 |
23.5±3.59 |
|
|
Positive | 57 |
21.9±2.78 |
| 27 |
22.4±2.81 |
|
| Venous
invasion |
|
| 0.7709 |
|
| 0.2139 |
|
Negative | 288 |
23.2±3.85 |
| 261 |
23.5±3.88 |
|
|
Positive | 215 |
22.9±3.06 |
| 152 |
23.5±2.92 |
|
| Growth pattern |
|
| 0.0453 |
|
| 0.6627 |
|
Expansive | 334 |
23.4±3.61 |
| 299 |
23.5±3.62 |
|
|
Infiltrative | 169 |
22.6±3.34 |
| 114 |
23.5±3.37 |
|
| ACS |
|
| <0.0001 |
|
| – |
|
Negative | 413 |
23.5±3.55 |
| – | – |
|
|
Positive | 90 |
21.5±2.98 |
| – | – |
|
Prognostic factors of RCC patients
without ACS
It is well known that patients with RCC and
accompanying ACS have a poor prognosis (14,15). In
the present study, the mean BMI of patients with ACS (21.5
kg/m2) was significantly lower than that of patients
without ACS (23.5 kg/m2; P<0.0001; Table II). OS was significantly shorter in
patients with ACS than it was in those without ACS (log-rank,
P<0.0001; Fig. 1B). On univariate
Cox proportional analysis, both low BMI and presence of ACS were
associated with shorter OS (P=0.0044 and P<0.0001, respectively;
Table III) and shorter CSS
(P=0.0081 and P<0.0001, respectively; Table III). Also significantly associated
with shorter OS and shorter CSS on univariate analysis were age
(P=0.0002 and P=0.0053, respectively), ECOG-PS (both P<0.0001),
pathological T stage (both P<0.0001), regional LN involvement
(both P<0.0001), distant metastasis (both P<0.0001), higher
tumor grade (both P<0.0001), infiltration pattern (both
P<0.0001) and venous invasion (both P<0.0001; Table III). On multivariate analysis, age
(P=0.0193), the presence of ACS (P=0.0089), pathological T stage
(P=0.0013), regional LN involvement (P=0.0129) and distant
metastasis (P<0.0001) were independent predictors of shorter OS;
however, BMI was not (P=0.5440). On multivariate analysis, the
presence of ACS (P=0.0308), pathological T stage (P=0.0011),
regional LN involvement (P=0.0062) and distant metastasis
(P<0.0001) were independent predictors of shorter CSS; however,
BMI was not (P=0.6804). Furthermore, the impact of BMI on
clinicopathological parameters in patients without ACS was also
analyzed. Although in overall patients BMI was significantly lower
in patients with aggressive forms of tumors (high grade, P=0.0027;
distant metastasis, P=0.0025; infiltrative growth pattern,
P=0.0453; Table II), in patients
without ACS there was no significant association between BMI and
any pathological parameters (Table
II). Additionally, BMI was not associated with OS in patients
without ACS (log-rank, P=0.4591; Fig.
1C).
 | Table III.Factors associated with shorter OS
and CSS in univariate and multivariate analysis. |
Table III.
Factors associated with shorter OS
and CSS in univariate and multivariate analysis.
|
| OS | CSS |
|---|
|
|
|
|
|---|
|
|
| Multivariate |
| Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | Univariate
P-value | HR | 95% CI | P-value | Univariate
P-value | HR | 95% CI | P-value |
|---|
| Sex
(females/males) |
0.0927 | – | – | – | 0.139 | – | – | – |
| Age (continuous
variables) |
0.0002 | 1.03 | 1.00–1.05 |
0.0193 |
0.0053 | 1.02 | 0.99–1.04 |
0.1958 |
| Eastern Cooperative
Oncology Group PS (PS2-4/PS0-1) | <0.0001 | 1.70 | 0.80–3.46 |
0.1611 | <0.0001 | 2.03 | 0.92–4.35 |
0.0766 |
| Body mass index
(continuous variables) |
0.0044 | 0.98 | 0.90–1.05 |
0.5440 |
0.0081 |
0.98 | 0.90–1.07 |
0.6804 |
| Anorexia-cachexia
syndrome (yes/no) | <0.0001 | 2.21 | 1.22–3.92 |
0.0089 | <0.0001 | 2.03 | 1.07–3.78 |
0.0308 |
| Pathological T
stage (pT3-4/pT1-2) | <0.0001 | 2.65 | 1.45–5.00 |
0.0013 | <0.0001 | 2.85 | 1.50–5.63 |
0.0011 |
| Regional lymph node
involvement (yes/no) | <0.0001 | 2.78 | 1.26–5.66 |
0.0129 | <0.0001 | 3.17 | 1.42–6.58 |
0.0062 |
| Distant metastasis
(yes/no) | <0.0001 | 7.47 | 4.25–13.2 | <0.0001 | <0.0001 | 7.46 | 4.09–13.8 | <0.0001 |
| Grade
(G3/G1-2) | <0.0001 | 1.49 | 0.85–2.66 |
0.1657 | <0.0001 | 1.44 | 0.78–2.72 |
0.2493 |
| Growth pattern
(infiltrative/expansive) | <0.0001 | 1.02 | 0.58–1.80 |
0.9557 | <0.0001 | 1.09 | 0.49–1.69 |
0.7781 |
| Venous invasion
(yes/no) | <0.0001 | 0.96 | 0.46–1.99 |
0.9084 | <0.0001 | 1.78 | 0.75–4.48 |
0.1949 |
| Histological type
(clear cell/other) |
0.1248 | – | – | – | 0.122 | – | – | – |
Most important constituent factor of
ACS associated with poor prognosis
We analyzed which constituent factor of ACS was more
important for the prediction of poor OS and CSS. On univariate Cox
proportional analysis, all factors were significantly associated
with shorter OS (all P<0.0001; Table
IV) and shorter CSS (all P<0.0001; Table IV). On multivariate analysis, the
presence of weight loss was the only significant predictor of
shorter OS [P=0.0004; hazard ratio (HR), 8.34; 95% confidence
interval (CI), 2.38–28.9] and shorter CSS (P=0.0009; HR, 8.26; 95%
CI, 2.21–30.5).
 | Table IV.Constituent factors of
anorexia-cachexia syndrome associated with shorter OS and CSS in
univariate and multivariate analysis. |
Table IV.
Constituent factors of
anorexia-cachexia syndrome associated with shorter OS and CSS in
univariate and multivariate analysis.
|
| OS | CSS |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Weight loss | 11.8
(6.99–19.5) | <0.0001 | 8.34
(2.38–28.9) | 0.0004 | 12.5
(7.13–21.2) | <0.0001 | 8.26
(2.21–30.5) | 0.0009 |
|
Hypoalbuminemia | 3.86
(2.29–6.31) | <0.0001 | 1.53
(0.78–2.90) | 0.2111 | 4.11
(2.36–6.92) | <0.0001 | 1.60
(0.79–3.16) | 0.1876 |
| Anorexia or
malaise | 8.37
(4.97–13.7) | <0.0001 | 1.12
(0.30–4.04) | 0.8721 | 8.92
(5.12–15.0) | <0.0001 | 1.16
(0.29–4.50) | 0.8366 |
Discussion
BMI has routinely been used as a convenient index of
obesity in several studies, and it has been suggested that
increased BMI is associated not only with increased risk of various
malignant neoplasms, but also with poor survival (2,3,6,7).
Although obesity has been reported to increase the risk of RCC, a
higher BMI is paradoxically associated with improved survival
following nephrectomy (10–13). Some studies in the United States have
demonstrated that patients who are overweight (25≤BMI<30
kg/m2) or obese (BMI≥30 kg/m2) are less
likely to present features of aggressive tumors (9,13). In
addition, a study by Haferkamp et al (10) indicated that, in Europe, being
underweight (BMI<18.5 kg/m2) at the time of
nephrectomy worsened the prognosis of patients with RCC more than
four-fold. This tendency is not limited to the Western world, but
is also seen in Asian populations (11,12). In
accordance with previous studies, the present study demonstrated
that a low BMI was significantly associated with increased tumor
aggressiveness and poor survival. The relationship between low BMI
and increased tumor aggressiveness and poor survival, however, was
inconsistent with the relationship between obesity and increased
risk of RCC, and the underlying mechanism remains unknown.
Low BMI in cancer patients could be explained by
several mechanisms. One is decreased weight due to cancer-related
ACS. Several studies have indicated ACS to be a strong predictor of
poor prognosis in RCC (14,15). ACS is one of the paraneoplastic
symptoms frequently observed in patients with RCC and is caused by
increased secretion of various cytokines and growth factors from
cancer cells, among which are interleukin-6, vascular endothelial
growth factor and platelet-derived growth factor (20–22). The
present study demonstrated that there was also a significant
association between low BMI and the presence of ACS, and that ACS
was an independent predictor of poor OS and poor CSS; however, it
also demonstrated that BMI was not an independent predictor of
survival. In addition, in patients without ACS, no significant
association was indicated between BMI and pathological parameters
and clinical outcome. These results suggested that the impact of
low BMI on aggressive clinicopathological parameters and poor
clinical outcome in patients with RCC could be due to ACS.
Haferkamp et al (10)
conjectured that their finding of being underweight to be a poor
predictor of RCC was partially due to cachexia. Their findings are
consistent with the present results.
The field of obesity has moved beyond simple
measurement of BMI, and the association of nutrition and body
composition with prognosis in RCC is an area of contemporary
interest (23). Although BMI is a
simple and useful parameter of obesity, it does not necessarily
reflect excessive adiposity because it is influenced by the amounts
of both muscle and fat (23). The
body fat distribution determined by measuring the visceral fat area
(VFA) and the subcutaneous fat area by computed tomography (CT) has
been used to assess adiposity not only in screening for
cardiovascular events and metabolic syndrome, but also in
monitoring clinical outcome in various types of cancer (24,25).
Visceral and subcutaneous fat have quite different properties in
terms of metabolic activity, sensitivity to lipolysis and
insulin-resistance (26). In
particular, increased visceral fat deposition is strongly
associated not only with increased risk of hyperglycemia,
hyperinsulinemia and cardiovascular events, but also with increased
risk of breast and colorectal cancer (26–28). The
relationship between VFA and clinicopathological parameters and
clinical outcome in RCC patients has also been investigated;
however, the clinical significance of CT-estimated VFA in
predicting clinicopathological parameters remains controversial
(23). The mechanism of
cancer-related ACS in patients with aggressive cancer is closely
related both to body composition and nutrition. Research has
demonstrated that ACS is characterized by preferential loss of
adipose tissue and that in progressive ACS, body fat is lost more
rapidly than lean tissue (29,30). The
clinical value of fat distribution pattern in predicting RCC
progression may be improved by combining the pattern with the
result of ACS assessment, which should be elucidated in future
research.
The present study had some limitations that must be
acknowledged. Firstly, the definition of ACS may include the impact
of factors such as poor psychological health, low physical activity
and low socioeconomic status (31).
The present study used hypoalbuminemia as an index of the
malnutrition in ACS; however, in the evaluation of malnutrition
there are individual differences depending on age, sex and previous
medical history. Secondly, the Asian body composition profile
differs from that of other races (19), and the present study used a
population comprised only of Japanese patients. Therefore, the BMI
of patients in the present study may have been different from that
of patients in most Western countries, and we would suggest that
this underlying influence, that is cancer-related ACS, applies only
to Asian populations. Thirdly, the patient selection was biased as
the present study was a retrospective and single-hospital
study.
Despite these limitations, in conclusion, the
results of the present study demonstrated that BMI is not
associated with tumor aggressiveness and prognosis of RCC when
patients with ACS are excluded, and that the previously reported
association between low BMI and poor prognosis of RCC could be due
to ACS. These results should be validated in a prospective
multi-institutional study conducted in Asian nations.
References
|
1
|
Ng M, Fleming T, Robinson M, Thomson B,
Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF,
et al: Global, regional, and national prevalence of overweight and
obesity in children and adults during 1980–2013: A systematic
analysis for the global burden of disease study 2013. Lancet.
384:766–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman JM: Obesity: Causes and control
of excess body fat. Nature. 459:340–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bjorge T, Tretli S and Engeland A:
Relation of height and body mass index to renal cell carcinoma in
two million Norwegian men and women. Am J Epidemiol. 160:1168–1176.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pischon T, Lahmann PH, Boeing H,
Tjønneland A, Halkjaer J, Overvad K, Klipstein-Grobusch K,
Linseisen J, Becker N, Trichopoulou A, et al: Body size and risk of
renal cell carcinoma in the European prospective investigation into
cancer and nutrition (EPIC). Int J Cancer. 118:728–738. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bardou M, Barkun AN and Martel M: Obesity
and colorectal cancer. Gut. 62:933–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cantarutti A, Bonn SE, Adami HO, Grönberg
H, Bellocco R and Bälter K: Body mass index and mortality in men
with prostate cancer. Prostate. 75:1129–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schips L, Lipsky K, Zigeuner R, Gidaro S,
Salfellner M, Rehak P, Pummer K and Hubmer G: Does overweight
impact on the prognosis of patients with renal cell carcinoma? A
single center experience of 683 patients. J Surg Oncol. 88:57–62.
2014. View Article : Google Scholar
|
|
9
|
Parker AS, Lohse CM, Cheville JC, Thiel
DD, Leibovich BC and Blute ML: Greater body mass index is
associated with better pathologic features and improved outcome
among patients treated surgically for clear cell renal cell
carcinoma. Urology. 68:741–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haferkamp A, Pritsch M, Bedke J, Wagener
N, Pfitzenmaier J, Buse S and Hohenfellner M: The influence of body
mass index on the long-term survival of patients with renal cell
carcinoma after tumour nephrectomy. BJU Int. 101:1243–1246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Awakura Y, Nakamura E, Ito N, Yamasaki T,
Kamba T, Kamoto T and Ogawa O: Influence of body mass index on
prognosis of Japanese patients with renal cell carcinoma. Urology.
70:50–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi Y, Park B, Jeong BC, Seo SI, Jeon SS,
Choi HY, Adami HO, Lee JE and Lee HM: Body mass index and survival
in patients with renal cell carcinoma: A clinical-based cohort and
meta-analysis. Int J Cancer. 132:625–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hakimi AA, Furberg H, Zabor EC, Jacobsen
A, Schultz N, Ciriello G, Mikklineni N, Fiegoli B, Kim PH, Voss MH,
et al: An epidemiologic and genomic investigation into the obesity
paradox in renal cell carcinoma. J Natl Cancer Inst. 105:1862–1870.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HL, Belldegrun AS, Freitas DG, Bui MH,
Han KR, Dorey FJ and Figlin RA: Paraneoplastic signs and symptoms
of renal cell carcinoma: Implications for prognosis. J Urol.
170:1742–1746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HL, Han KR, Zisman A, Figlin RA and
Belldegrun AS: Cachexia-like symptoms predict a worse prognosis in
localized t1 renal cell carcinoma. J Urol. 171:1810–1813. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolf I, Sadetzki S, Kanety H, Kundel Y,
Pariente C, Epstein N, Oberman B, Catane R, Kaufman B and Shimon I:
Adiponectin, ghrelin, and leptin in cancer cachexia in breast and
colon cancer patients. Cancer. 106:966–973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
WHO Expert Consultation, . Appropriate
body-mass index for Asian populations and its implications for
policy and intervention strategies. Lancet. 363:157–163. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horiguchi A, Oya M, Marumo K and Murai M:
STAT3, but not ERKs, mediates the IL-6-induced proliferation of
renal cancer cells, ACHN and 769P. Kidney Int. 61:926–938. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding GX, Feng CC, Song NH, Fang ZJ, Xia
GW, Jiang HW, Hua LX and Ding Q: Paraneoplastic symptoms: Cachexia,
polycythemia, and hypercalcemia are, respectively, related to
vascular endothelial growth factor (VEGF) expression in renal clear
cell carcinoma. Urol Oncol. 31:1820–1825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oya M: Renal cell carcinoma: Biological
features and rationale for molecular-targeted therapy. Keio J Med.
58:1–11. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YH, Lee JK, Kim KM, Kook HR, Lee H,
Kim KB, Lee S, Byun SS and Lee SE: Visceral obesity in predicting
oncologic outcomes of localized renal cell carcinoma. J Urol.
192:1043–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fox CS, Massaro JM, Hoffmann U, Pou KM,
Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB,
Cupples LA, et al: Abdominal visceral and subcutaneous adipose
tissue compartments: Association with metabolic risk factors in the
framingham heart study. Circulation. 116:39–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doyle SL, Donohoe CL, Lysaght J and
Reynolds JV: Visceral obesity, metabolic syndrome, insulin
resistance and cancer. Proc Nutr Soc. 71:pp. 181–189. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ibrahim MM: Subcutaneous and visceral
adipose tissue: Structural and functional differences. Obes Rev.
11:11–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schapira DV, Clark RA, Wolff PA, Jarrett
AR, Kumar NB and Aziz NM: Visceral obesity and breast cancer risk.
Cancer. 74:632–639. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh TH, Byeon JS, Myung SJ, Yang SK, Choi
KS, Chung JW, Kim B, Lee D, Byun JH, Jang SJ and Kim JH: Visceral
obesity as a risk factor for colorectal neoplasm. J Gastroenterol
Hepatol. 23:411–417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dahlman I, Mejhert N, Linder K, Agustsson
T, Mutch DM, Kulyte A, Isaksson B, Permert J, Petrovic N,
Nedergaard J, et al: Adipose tissue pathways involved in weight
loss of cancer cachexia. Br J Cancer. 102:1541–1548. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fouladiun M, Körner U, Bosaeus I, Daneryd
P, Hyltander A and Lundholm KG: Body composition and time course
changes in regional distribution of fat and lean tissue in
unselected cancer patients on palliative care-correlations with
food intake, metabolism, exercise capacity, and hormones. Cancer.
103:2189–2198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ali SM and Lindström M: Socioeconomic,
psychosocial, behavioural, and psychological determinants of BMI
among young women: Differing patterns for underweight and
overweight/obesity. Eur J Public Health. 16:325–331. 2006.
View Article : Google Scholar : PubMed/NCBI
|